Eye movement indices in the study of depressive disorder
2016-12-09YuLIYangyangXUMengqingXIATianhongZHANGJunjieWANGXuLIUYongguangHEJijunWANG
Yu LI, Yangyang XU, Mengqing XIA, Tianhong ZHANG, Junjie WANG, Xu LIU, Yongguang HE*,Jijun WANG*
Eye movement indices in the study of depressive disorder
Yu LI1, Yangyang XU1, Mengqing XIA1, Tianhong ZHANG1, Junjie WANG1, Xu LIU2, Yongguang HE1*,Jijun WANG1*
depression disorder; eye movement; saccade
1. Introduction
Depressive disorder is a kind of mental disorder which can be caused by a number of factors, and that mainly presents as a loss of functioning with prominent depressed mood. In recent years, depressive disorder has caused the most social burden amongst all the neural and mental diseases;[1]but its pathogenesis and objective diagnostic methods are still not clear.Eye movements can be measured with eye-tracking devices. The advantage with these devices is that they are easy to operate and save time. Also inhibition function, attention and other cognitive activities can be studied with eye movements through simple paradigms.Therefore, more research studying biological markers and the pathogenesis of mental disorders uses eye tracking. The common simple eye movement tasks used in studying mental disorders include the antisaccade task, the free-view task, the fixation task and so forth. Most previous eye-tracking studies into depressive disorder focused on the anti-saccade task,and they found that patients with depressive disorder performed worse in the anti-saccade task than did controls, which presented as difficulties in inhibiting reflex jumps[2]and increases in anti-saccade reaction time.[3]Hence, some scholars have suggested that the anti-saccade task performance can be used as a state biomarker for depressive disorder.[4]In the depressive disorder studies conducted by Kellouh and colleagues,it was indicated that patients had abnormal freeview processes with longer fixation duration as the most obvious abnormality.[5]However, there is still relatively little research about the fixation stability of patients with depressive disorder. Previous eye tracking studies of depressive disorders mostly focused on a certain single eye movement task, and the course of disease, medication and other patient conditions differed between studies. Therefore, whether or not individuals with depressive disorder have abnormal eye movement functions requires further exploration, and the relationship between these eye movement indices and symptoms also deserves further research. The present study used three simple eye movement tasks to examine eye movement features in a group of patients with depressive disorder, and analyzed the relationship between these eye movement indices and patients clinical symptoms scores. This study used eye tracking tests to provide further evidence in the ongoing search of an objective biomarker for depressive disorder.
2. Methods
2.1 Participants
The depression disorder group was made up of patients with depressive disorder were recruited at the outpatient clinic of the Shanghai Mental Health Center from September 2015 to April 2016. Inclusion criteria were the following: (a) meeting DSM-IV[6]diagnostic criteria for depressive disorder; (b) aged between 15 to 45 years old;(c) total score on the Hamilton Depression Scale (HAMD)[7]was above 8; (d) current episode was patients’ first episode of depression and they had never taken medication; (e) patients were right handed. Exclusion criteria were the following: (a)patients had comorbidity with another mental disorder,including disorders listed in DSM-IV Axis I criteria and mental retardation; (b) being ruled out due to antisocial personality disorder after conducting Mini-International Neuropsychiatric Interview (MINI); (c) individuals with organic brain disease, nervous system disease, severe endocrine disease or metabolic disease;(d) people who have taken medication which could affect mental and cognitive functions; (e) those who previously or currently have a nervous system disorder that could cause a depressive disorder or impair their judgment; (f)those who have taken anti-depressants; (g) those with poor vision due to an eye disease. In total we collected and carried out analysis on data from 60 patients.
The control group was made up of volunteers who were recruited from around Shanghai via posted advertisements, the internet, and using the social media app Wechat. The control group and depressive disorder group were matched for gender, age (15-45)and educational level. In addition, participants were evaluated by trained psychiatrists using the MINI in order to rule out any other mental disorders. All other inclusion/exclusion criteria were the same as those for the depression disorder group. All healthy controls volunteered to participate in this study. In total, the eye movement data for 60 participants in the control group was collected and analyzed. All participants in both the control and study group provided written informed consent to participate in this study (for those participants under the age of 18, informed consent was provided by their parents or guardians). This study was approved by the Institution Review Board of the Shanghai Mental Health Center.
2.2 Methods
2.2.1 Clinical evaluation
Psychiatrists from our group conducted MINI interviews,and used HAMD to assess depressive symptoms. In addition, the Hamilton Anxiety Scale (HAMA)[9]was used to evaluate anxiety symptoms.
2.2.2 Experiment tasks:
(1) Fixation stability task: before the task began,participants fi xed their eyes on a black cross “+” in the center of the white screen. After the task began, they were instructed to continue looking at the black dot(the size was 0.50) in the middle of the white screen during the whole process, and ignore the disturbances of “*” and other marks on the side. Within 5000ms after the task began, there was only the central black solid dot on the screen. But between the 5000ms and 10000ms after the task began, “*” interference marks blinked on the left or right side of the solid dot in the 60and 120positions for a certain period of time. There were 10 trials in total. The positions of the interference stimulations were randomized so that the participants could not predict them. (2) The saccade task:participants first fixed their eyes on the black fixationpoint “+” in the center of the white screen. When the target stimulation (a solid dot or a hollow dot) appeared(at the same time, “+” disappeared), the participants were instructed to look at the target as fast and accurately as possible (if the target was a solid dot), or look at the opposite side of the target (if the target was a hollow dot). The duration of the target presentation was 1000ms, and the size was 0.50. The angles of presentation were 60and 120on the left or right. There were 8 pro-saccades and 8 anti-saccades in total. The type of targets, duration and angle were all randomized.(3) The free-view task: participants observed the pictures freely under the experiment conditions. There were 35 black and white pictures serving as stimulation,including pictures of nature, social situations, still objects and meaningless images (e.g. arrayed lines,blurred noise, etc.). All pictures were presented in a random order with a duration of 10s. There were white screens with a black cross “+” in the center between pictures, and the participants were instructed to fi x their eyes on the black cross “+” during this period of time.The total duration of three tasks was 15 minutes. All participants completed the eye movement tasks in the same order: fixation task-saccade, and then task-freeview task.
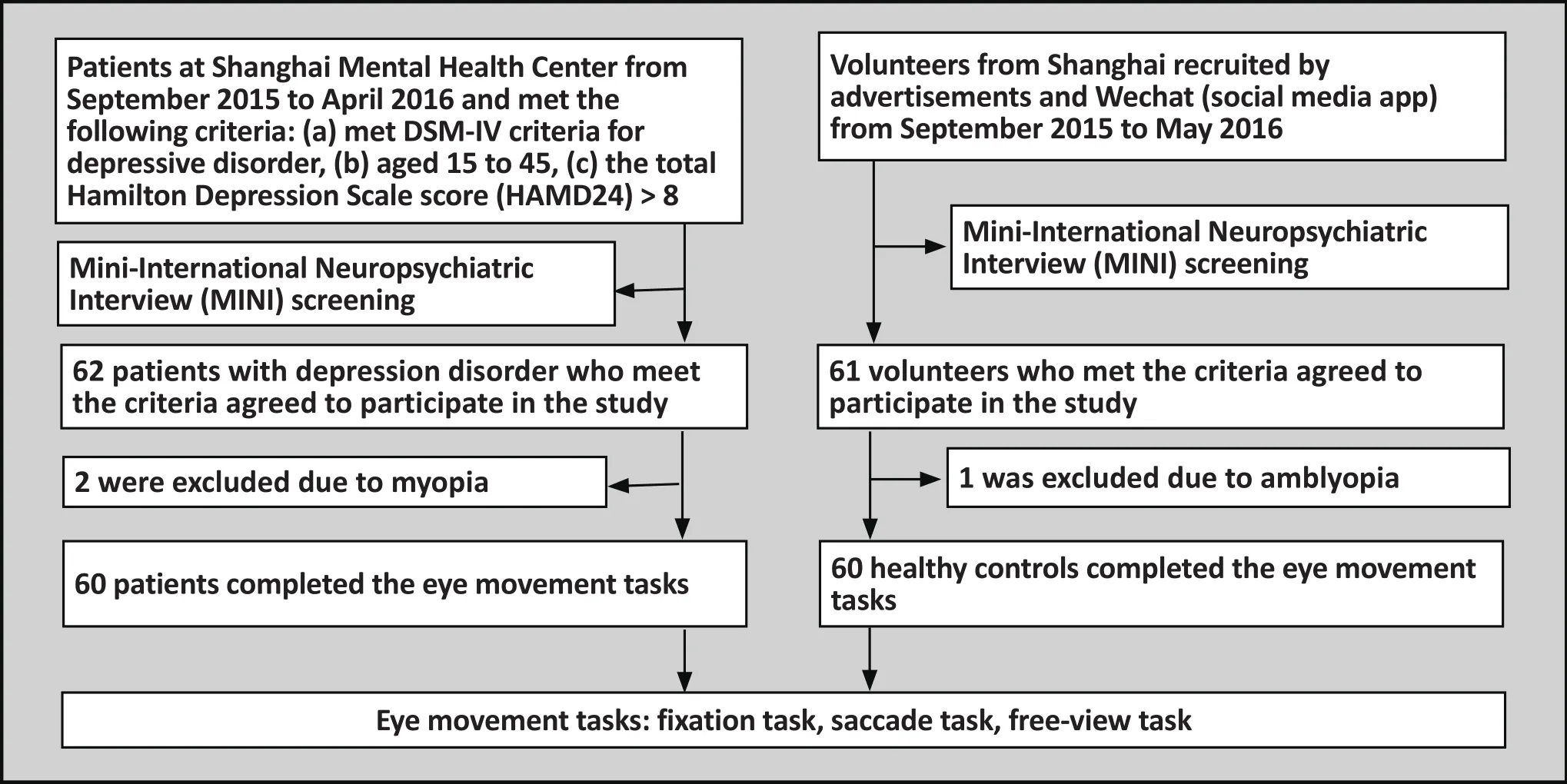
Figure 1. Flow chart of the study
2.2.3 Eye movement data collection equipment
Eye movements were examined and recorded automatically using the Canadian SR Research Limited Co.’s EYELINK 200 desktop eye tracking device for highspeed infrared pupil tracking imaging and corneal ref l ection model. Participants were seated in a speciallydesigned chair with adjustable height. Their eyes were at the same height as the center of the screen, and they were 50cm away from the screen. They placed their heads on the special jaw holder so that eye movements in the horizontal (±30o)and vertical (±30o) positions could be tracked. The screen size was 17 inches and the resolution rate was 1024*768 pixels. The current study collected data from the dominant eyes with the 9-point calibration mode. The program used in the experiment was written with Experiment Builder –visualized experiment design software, and the data were analyzed with Data Viewer.
2.3 Data analysis
SPSS version 19.0 statistics software package was used in the data analysis. For the no disturbance and disturbance conditions, the mean fi xation times, mean fixation duration, mean saccade times and mean saccade path were analyzed for each test. The saccade latency, saccade duration, saccade amplitude, peak saccade velocity and the mean saccade velocity were analyzed for both pro-saccades and anti-saccades in the saccade task. The fi xation times (i.e., the mean number of fi xation times in each picture), mean fi xation duration(i.e., the mean fi xation duration for each fi xation point),saccade times (i.e., the mean saccade times for each picture), saccade amplitude (i.e. the mean amplitude of each saccade), fixation duration (i.e. the total fixation duration for each picture) and saccade path (i.e. the mean length of the saccades for each picture) were analyzed in the free-view task. The measurement unit of duration was millisecond (ms). Degree (0) was employed to measure saccade lengths, and the unit of measurement of velocity was degree/second (0/s). Independent sample t-test was applied to compare the means of fixation stability task and saccade task.Because blinking could affect the free-view task more,blinking time was used as a covariant in the covariant analysis to compare the means. Independent sample t-test was used for the between-group comparison of quantitative demographic data and chi squared was used for between group comparison of the enumeration data. Pearson correlation analysis and partial correlation analysis were conducted on the HAMD scores and HAMA scores with eye movement indexes respectively,and the two-tailed test’s signif i cance level α was 0.05.
3. Results
3.1 Clinical data
There were no statistically significant differences in the age, gender or education level between the 60 participants with depressive disorder and the 60 control group participants. Table 1 shows the clinical demographic data and clinical symptoms rating scores.
3.2 Fixation stability task analysis
The results of independent sample t-test indicated that compared with controls, patients with depressive disorder showed more fixation times (7.4[3.4] vs.6.1[2.6], t= -2.23, p=0.028), shorter fixation durations(6195.2[1292.8] vs. 6806.7[1118.2], t=2.70, p=0.008),more saccade times (6.5(3.5) vs. t=-2.10, p=0.038)and longer saccade paths in the no disturbance tasks(11.6[13.6] vs. 5.3[4.5], t=-3.41, p=0.001), and the differences were all statistically significant. Similarly,in the disturbance tasks, patients with depressive disorder also showed more fixation times (7.5[3.4] vs.6.1[2.5], t=-2.53, p=0.013), shorter fixation durations(5728.0[1172.1] vs. 6151.6[1043.9], t=2.09, p=0.039),more saccade times (6.6[3.5] vs. 5.2[2.5], t=-2.57,p=0.011) and longer saccade paths (10.2[8.4] vs.6.1[4.9], t=-3.27, p=0.001), and the differences were statistically significant. Table 2 shows the results of fi xation stability tasks.
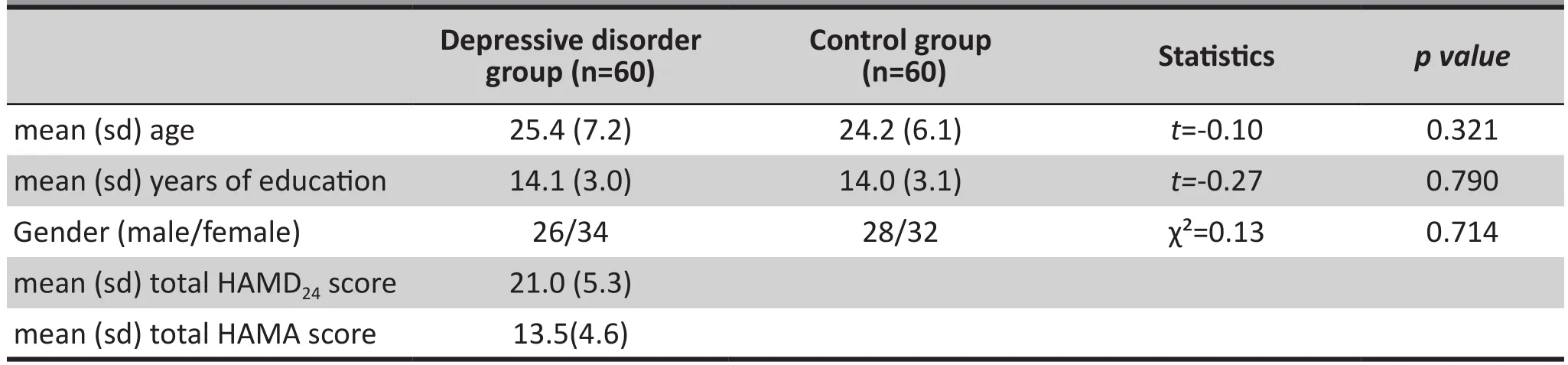
Table 1. Clinical demographic data and clinical symptoms ratings
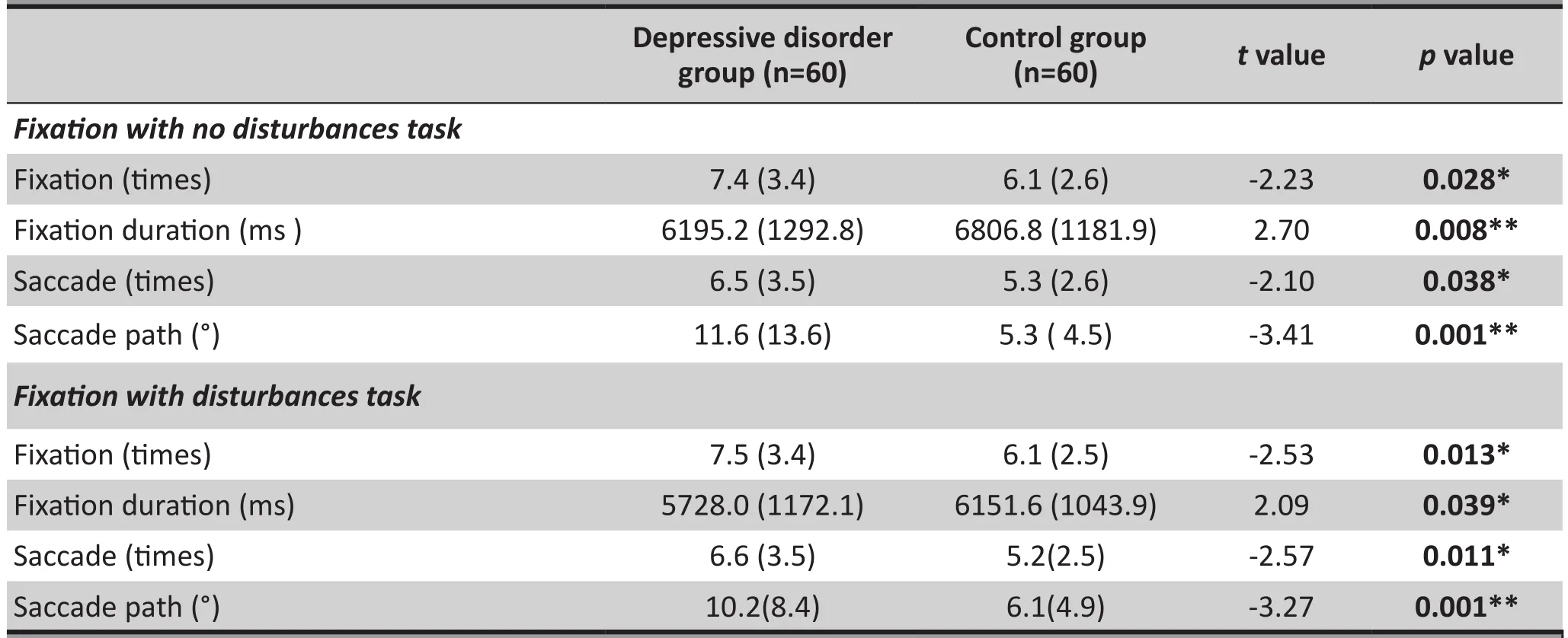
Table 2. Results of the fi xation stability task (mean [sd])
3.3 Saccade task analysis
Results of the independent sample t-test showed that for patients with depressive disorder there were no signif i cant differences, when compared with controls, in pro-saccade latency, pro-saccade duration, pro-saccade amplitude, peak pro-saccade velocity and mean prosaccade velocity in the pro-saccade task. However, in the anti-saccade task, patients with depressive disorder showed significantly longer anti-saccade latencies(553.5[108.5] vs. 491.2[49.0], t=-2.75, p=0.007) and smaller peak anti-saccade velocity (334.2[77.6] vs.381.7[83.3], t=3.23, p=0.002). There were no statistically signif i cant differences in the anti-saccade duration, the anti-saccade amplitude and the mean anti-saccade velocity between the two groups. Table 3 shows the results of the saccade task.
3.4 Free-view task analysis
Compared with the control group, patients with depressive disorder showed less saccade times(25.0[4.55] vs. 26.3[3.6], F=3.97, df=118, p=0.049)and longer mean fixation duration (322.2[67.8] vs.308.4[50.8], F=5.98, df=118, p=0.016) in the free-view task, and the results were statistically significant. In contrast, the fixation times, saccade amplitude and saccade path were not significantly different between the two groups. Table 4 shows the results of the freeview task.
3.5 Eye movement indices relationship with depression and anxiety
According to the results of correlation analyses for eye movement indices on the fixation stability taskand the saccade task, HAMD scores and HAMA scores,the pro-saccade amplitude was negatively correlated with HAMA scores (r=-0.232, p=0.045), and the antisaccade latency was positively correlated with HAMA scores (r=0.316, p=0.014). The results of the partial correlation (with blinking as the control factor) for the eye movement indices in the free-view task with HAMD scores and HAMA scores indicated that fi xation time, saccade time and saccade path were negatively correlated with HAMD scores (r=-0.362, p=0.005; r=-0.358, p=0.005; r=-0.384, p=0.003); while the mean fixation duration was positively correlated with HAMD scores (r=0.343, p=0.008). The remaining indices did not show any statistically significant correlations (all were p>0.05).
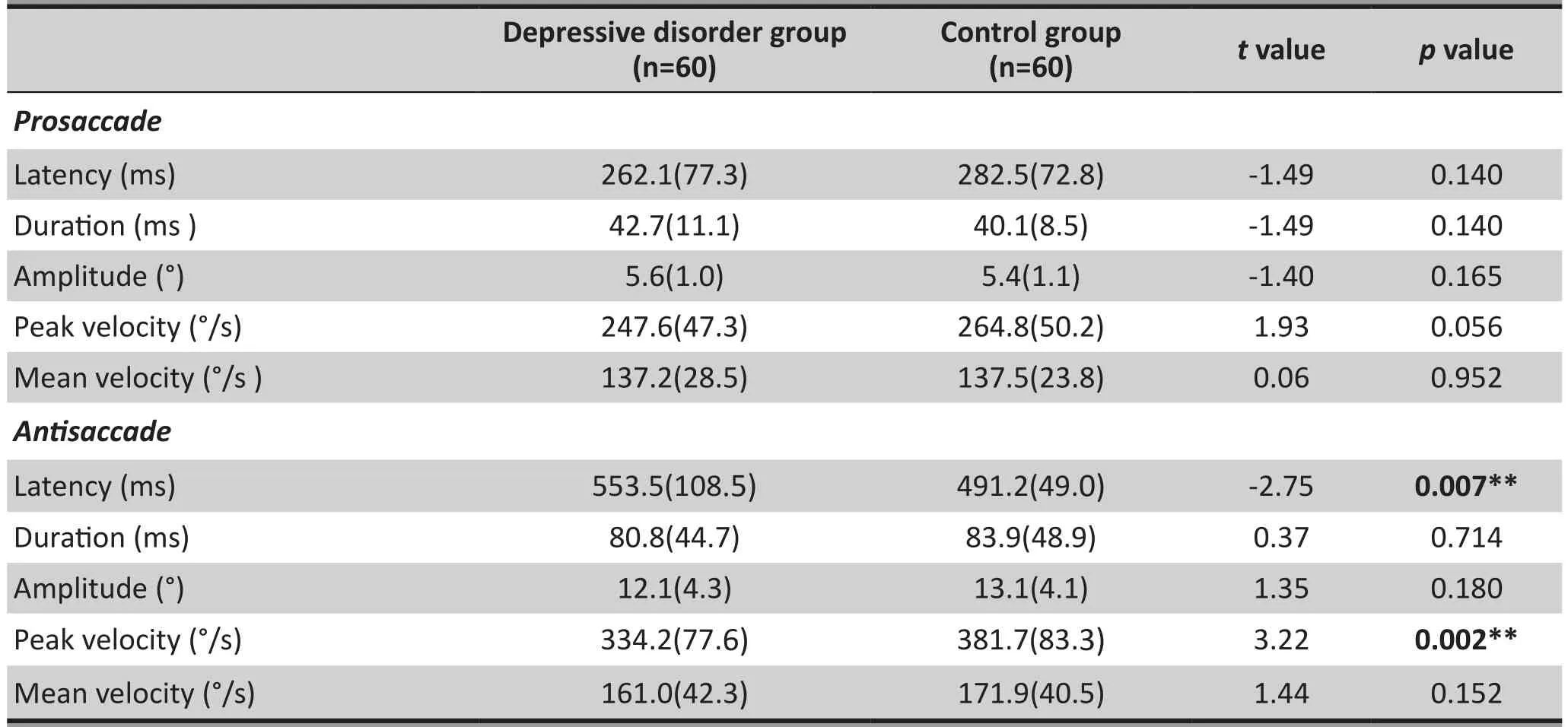
Table 3. Results of the saccade task (mean [sd])
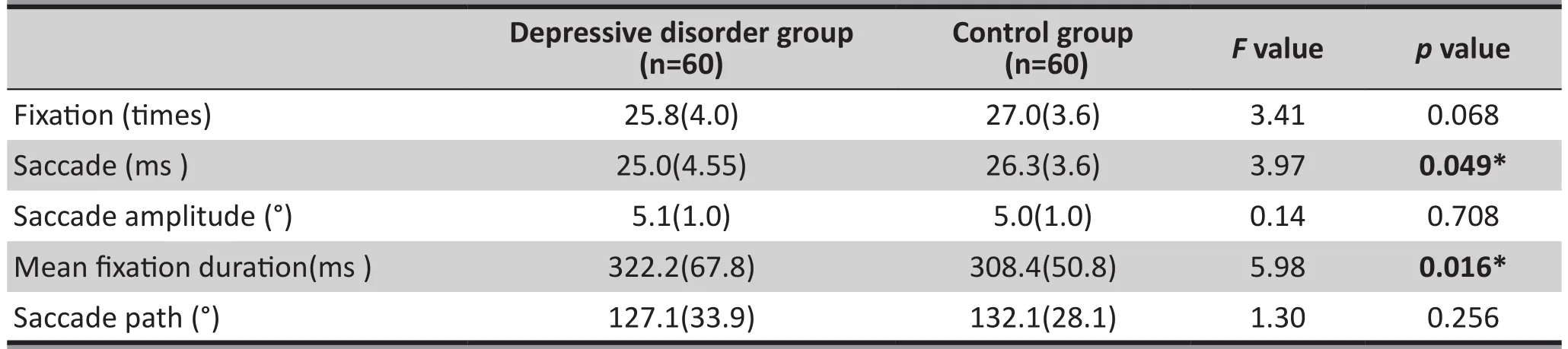
Table 4. Results of the free-view task (mean [sd])
4. Discussion
4.1 Main findings
The present study employed three simple eye movement tasks (the fi xation stability task, the saccade task, the free-view task) to compare the differences in eye movements between patients with depressive disorder and healthy controls. The results indicate that patients with depressive disorder have abnormal eye movements when compared to non-depressed controls. Those with depressive disorder showed poorer fixation stability than healthy controls. In the saccade task, depression group participants’ antisaccade latencies were longer, and the peak velocity was smaller, suggesting that the abnormal brain circuits related to anti-saccade led to impairment in eye movement inhibition functioning. Similarly, the fi xationtimes, saccade times and mean fi xation duration were abnormal in the free-view task as well. The pro-saccade amplitude and the anti-saccade latency were correlated with anxiety symptoms; while the free-view task was correlated with depression symptoms.
The saccade task can examine and reflect individuals’ cognitive inhibition function.[10]Cognitive inhibition is referred to as a restrictive effect that an individual’s cognitive status has, which adversely influences current cognition and problem solving. The pro-saccade task requires participants to pay attention to new stimuli that occur; while the anti-saccade task requires participants to ignore new stimuli, which inhibits the pro-saccade reflex.[11]Based on the results of the present study, those with depression showed longer latencies and smaller peak velocity in the antisaccade task, which suggests that they needed moretime to trigger the inhibition function and more time to coordinate the transaction from the ref l ective process to the inhibition process. This is indicative of impairment in cognitive inhibition. The results of this study illustrate that advanced cognitive inhibition in patients with depressive disorder can cause difficulties in excluding and processing interference information.[12]Previous research has shown that the impairment in the cognitive inhibition function is correlated with suicide risk in those with depressive disorder, and it is also likely correlated with the motives for suicide and the final decision to commit suicide.[13]In addition, rTMS intervention studies have indicated that after rTMS treatment to the left brain area DLPFC (which is closely related to antisaccades) for 20 days, patients with depression showed improvements not only in mood but also in cognitive functioning.[14]Carvalho and colleagues found that those with depressive disorder showed prolonged latencies in the pro-saccade task.[3]However, in the current study,patients with depressive disorder showed no differences in the pro-saccade task performance when compared with controls. Discrepancies between these results could be attributed differences in sample size, age of participants and participants severity of symptoms in the two studies. It is possible that abnormality of saccade tasks could serve as a status index or feature index for mood disorders. Future studies can continue to explore the pathogenesis of depressive disorder.The fi xation stability task requires participants to stably fix their eyes on a fixed point on the screen, and to inhibit the possible pro-saccades and remain staring at the fixed point when interference factors appear. The fixation stability task can reflect the inhibition control function of the brain more easily[15]; and the ability for high spatial frequency detail recognition reflected by this task can affect people’s cognition and recognition of things. Currently, there is little research about fixation stability for those with depressive disorder. Based on the studies about the fi xation stability of patients with schizophrenia, it was found that their fixation stability is vastly different from normal people’s[16], and it has been suggested that this abnormality could be a special feature of schizophrenia.[17]The results of the present study show that patients with depressive disorder showed poorer fixation stability than normal controls,indicating that the abnormal fi xation stability is not only seen in schizophrenia[18], but that those with depressive disorder may share similarities in abnormal neural circuitry. Some scholars believe that the impairments in brain inhibition function suggest that individuals may also have attention def i cit, working memory def i cit and motivation attenuation[17]; and indeed these def i cits are clearly displayed as symptoms of depressive disorder.
The fixation and the saccade tasks constitute the free-view process; the details are obtained by fi xations,and the saccades reflect the visual orientation and eye movement control ability.[19]The present study indicates that patients with depressive disorder show longer mean fixation durations and less saccades,suggesting that within the same period of time, those with depressive disorder capture less information than normal controls, that their relevant brain areas in the front lobe process information more slowly than normal controls’[20], and that their ability to process the physical properties and sensation features of images is different from the normal controls’.[21]Many studies have used positive and negative image content testing to explore how those with depressive disorder process emotional images[22], and they have found differences in how emotional content is process between those with depression and those with bipolar disorder.[23,24]Patients with different mental disorders apply different strategies in the free-view task. Research has shown that those with schizophrenia, depressive disorder and bipolar disorder show different attention preferences and fi xation strategies in the free-view task, suggesting that the capturing and processing of information are different in different disorders. Therefore, the free-view task deserves further exploration.
In the present study, the correlation analyses showed that pro-saccade and anxiety symptoms were negatively correlated, while anti-saccade and anxiety symptoms were positively correlated. This suggests that the more anxious patients are, the worse their inhibition control functions are. Besides this, fixation times,saccade times and saccade path in the free-view task are negatively correlated with depressive symptoms,while the mean fi xation duration is positively correlated with depressive symptoms. This indicates that the abnormality ref l ected in the free-view task is correlated with the severity of depressive symptoms. Previous research has also indicated that depressive symptoms and free-view processes improve after successful treatment of depression[25], which supports the results of the present study.
4.2 Limitations
The present study has several limitations. First of all, the design of the tasks should have been more pertinent by including content related to the emotions of depressive disorder. Secondly, future research needs to include more cohort studies which make it possible to longitudinally observe the dynamic changes of eye movements of patients with depressive disorder.Besides these, in order to explore the specificity and sensitivity of abnormal eye movements on depression,future studies should include comparison groups with other mental disorders.
4.3 Implications
The present study used the fixation stability task, the saccade task and the free view task and found that patients with depressive disorder had abnormalities in their eye movements. These eye tracking tests examine cognitive function, psychomotor function and reaction movement inhibition function, and can also evaluate visual exploration ability, spatial working memory, and emotional reaction inhibition ability.[26]Eye movement studies about depression can explore the pathogenesis,clinical biomarkers, and the methods used to clinically distinguish depression from other mental disorders.[27,28]
Funding
National Clinical Key Specialty Construction Project(2014; 2011873); Shanghai Mental Health Centre International Collaboration Project (2013-YJGJ-05)
Conflict of interest
The authors declared no conflict of interest related to this manuscript.
Informed consent
All participants and their legal guardians provided signed informed consent to participate in this study.
Ethics approval
The study was approved by the Ethics Committee of Shanghai Mental Health Centre.
Author contributions
Li Yu completed the first draft. Wang Jijun and He Yongguang designed the study and helped with this revision of the first draft. Li Yu and Xia Mengqing participated in the recruiting and evaluation of participants. Li Yu, Xu Yangyang and Zhang Tianhong analyzed the data. Liu Xu and Wang Junjie designed and wrote the data analysis. All authors read and agreed upon the final version of this article.
Reference:
1 Collins PY, Insel TR, Chockalingam A, Daar A, Maddox YT.Grand challenges in global mental health: integration in research, policy, and practice. PLoS Med. 2013; 10(4):e1001434. doi: http://dx.doi.org/ 10.1371/journal.pmed.1001434
2 Harris MS, Reilly JL, Thase ME,Keshavan MS, Sweeney JA.Response suppression deficits in treatment-naive firstepisode patients with schizophrenia, psychotic bipolar disorder and psychotic major depression. Psychiatry Res.2009; 170(2-3): 150-156. doi: http://dx.doi.org/ 10.1016/j.psychres.2008.10.031
3 Carvalho N, Noiret N, Vandel P, Monnin J, Chopard G,Laurent E.Saccadic eye movements in depressed elderly patients. PLoS One. 2014; 9(8): e105355. doi: http://dx.doi.org/ 10.1371/journal.pone.0105355
4 Malsert J, Guyader N, Chauvin A, Polosan M, Poulet E,Szekely D, Bougerol T, et al. Antisaccades as a follow-up tool in major depressive disorder therapies: a pilot study.Psychiatry Res. 2012; 200(2-3): 1051-1053. doi: http://dx.doi.org/ 10.1016/j.psychres.2012.05.007
5 Kellough JL, Beevers CG, Ellis AJ, Wells TT. Time course of selective attention in clinically depressed young adults: an eye tracking study. Behav Res Ther. 2008; 46(11): 1238-1243.doi: http://dx.doi.org/ 10.1016/j.brat.2008.07.004
6 American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington,DC: American Psychiatric Association; 1990
7 Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry; 1960. 23: 56-62
8 Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998; 59(Suppl 20): 22–33
9 Shear MK, Vander Bilt J, Rucci P, Endicott J, Lydiard B, Otto MW, et al. Reliability and validity of a structured interview guide for the Hamilton Anxiety Rating Scale (SIGH-A).Depression & Anxiety. 2001; 13(4): 166–178. doi: http://dx.doi.org/ 10.1002/da.1033.abs
10 Henderson JM, Shinkareva SV, Wang J, Luke SG, Olejarczyk J.Predicting cognitive state from eye movements. PLoS One.2013; 8(5): e64937. doi: http://dx.doi.org/ 10.1371/journal.pone.0064937
11. Garcia-Blanco AC, Perea M, Salmeron L. Attention orienting and inhibitory control across the different mood states in bipolar disorder: an emotional antisaccade task. Biol Psychol.2013; 94(3): 556-561. doi: http://dx.doi.org/10.1016/j.biopsycho.2013.10.005
12. Richard-Devantoy S, Jollant F, Kefi Z,Turecki G, Olie JP, Annweiler C, et al. Deficit of cognitive inhibition in depressed elderly: a neurocognitive marker of suicidal risk.J Affect Disord. 2012; 140(2): 193-199. doi: http://dx.doi.org/10.1016/j.jad.2012.03.006
13. Dombrovski AY, Butters MA, Reynolds CF, Houck PR, Clark L, Mazumdar S, et al. Cognitive performance in suicidal depressed elderly: preliminary report. Am J Geriatr Psychiatry. 2008; 16(2): 109-115. doi: http://dx.doi.org/10.1097/JGP.0b013e3180f6338d
14. Beynel L, Chauvin A, Guyader N,Harquel S, Szekely D,Bougerol T, et al. What saccadic eye movements tell us about TMS-induced neuromodulation of the DLPFC and mood changes: a pilot study in bipolar disorders. Front Integr Neurosci. 2014; 8: 65. doi: http://dx.doi.org/10.3389/fnint.2014.00065
15. Barton JJ, Pandita M, Thakkar K,Gof DC, Manoach DS. The relation between antisaccade errors, fixation stability and prosaccade errors in schizophrenia. Exp Brain Res. 2008;186(2): 273-282. doi: http://dx.doi.org/ 10.1007/s00221-007-1235-2
16. Benson PJ, Beedie SA, Shephard E,Giegling I, Rujescu D,St Clair D. Simple viewing tests can detect eye movement abnormalities that distinguish schizophrenia cases from controls with exceptional accuracy. Biol Psychiatry.2012; 72(9): 716-724. doi: http://dx.doi.org/ 10.1016/j.biopsych.2012.04.019
17. Amador XF, Malaspina D, Sackeim HA,Coleman EA, Kaufmann C A, Hasan A, et al. Visual fi xation and smooth pursuit eye movement abnormalities in patients with schizophrenia and their relatives. J Neuropsychiatry Clin Neurosci. 1995; 7(2):197-206. doi: http://dx.doi.org/10.1176/jnp.7.2.197
18. Gooding DC, Grabowski JA, Hendershot CS. Fixation stability in schizophrenia, bipolar, and control subjects. Psychiatry Res. 2000; 97(2-3): 119-128
19. Mele ML, Federici S. Gaze and eye-tracking solutions for psychological research. Cogn Process. 2012; 13 (Suppl 1):S261-265. doi : http://dx.doi.org/10.1007/s10339-012-0499-z
20. Chen JH, Yao ZJ, Zhao K, Yan R, Hua LL, Jia FN, et al.. [The global efficiency of brain white matter networks in the unipolar and bipolar depression patients and its relationship with the clinical features]. Zhong Hua Jing Shen Ke Za Zhi.2015; 48(5): 271-278. Chinese. doi: http://dx.doi.org/10.3760/cma.j.issn.1006-7884.2015.05.004
21. Roux P, Brunet-Gouet E, Passerieux C, Ramus F. Eye-tracking reveals a slowdown of social context processing during intention attribution in patients with schizophrenia. J Psychiatry Neurosci. 2016; 41(2): E13-E21
22. Noiret N, Carvalho N, Laurent É, Vulliez L, Bennabi D,Chopard G, et al. Visual scanning behavior during processing of emotional faces in older adults with major depression.Aging Ment Health. 2015; 19(3): 264-273. doi: http://dx.doi.org/ 10.1080/13607863.2014.926473
23. Sanchez A, Vazquez C, Marker C, LeMoult J, Joormann J, et al. Attentional disengagement predicts stress recovery in depression: an eye-tracking study. J Abnorm Psychol. 2013;122(2): 303-313. doi: http://dx.doi.org/ 10.1037/a0031529
24. Garcia-Blanco A, Salmeron L, Perea M, Livianos L. Attentional biases toward emotional images in the different episodes of bipolar disorder: an eye-tracking study. Psychiatry Res.2014; 215(3): 628-633. doi: http://dx.doi.org/ 10.1016/j.psychres.2013.12.039
25. Wells TT, Clerkin EM, Ellis A J, Beevers CG. Effect of antidepressant medication use on emotional information processing in major depression. Am J Psychiatry. 2014;171(2): 195-200. doi: http://dx.doi.org/ 10.1176/appi.ajp.2013.12091243
26. Li M, Lu S, Wang G, Feng L, Fu B, Zhong N. Emotion, working memory, and cognitive control in patients with first-onset and previously untreated minor depressive disorders. J Int Med Res. 2016; 44(3): 529-541. doi: http://dx.doi.org/10.1177/0300060516639169
27. Bittencourt J, Velasques B, Teixeira S, Basile LF, Salles JI, Nardi AE, et al. Saccadic eye movement applications for psychiatric disorders. Neuropsychiatr Dis Treat. 2013; 9: 1393-1409. doi:http://dx.doi.org/ 10.2147/ndt.s45931
28. Carvalho N, Laurent E, Noiret N,Chopard G, Haffen E,Bennabi D, et al. Eye movement in unipolar and bipolar depression: a systematic review of the literature. Front Psychol. 2015; 6: 1809. doi: http://dx.doi.org/ 10.3389/fpsyg.2015.01809

Li Yu graduated with a bachelor’s degree in medicine from Jining Medical School in 2014. She has been studying for a master’s degree at Shanghai Jiaotong University since 2014, and currently works at the EEF Lab of Shanghai Jiaotong University’s medical school. Her current research interest is using eye tracking methods to find biomarkers for mental disorders.
抑郁障碍的眼动指标研究
李瑜,许扬扬,夏梦青,张天宏,王俊杰,刘旭,何永光,王继军
抑郁障碍、眼动、跳视
Background:Impaired cognition is one of the most common core symptoms of depressive disorder. Eye movement testing mainly reflects patients’ cognitive functions, such as cognition, memory, attention,recognition, and recall. This type of testing has great potential to improve theories related to cognitive functioning in depressive episodes as well as potential in its clinical application.Aims:This study investigated whether eye movement indices of patients with unmedicated depressive disorder were abnormal or not, as well as the relationship between these indices and mental symptoms.Methods:Sixty patients with depressive disorder and sixty healthy controls (who were matched by gender,age and years of education) were recruited, and completed eye movement tests including three tasks:fixation task, saccade task and free-view task. The EyeLink desktop eye tracking system was employed to collect eye movement information, and analyze the eye movement indices of the three tasks between the two groups.Results:(1) In the fixation task, compared to healthy controls, patients with depressive disorder showed more fixations, shorter fixation durations, more saccades and longer saccadic lengths; (2) In the saccade task, patients with depressive disorder showed longer anti-saccade latencies and smaller anti-saccade peak velocities; (3) In the free-view task, patients with depressive disorder showed fewer saccades and longer mean fi xation durations; (4) Correlation analysis showed that there was a negative correlation between the pro-saccade amplitude and anxiety symptoms, and a positive correlation between the anti-saccade latency and anxiety symptoms. The depression symptoms were negatively correlated with fi xation times, saccades,and saccadic paths respectively in the free-view task; while the mean fixation duration and depression symptoms showed a positive correlation.Conclusion:Compared to healthy controls, patients with depressive disorder showed signif i cantly abnormal eye movement indices. In addition patients’ anxiety and depression symptoms and eye movement indices were correlated. The pathological meaning of these phenomena deserve further exploration.
[Shanghai Arch Psychiatry. 2016; 28(6): 326-334.
http://dx.doi.org/10.11919/j.issn.1002-0829.216078]
1Shanghai Mental Health Center, Medical School, Shanghai Jiao Tong University, Shanghai, China
2Shanghai Institutes for Neuroscience, Chinese Academy of Sciences, Shanghai, China
*correspondence: Professor Jijun Wang. Mailing address: Electroencephalography Imaging Lab, B2303, Building No.2, 600 South Wanping RD, Shanghai,China. Postcode: 200030. E-Mail: jijunwang27@163.com
*correspondence: Professor Yongguang He. Mailing address: Shanghai Mental Health Center, 3210 Humin RD, Shanghai, China. Postcode: 201108. E-Mail:hyg_512@126.coms
背景:认知受损是抑郁障碍常见的核心症状之一,眼动(eye movement)检查主要反映患者的认识、记忆、注意及再认、回忆比较等方面的认知功能,对研究抑郁发作的认知功能有一定的理论和实践意义。目的:探究从未服药抑郁障碍患者眼动指标是否异常以及这些指标与精神症状的关系。方法:纳入60例抑郁障碍患者和性别、年龄及教育年限匹配的60例健康志愿者进行注视稳定性、跳视、自由视图三个任务的眼球运动测试,应用EyeLink桌面式眼动仪收集眼动信息,分析比较两组三个任务的眼动指标。结果:(1)注视稳定性任务中,相对于健康对照,抑郁障碍患者在无干扰任务和有干扰任务中都呈现注视次数增加、注视时间缩短、跳视次数增加、跳视路径增加;(2)跳视任务中,抑郁障碍患者反向跳视潜伏期延长,反向跳视峰速度减小;(3)自由视图任务中,抑郁障碍患者扫视次数减少、平均注视时间延长;(4)相关分析显示:朝向跳视幅度与焦虑症状呈负相关,反向跳视潜伏期与焦虑症状呈正相关,自由视图任务的注视次数、跳视次数、扫视路径与抑郁障碍症状呈负相关,而平均注视时间与抑郁障碍症状呈正相关。结论:抑郁障碍患者较健康对照眼动指标存在明显异常,且患者的焦虑、抑郁症状与眼动指标有相关性,其病理生理学意义值得进一步探究。
猜你喜欢
杂志排行
上海精神医学的其它文章
- Effects of transcranial direct current stimulation (tDCS) for auditory hallucinations: A systematic review
- Effect of repetitive transcranial magnetic stimulation on cigarette smoking in patients with schizophrenia
- Effect of clonazepam co-administered with clozapine on the serum clozapine and norclozapine concentration of patients with schizophrenia: A Retrospective Survey
- Comparative analysis of results from a cognitive emotion regulation questionnaire between international students from West Asia and Xinjiang college students in China
- Why is diagnosing MDD challenging?
- Psychogenic blepharospasm: a diagnostic dilemma
