Prospects for bone marrow cell therapy in amyotrophic lateral sclerosis: how far are we from a clinical treatment?
2016-12-01FernandaGubertMarceloSatiagoInstitutodeBiofsicaCarlosChagasFilhoUniversidadeFederaldoRiodeJaneiroRiodeJaneiroRJBrasil
Fernanda Gubert, Marcelo F. SatiagoInstituto de Biofísica Carlos Chagas Filho, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brasil
Prospects for bone marrow cell therapy in amyotrophic lateral sclerosis: how far are we from a clinical treatment?
Fernanda Gubert*, Marcelo F. Satiago
Instituto de Biofísica Carlos Chagas Filho, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brasil
How to cite this article: Gubert F, Satiago MF (2016) Prospects for bone marrow cell therapy in amyotrophic lateral sclerosis∶ how far are we from a clinical treatment? Neural Regen Res 11(8)∶1216-1219.
Funding: This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (www.cnpq.br), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (www.capes.gov.br) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (www.faperj.br).
Fernanda Gubert, Ph.D.,
fegubert@biof.ufrj.br.
orcid:
0000-0002-0568-7935
(Fernanda Gubert)
Accepted: 2016-08-15
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease that causes progressive muscular atrophy and death within 3-5 years after its onset. Despite the significant advances in knowledge of ALS pathology, no effective treatment is available. Therefore, it is imperative to search for new alternatives to treat ALS. Cell therapy, especially using bone-marrow cells, has showed to be very useful to protect the neural tissue in different brain disease or traumatic lesions. In ALS, most published results show beneficial effects of the use bone marrow cells, especially mesenchymal stromal cells. However, until now, the best outcome extends animal’s lifespan by only a few weeks. It is essential to continue the search for a really effective therapy, testing different cells, routes and time-windows of administration. Studying the mechanisms that initiate and spread the degenerative process is also important to find out an effective therapy. Therefore, we discussed here some progresses that have been made using bone-marrow cell therapy as a therapeutic tool for ALS.
amyotrophic lateral sclerosis; cell therapy; bone-marrow mononuclear cells; mesenchymal stromal cells; neuroinflammation; motor neurons
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease that causes progressive muscular atrophy and death within 3-5 years after its onset. Most patients have the so-called sporadic ALS, with unknown etiology; and approximately 10% of the patients have the familial form of the disease, when more than one individual from the same family is affected. In recent years knowledge of the disease physiopathology has increased greatly, and different genes have been associated with the familial form. Currently, the gene most commonly associated with ALS is the C9ORF72 gene, which affects approximately 40% of cases of familial ALS, followed by Cu/Zn-superoxide dismutase1 (SOD1), TAR DNA-binding protein 43 (TARDBP), fused in sarcoma (FUS) and other less-frequent mutations (Su et al., 2014). Despite the significant advances in knowledge of ALS pathology, at the moment the only available treatment is Riluzole, which extends the survival time by only three months, with no improvement in the quality of life. Therefore, it is imperative to search for new alternatives to treat ALS, and cell therapy appears to be a promising therapy.
In 1994, the first ALS mouse model was described, leading to enormous breakthroughs in this field (Gurney, 1994). In this mouse model, the genome carries multiple copies of the mutant SOD1 human gene, which contains a substitution of glycine for alanine at position 93 in the amino acid chain (SOD1G93A). Although this mouse model represents only a small percentage (2%) of ALS patients, it shows a progressive degeneration, with muscle atrophy and loss of motorneurons in the ventral horn of the spinal cord, culminating in the animal’s death, which is similar to the human pathology. Subsequently, other mutations have been used to produce different animal models of ALS, but most of the disease mechanisms were elucidated in the G93A model. Researchers have formed a consensus that ALS is a multifactorial disease, in which other cells beyond the motor neurons are involved, such as astrocytes and microglia (McGoldrick et al., 2013). The multifactorial feature of the disease leads researchers to believe that in order to develop an effective treatment it is necessary to address multiple compromised pathways. In this respect, unlike a canonical pharmacological approach, cell therapy, for instance, could produce multiple clinical effects regulating these disturbed pathways.
Cell Therapy
Initially, the main goal of cell therapy was to replace lost or damaged tissue. The discovery of stem cells in differentniches, including the central nervous system, and the isolation of pluripotent embryonic stem cells contributed to this prospect. Although cell replacement is possible in some damaged tissues such as bone marrow, in the central nervous system this goal is still far from being achieved, especially concerning lost motor neurons, the affected cells in ALS patients. Considering the multifactorial nature of ALS, the limited knowledge of the mechanisms that initiate neuronal death, and the necessity for some kind of axonal guidance to direct long-distance and specific axonal innervation, it appears to be a difficult matter to use cell replacement for this purpose. A second possibility may be to replace cells other than the motorneurons, such as astrocytes. SOD1 mutated astrocytes show a harmful profile, being toxic to motor neurons. In this respect, the injection of glial restricted precursors (GRP) into the spinal cord of SOD1G93Amice has been tested, and resulted in the engraftment of normal astrocytes, the protection of surrounding motor neurons, and an increase in the animals’ lifespan (Rizzo et al., 2014). In addition, cell therapy, especially using bone marrow cells, also proved to be very useful to protect the neural tissue. This approach has been tested in different types of brain disease or traumatic lesions with promising results. It has been demonstrated, for instance, that bone marrow transplantation, either intravenously or locally, stimulates angiogenesis, neurogenesis and axonal outgrowth, and decreases inflammation and neuronal death (Mesentier-Louro et al., 2016). Another advantage of the use of bone marrow cells is the possibility to perform autologous transplantation, reducing the risk of adverse effects triggered by immune responses. However, it is necessary to determine whether the autologous transplanted cells maintain the same beneficial potential, especially in genetic ALS cases.
Bone Marrow Cell Therapy
Bone marrow cell therapy includes the use of the mononuclear fraction of bone marrow cells (BMMC - bone marrow mononuclear cells) or mesenchymal stromal cells (MSC) resident in this region. BMMC comprise both hematopoietic stem cells and MSC in a small percentage, several hematopoietic progenitors, and differentiated bone marrow cells such as lymphocytes and monocytes. Both MSC and BMMC have been used in cell therapy. In a rat model of focal ischemia, these cell populations showed similar beneficial effects on the animals’ functional recovery, although only one-tenth the number of MSC was needed to achieve the same effect as BMMC (de Vasconcelos Dos Santos et al., 2010). In addition, Pastor et al. (2012) obtained a better outcome using whole bone marrow cells, compared to MSC transplant, in the mdf/ ocd mouse model of motoneuron degeneration. Nevertheless, for clinical use, BMMC therapy is safer than MSC, since the latter cells require extensive laboratory manipulation and weeks of culturing procedures, increasing the possibilities of cell contamination or even chromosomal alterations. On the other hand, BMMC could be injected in the same day of their isolation.
In SOD1G93Amice models of ALS, many research groups have begun to test bone marrow-derived cells. Initially, the strategy aimed toward bone marrow replacement, using high doses of irradiation. Although some groups observed an increase in animal survival, the use of irradiation in humans could be a dangerous procedure (Corti et al., 2004). After this finding, many groups focused on the use of MSC. Different routes of injection, either intraspinally or systemically, and different doses have been tested. Most published results show beneficial effects of the use of MSC, demonstrating an increase in animal survival, protection of motorneurons, and decreases in inflammation and in the number of microglia and astrocytes (Lewis and Suzuki, 2014). Low doses of MSC (< 500,000 cells), especially when administered intrathecally, showed no beneficial effects.
Therapeutic Window of Administration
Another important issue that should be addressed in pre-clinical studies is the timing of the treatment. Currently, pre-clinical studies have shown a beneficial effect of treating animals in the presymptomatic phase or at the onset of the disease. It is known that by the time the symptoms become apparent, a significant number of motor neurons have already died, supporting the importance of this approach. However, most patients have sporadic ALS, and even those with the familial form of the disease are not necessarily diagnosed early. Added to this, the diagnosis is usually difficult and may take months to become conclusive. For these reasons it is important to be certain that therapies are beneficial even when the disease is advanced.
In our work, we demonstrated, for the first time, different outcomes when we treated ALS mice with intraspinal injection of BMMC. As mentioned previously, it has been shown that therapy with these cells is capable of reducing cell death, microglial activation and to enhance the axonal regeneration. Bone marrow cells were showed to protect neurons from apoptosis through growth factors releasing, such as GDNF (Pastor et al., 2012). Others growth factors and cytokines also seems important to keep a protective and regenerative environment, such as fibroblast growth factor 2 (FGF-2) (Mesentier-Louro et al., 2016). In addition, clinical trials with BMMC demonstrated that they are safe and feasible for human use as well as being easy to isolate requiring little manipulation, a clear advantage comparing to MSC. Therefore, it is important to test this therapy in pre-clinical models. Although we did not observe an increase in the animals’ survival using this approach, animals treated in the presymptomatic phase showed delays in the disease progression, analyzed by their functional motor performances, using rotarod and hanging wire tests. When we treated symptomatic animals, no modification of the progression of the diseasewas observed, demonstrating that BMMC could not reverse the established damage (Gubert et al., 2016). It is important to point out that, only in BMMC-treated animals in the symptomatic phase, we observed a couple of animals that survive for a longer period, more than 160 days, while non-treated animals did not exceeds 150 days. Therefore, it would be necessary to test if increasing the number of cells would result in a positive effect in lifespan. These results highlight the importance of preclinical studies.
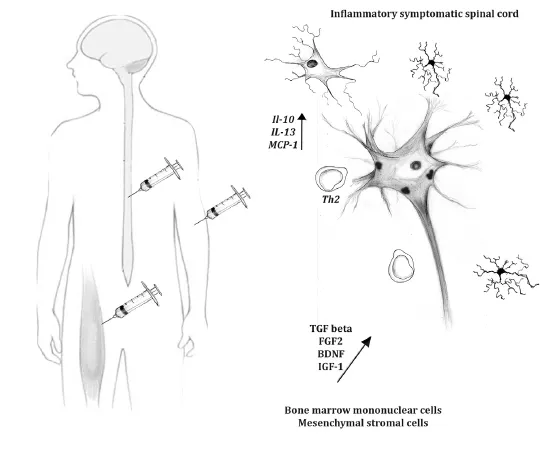
Figure 1 Future of cell therapy for amyotrophic lateral sclerosis.
Route of Administration
Many groups have investigated the best route of injection for cellular therapies. Local injections have the advantage of ensuring that the injected cells are near the region of interest. Although spinal-cord injections involve some risk, intra-parenchymal transplants are feasible. MSC injected into the spinal cord seem to survive in the injection site for at least 10 weeks in ALS animal models (Vercelli et al., 2008). However, testing different strategies to track BMMC, we demonstrated that these cells did not remain for a long time in this region (Gubert et al., 2016). This characteristic could explain the transitory effects of these cells in ALS mice observed by our group. The hazardous environment of the spinal cord could impair the transplanted cells and be responsible for damage/disappearance of the injected. In this respect, it has been shown that MSC have both antioxidant and immunomodulatory potentials that could protect them from the harmful environment. Intravenous treatment using MSC or human umbilical-cord cells also showed positive outcomes (Lewis and Suzuki, 2014). These results are very promising, since this approach is less invasive, allowing multiple injections in a potential clinical trial. Concerning this aspect, ongoing studies in our laboratory indicates a better outcome when the BMMC were injected intravenously. It is known that bone marrow cells migrate to the damaged area, even in neurodegenerative diseases such as ALS (Corti et al., 2004). However, the main contribution of these cells in slowing the progression of the disease may be through modulating inflammation. The contribution of immune cells, especially T lymphocytes and microglia, has been demonstrated, and the differentiation of these cells to an anti-inflammatory profile could delay the disease progression (Figure 1). Intramuscular and intracerebroventricular transplants are other potential routes of injection that have already been tested (Lewis and Suzuki, 2014).
Bone Marrow Cell Therapy as a Possible Inflammation Modulator
As mentioned above, modulation of inflammation is a possible strategy for bone marrow therapy. In recent years, many groups have demonstrated a relationship between an inflammatory pathway and the progression of the disease, in both human patients and animal models. It has been demonstrated in the animal model that the disease progresses more slowly during its onset than in a more-advanced phase (Beers et al., 2011). This initial stable phase was associated with the presence of regulatory T cells and with an anti-inflammatory profile of the microglia. In contrast, the rapid-progression phase wasassociated witha decrease of regulatory T cells, an increase of T cytotoxiclymphocytes, and with a pro-inflammatory microglia. In human patients, the infiltration of lymphocytes in the spinal cord was also observed (Rizzo et al., 2014). In this respect, BMMC could contribute to regulate the inflammatory profile, especially considering the presence of lymphocytes and monocytes in this population. MSC has also been shown to regulate the immune system, e.g., by reducing the proliferation of CD8+T cells through transforming growth factor beta (TGF beta) secretion and downregulating the production of IFNγ of natural killer cells (Chen et al., 2006).
Clinical Trials Using Bone Marrow Cells
The promising results using bone marrow cells have encouraged clinical trials for ALS patients. At the moment, most trials seek to determine if bone marrow therapy is safe and feasible, although the clinical outcome may also be analyzed. BMMC were injected both intraspinally and intrathecally, with no major adverse response. A retrospective analysis found that BMMC therapy had a significant effect on patient survival (Sharma et al., 2015). However, a randomized study is still necessary, analyzing a larger cohort to prove the efficacy of BMMC transplants. MSC are also being tested, and were found to be safe when injected intrathecally, intraspinally and intravenously.
Conclusion
Bone marrow cell therapy for ALS is in its infancy, and much has to be done before we can determine the true potential of this approach. Many groups, including ours, are working intensively to find the best protocol that could be translated to the clinic. However, until now, the best outcome extends an animal’s lifespan by only a few weeks. It is essential to continue to investigate the mechanisms that initiate and spread the degenerative process, in order to fight them and further delay the progression of the disease (Figure 1). Multiple injections, especially intravenously, could sustain the effect longer, but it is important to think in broad terms to deal with ALS. A combination of cell therapy with gene therapy could potentiate the results, and pharmacological approaches should be added to treat specific targets. Finally, inflammation seems to be a potential issue in ALS and should also be considered a potential target in the investigation of ALS disease treatment.
Acknowledgments: We thank Louise Mesentier-Louro and Igor Bonacossa-Pereira for help with the art work and together with Camila Zaverucha-do-Valle and Janet Reid for comments and English revision. We apologize to all authors whose work could not be cited here due to paper length restrictions.
Author contributions: FG was responsible for development and manuscript writing. MFS was responsible for manuscript writing and revision.
Conflicts of interest: None declared.
References
Beers DR, Zhao W, Liao B, Kano O, Wang J, Huang A, Appel SH, Henkel JS (2011) Neuroinflammation modulates distinct regional and temporal clinical responses in ALS mice. Brain Behav Immun 25:1025-1035.
Chen X, Armstrong MA, Li G (2006) Mesenchymal stem cells in immunoregulation. Immunol Cell Biol 84:413-421.
Corti S, Locatelli F, Donadoni C, Guglieri M, Papadimitriou D, Strazzer S, Del Bo R, Comi GP (2004) Wild-type bone marrow cells ameliorate the phenotype of SOD1-G93A ALS mice and contribute to CNS, heart and skeletal muscle tissues. Brain 127:2518-2532.
de Vasconcelos Dos Santos A, da Costa Reis J, Diaz Paredes B, Moraes L, Jasmin, Giraldi-Guimaraes A, Mendez-Otero R (2010) Therapeutic window for treatment of cortical ischemia with bone marrow-derived cells in rats. Brain Res 1306:149-158.
Gubert F, Decotelli AB, Bonacossa-Pereira I, Figueiredo FR, Zaverucha-do-Valle C, Tovar-Moll F, Hoffmann L, Urmenyi TP, Santiago MF, Mendez-Otero R (2016) Intraspinal bone-marrow cell therapy at pre- and symptomatic phases in a mouse model of amyotrophic lateral sclerosis. Stem Cell Res Ther 7:41.
Gurney ME (1994) Transgenic-mouse model of amyotrophic lateral sclerosis. N Engl J Med 331:1721-1722.
Lewis CM, Suzuki M (2014) Therapeutic applications of mesenchymal stem cells for amyotrophic lateral sclerosis. Stem Cell Res Ther 5:32.
McGoldrick P, Joyce PI, Fisher EM, Greensmith L (2013) Rodent models of amyotrophic lateral sclerosis. Biochim Biophys Acta 1832:1421-1436.
Mesentier-Louro LA, Zaverucha-do-Valle C, Rosado-de-Castro PH, Silva-Junior AJ, Pimentel-Coelho PM, Mendez-Otero R, Santiago MF (2016) Bone marrow-derived cells as a therapeutic approach to optic nerve diseases. Stem Cell Int 2016:5078619.
Pastor D, Viso-Leon MC, Jones J, Jaramillo-Merchan J, Toledo-Aral JJ, Moraleda JM, Martinez S (2012) Comparative effects between bone marrow and mesenchymal stem cell transplantation in GDNF expression and motor function recovery in a motorneuron degenerative mouse model. Stem Cell Rev 8:445-458.
Rizzo F, Riboldi G, Salani S, Nizzardo M, Simone C, Corti S, Hedlund E (2014) Cellular therapy to target neuroinflammation in amyotrophic lateral sclerosis. Cell Mol Life Sci 71:999-1015.
Sharma AK, Sane HM, Paranjape AA, Gokulchandran N, Nagrajan A, D’sa M, Badhe PB (2015) The effect of autologous bone marrow mononuclear cell transplantation on the survival duration in Amyotrophic Lateral Sclerosis - a retrospective controlled study. Am J Stem Cells 4:50-65.
Su XW, Broach JR, Connor JR, Gerhard GS, Simmons Z (2014) Genetic heterogeneity of amyotrophic lateral sclerosis: implications for clinical practice and research. Muscle Nerve 49:786-803.
Vercelli A, Mereuta OM, Garbossa D, Muraca G, Mareschi K, Rustichelli D, Ferrero I, Mazzini L, Madon E, Fagioli F (2008) Human mesenchymal stem cell transplantation extends survival, improves motor performance and decreases neuroinflammation in mouse model of amyotrophic lateral sclerosis. Neurobiol Dis 31:395-405.
10.4103/1673-5374.189167
*Correspondence to:
杂志排行
中国神经再生研究(英文版)的其它文章
- Secondary parkinsonism induced by hydrocephalus after subarachnoid and intraventricular hemorrhage
- Volume transmission and receptor-receptor interactions in heteroreceptor complexes: understanding the role of new concepts for brain communication
- Uncoupling protein 2 in the glial response to stress: implications for neuroprotection
- Selective neuronal PTEN deletion: can we take the brakes off of growth without losing control?
- TRPV1 may increase the effectiveness of estrogen therapy on neuroprotection and neuroregeneration
- Tamoxifen: an FDA approved drug with neuroprotective effects for spinal cord injury recovery
