难熔金属碳化物ZrC对树脂基炭微观结构的影响
2016-10-31张中伟南策文王俊山许正辉
张中伟, 梅 敏, 李 亮, 南策文, 王俊山, 许正辉
(1.清华大学 材料科学与工程系新型陶瓷与精细加工国家重点实验室,北京100084;2.航天材料及工艺研究所 先进功能复合材料技术重点实验室,北京100076;3.中国运载火箭技术研究院,北京100076)
难熔金属碳化物ZrC对树脂基炭微观结构的影响
张中伟1,2,梅敏2,李亮3,南策文1,王俊山2,许正辉2
(1.清华大学 材料科学与工程系新型陶瓷与精细加工国家重点实验室,北京100084;2.航天材料及工艺研究所 先进功能复合材料技术重点实验室,北京100076;3.中国运载火箭技术研究院,北京100076)
在微纳观尺度,利用SEM、TEM和HR-TEM观察了难熔碳化物(ZrC)和树脂基炭经2 500 ℃以上高温处理后的显微组织结构。结果表明:ZrC颗粒表面包裹了一层取向和生长良好的石墨微晶炭过渡层。其中炭过渡层中晶体的层间距d002为0.337 5 nm,而没有包裹ZrC颗粒的树脂基炭仍然是各向同性结构。结合选区电子衍射结果可知,ZrC颗粒在高温下对树脂基炭有催化石墨化和诱导石墨化效应。
难熔碳化物; 碳; 微观结构; 石墨化
1 Introduction
Carbon materials, including graphite and carbon/carbon composites, have been widely applied in the astronautic and aerospace fields such as airplane brakes, nose tips and rocket motor nozzles and are considered to be the ideal thermal protection materials owing to their excellent mechanical and thermo-physical properties at high temperatures[1]. However, their applications have been restricted at high temperature to an inert environment, because of their susceptivity to oxidation above 500 ℃. Therefore, the improvement of oxidation protection of carbon materials is one of the most important work to be done. The matrix modification and external coating as effective methods have been proposed. Ceramic coating is limited to low temperatures below 1 800 ℃ due to the relatively low service temperature and poor usage reliability[2]. Recently, researchers are trying to improve the anti-ablation and oxidation resistance of carbon materials by doping phosphorus, boron, carbides, borides, refractory metals and their oxides into their matrix to apply carbon materials in severe service conditions[3-18]. Many research results indicated that the thermal protection performance of carbon materials was improved evidently by this way.
It has been identified that doping refractory metal compounds can obviously enhanced the ablation resistance of carbon materials above 2 000 ℃ in an oxidizing atmosphere[6]. Nevertheless, the influence of refractory metal compounds on the microstructure of carbon matrix is rarely reported, which is fairly important to the properties of the whole carbon materials. The aim of this work is to study the microstructures of refractory metal compounds and resin-based carbon treated at high temperature above 2 500 ℃, and to discuss the mechanism of the interaction between the refractory metal compounds and carbon.
2 Experimental
2.1Preparation
The starting materials were mixed, mechanically ball-milled at high speed, cured, carbonized, and heat treated. The phenolic resin with about 70% in solid content (30% alcohol remains), and the refractory metal zirconium oxide (ZrO2) with 99% in pure and 5m in size, were used as the starting materials. Prior to curing, the phenolic resin was mixed with ZrO2powder with a mass ratio of 80∶20. The solid-liquid mixture was milled for 8 h by attrition milling with an agating media. The volume of mixture was fivefold to that of milling balls, the milling speed was 150 r/min. The slurry was set in a graphite mould and heated up to 180 ℃ under a pressure of 3 MPa and Ar gas. At last, the block after cured was carbonized at 1 000 ℃ and graphitized at 2 500 ℃ in Ar.
2.2Characterization
The phase composition of the samples was analyzed by XRD using a Rigaku D/max-2400, and the microstructure of the samples was inspected by SEM using a APOLLO 300,by TEM using a JEOL 2010 and by HR-TEM using a LEO 1530 XB.
3 Results and discussion
The composite consists of resin-based carbon and ZrC since ZrC has a good thermal stability and chemical inertness. ZrC is converted from the chemical reaction of ZrO2with C after high temperature treatment (HTT), which is shown in Fig. 1. It can be known that ZrO2as the starting material has been completely reacted with C during the HTT.
Fig.2 shows the micro-morphology of carbon around a ZrC particle. As shown in Fig. 2, there is not only resin carbon but also “transition carbon” around the ZrC particle. The transition carbon is different from resin carbon and has not extended into the resin carbon matrix. It was well-known that the thermosetting resins transforms into amorphous isotropic carbon after HTT[7]. The amorphous isotropic carbon is also called “glassy carbon” because of its high vitreous luster and glassy status. The glassy carbon, with a network micro-structure (Fig. 3), is non-graphitizing carbon and hard to be graphitized by heat treatment even at 3 000 ℃. However, as shown in Fig. 4, resin-based carbon with a good graphite structure can be observed around the ZrC particle by HR-TEM.

Fig. 1 XRD pattern of the sample after HTT.
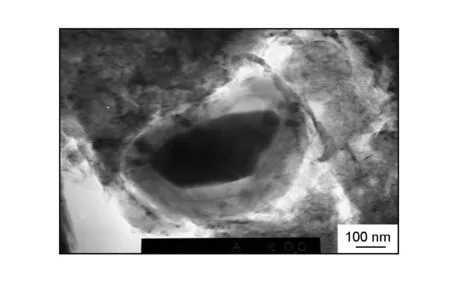
Fig. 2 TEM micrograph of carbon around a ZrC particle.

Fig. 3 A non-graphitizing carbon structure.
From Fig. 4, it can be seen that the resin carbons around ZrC particle consists of the crystal area (A) and the amorphous area (B). In the crystal area, the regular lattice stripes of graphite with 58 layers can be observed distinctly and the distance between two close layers is equivalent and measured to be 0.337 5 nm. Moreover, it should be noted that all lattice stripes stack in parallel along the edge of the ZrC particle.

Fig. 4 HR-TEM morphologies of carbon around the ZrC particle.
These results indicate that the refractory carbide ZrC has a catalytic graphitization effect on the resin-based carbon around it and this effect is more remarkable during HTT. This graphitization phenomenon is different from the former description that the resin carbon is amorphous and hard to be graphitized by heat treatment even at 3 000 ℃. On the other hand, the high thermal stress will be yielded at the interface between ZrC and carbon during HTT due to the far larger linear coefficient of thermal expansion (CTE) of ZrC (CTE is 6×10-7-7×10-6/℃) than that of carbon (CTE is 3×10-6- 4×10-6/℃)[8], which will induce the transformation of carbon from amorphous to graphite at the edge of ZrC particle (Fig. 2 and 4). Ultimately, the resin carbon was transformed into the graphite structure with a whole layer-structure, perfect crystal tropism and regular stack by the integrative effects of catalytic graphitization and induced graphitization.
Fig. 5 shows the selected area electron diffractions of carbon around the ZrC particle. It can be seen that the diffraction pattern of the carbon away from ZrC (Fig.5 (a)) exhibits as typical diffraction rings, which means a low crystal orientation. In contrast, the diffraction pattern of the carbon at the edge of ZrC particle (Fig.5 (b)) shows the characteristics of a preferred orientation. The results above could also prove that the ZrC particle has an effect on the structure transformation of resin carbon.
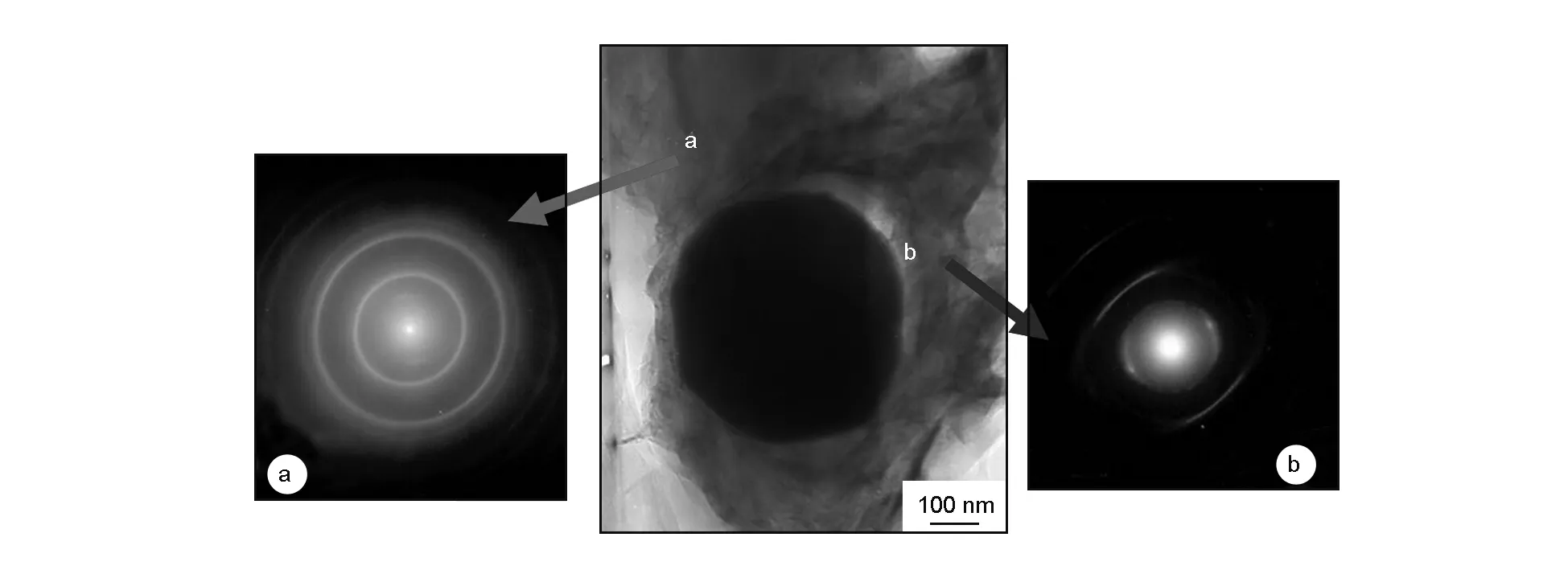
Fig. 5 Selected area electron diffraction patterns of carbon around a ZrC particle.
The catalytic graphitization is a complex process with both physical and chemical changes. Based on the above analysis, the Zr element has an obvious diffusion behavior in the C matrix. Besides, the diffusion behavior changes significantly at high temperature. The phase diagram of Zr-C binary system is given in Fig. 6. A relatively wide ZrCxsolid solution region could be observed. The Zr element diffuses and then the concentration of Zr reaches a low level, which results in the precipitation of elemental carbon with the formation of graphite. In addition, it also can be seen that the ZrCxsolid solution region decreases with the increasing of the temperature, which indicates that the carbon is easy to reach supersaturation at high temperature and then precipitates from the ZrCxsolid solution region.
The catalytic graphitization mechanism of ZrC to carbon could be explained by the liquating and separating theory. The schematic of catalytic graphitization by Zr components is shown in Fig. 7. At high graphitization temperature, Zr diffuses to carbon matrix and reacts with amorphous carbon to form carbide. With the continual diffusion of Zr, the graphite carbon and elemental Zr are generated. Then the Zr diffuses along with the surface of carbide particles to the side of amorphous carbon and reacts with it to form more carbide. The entire process is repeated as the Zr migration, which results in catalytic graphitization. It is worth noting that the graphitization degree of carbon matrix could be improved as the diffusion rate of Zr element is accelerated at high temperatures.

Fig. 6 Binary phase diagram of ZrC.
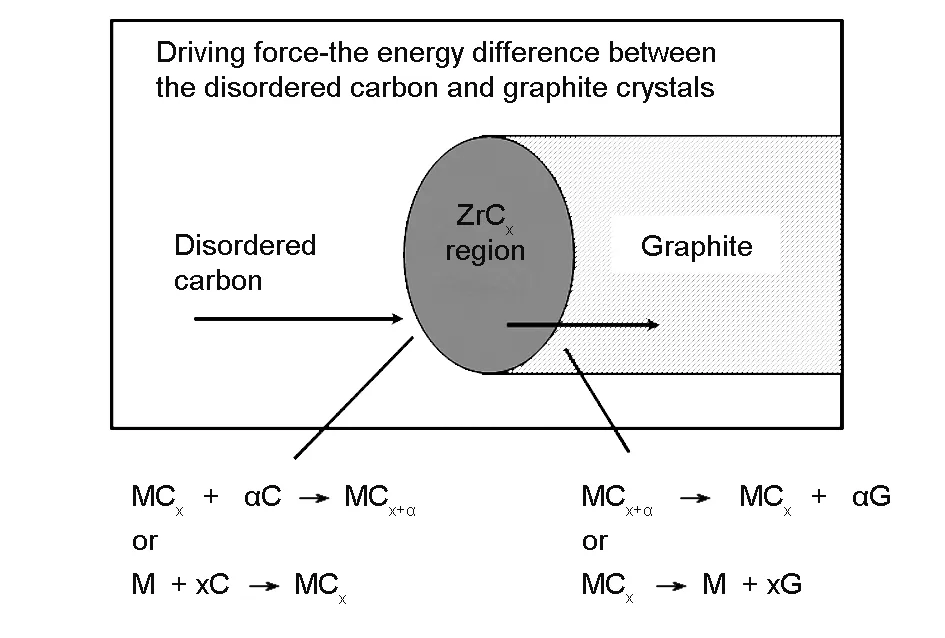
Fig. 7 A schematic illustration of
4 Conclusions
The studies on microstructure of resin carbon around a ZrC particle at micro- and nano-scale demonstrate that the refractory carbide ZrC has an obvious catalytic graphitization effect on the carbon around it, this effect will result in the growth of graphitic micro-crystal and regular stack of graphitic layers to improve the graphitization degree of carbon materials. Finally, the mechanism of the graphitization is explained.
[1]Savage G. Carbon-Carbon Composites[M]. London: Chapman and Hall, 1993: 193-225.
[2]Buckley J D. Carbon-Carbon Material and Composites[M]. New Jersey: Noyes Publications, 1993: 229-262.
[3]Choury J J. Carbon-carbon materials for nozzles of solid propellant rocket motors[P]. AIAA Paper, No.76-609, 1976.
[4]Zhang Z W, Fang X L, Xu Z H. Studies on the oxidation and ablation behavior of low-ablation C/C-ZrC composite at high temperature above 2 200 ℃[C]. The 84th Academic Seminar on New Carbon Materials, 2007: 477-480.
[5]Cui H. Analysis on microstructure of anti-ablation multi-matrix C/C composite[J]. Journal of Solid Rocket Technology, 2001, 24(3): 63-67.
[6]R Imamura, K Matsui, S Takeda, et al. A new role for phosphorus in graphitization of phenolic resin[J]. Carbon, 1999, (37): 261-267.
[7]Mohamed O. Abdalla, Adriane Ludwick, Temisha Mitchell. Boron-modified phenolic resins for high performance applications[J]. Polymer, 2003, (44): 7353-7359.
[8]Asa Oya, Sugi Otani. Catalytic graphitization of carbons by various metals[J]. Carbon, 1979, (17): 131-137.
[9]Shinn-Shyong Tzeng. Catalytic graphitization of electroless Ni-P coated PAN-based carbon fibers[J]. Carbon, 2006, (44): 1986-1993.
[10]YI Shoujun , CHEN Jinhua , XIAO Xiong, LIU Lu, et al. Effect of praseodymium on catalytic graphitization of furan resin carbon[J]. Jounal of Rare Earths, 2010, 28(1): 69-71.
[11]Ya Wen, Yonggen Lu, Hao Xiao, et al. Further investigation on boric acid catalytic graphitization of polyacrylonitrile carbon fibers: Mechanism and mechanical properties[J]. Materials and Design, 2012, (36): 728-734.
[12]Jigang Wang, Nan Jiang, Haiyun Jiang. Micro-structural evolution of phenol-formaldehyde resin modified by boron carbide at elevated temperatures[J]. Materials Chemistry and Physics, 2010, (120): 187-192.
[13]A . OYA. Phenomena of catalytic graphitization[J]. Jounal of Material Science, 1982, 17: 309-322.
[14]Shihai Xu, Fengying Zhang, Qing Kang, et al. The effect of magnetic field on the catalytic graphitization of phenolic resin in the presence of Fe-Ni[J]. Carbon, 2009, (47): 3233-3237.
[15]Changqing Liu, Kezhi Li, Hejun Li, et al. The effect of zirconium incorporation on the thermal stability and carbonized product of phenoleformaldehyde resin[J]. Polymer Degradation and Stability, 2014, (102): 180-185.
[16]Li C Y, Li K Z, Li H J, et al. Microstructure and ablation resistance of carbon/carbon composites with a zirconium carbide rich surface layer[J]. Corrosion Science, 2014, 85: 160-166.
[17]Gao X Q, Liu L, Guo Q G, et al. The effect of zirconium addition on the microstructure and properties of chopped carbon fiber/carbon composites[J]. Composites Science and Technology, 2007, 67: 525-529.
[18]Gao X Q, Guo Q G, Shi J L, et al. The fabrication of chopped carbon fiber-carbon composites and their thermal/electrical conductivity and microstructure[J]. New Carbon Materials, 2005, 20(1): 18-22.
Influence of ZrC on the microstructure of its surrounding resin-based carbon at high temperature
ZHANG Zhong-wei1, 2,MEI Min2,LI Liang3,NAN Ce-wen1,WANG Jun-shan2,XU Zheng-hui2
(1.StateKeyLaboratoryofNewCeramics&FineProcessing,DepartmentofMaterialsScienceandEngineering,TsinghuaUniversity,Beijing100084,China;2.ScienceandTechnologyonAdvancedFunctionalCompositesLaboratory,AerospaceResearchInstituteofMaterialsandProcessingTechnology,Beijing100076,China;3.ChinaAcademyofLaunchVehicleTechnology,Beijing100076,China)
The microstructures of a refractory ZrC particle and its surrounding resin-based carbon treated above 2500°C were investigated by SEM, TEM, HR-TEM and selected area electron diffraction. Results show that the ZrC particle is enclosed by a transition carbon layer that has ad002of 0.3375 nm, close to that of ideal graphite, indicating the formation of a well graphitized structure. The carbon far away from the ZrC particle remains isotropic and amorphous. These results are verified by selected area electron diffraction, and can be attributed to catalytic graphitization and stress-induced graphitization.
Refractory carbide; Carbon; Microstructure; Graphitization
ZHANG Zhong-wei, Ph. D. E-mail: zhangzhongw@163.com
date: 2016-05-10;Reviseddate: 2016-07-20
10.1016/S1872-5805(16)60024-0
1007-8827(2016)04-0451-04
TB332
A
张中伟,博士. E-mail: zhangzhongw@163.com
English edition available online ScienceDirect ( http:www.sciencedirect.comsciencejournal18725805 ).
