The characterisation and cryopreservation of Venda chicken semen
2016-10-18MasindiMphaphathiMokgadiSeshokaDibungiLusebaBenjaminSutherlandTshimangadzoNedambale
Masindi L. Mphaphathi, Mokgadi M. Seshoka, Dibungi Luseba, Benjamin Sutherland, Tshimangadzo L. Nedambale,3*
1Agricultural Research Council, Animal Production, Germplasm Conservation & Reproduction Biotechnologies, Private Bag X 2, Irene, 0062, Republic of South Africa
2Tshwane University of Technology, Faculty of Science, Department of Animal Sciences, Private Bag X 680, Pretoria, 0001, Republic of South Africa
3University of the Free State, Department of Animal, Wildlife and Grassland Sciences, P.O Box 339, Bloemfontein, 9300, Republic of South Africa
The characterisation and cryopreservation of Venda chicken semen
Masindi L. Mphaphathi1,2, Mokgadi M. Seshoka1,2, Dibungi Luseba2, Benjamin Sutherland2, Tshimangadzo L. Nedambale1,2,3*
1Agricultural Research Council, Animal Production, Germplasm Conservation & Reproduction Biotechnologies, Private Bag X 2, Irene, 0062, Republic of South Africa
2Tshwane University of Technology, Faculty of Science, Department of Animal Sciences, Private Bag X 680, Pretoria, 0001, Republic of South Africa
3University of the Free State, Department of Animal, Wildlife and Grassland Sciences, P.O Box 339, Bloemfontein, 9300, Republic of South Africa
ARTICLE INFO
Article history:
Semen
Venda cocks
Cryopreservation
Cryoprotectant
DMSO
Ethylene glycol
ABSTRACT
Objective: To characterize Venda cocks semen, find a suitable short-term diluted semen storage temperature, find a suitable cryopreservation cryoprotectant and to investigate cryoprotectant toxicity. Methods: Semen was collected from six Venda cocks and evaluated macroscopically for semen volume, pH and sperm concentration. Microscopic sperm characteristics examined included were total motility (rapid, medium and slow) progressive and non-progressive motility. Velocity characteristics included curvilinear and straight-line velocity, average path velocity, linearity, straightness, wobble, amplitude of lateral head displacement and beat cross frequency. Results: Results showed that the average semen volume was (0.3±0.1) mL, the pH (6.9±0.4) and the sperm concentration (6.8±79.8) 伊109/mL. A positive correlation was observed between body weight and semen volume (r=0.38). Similarly a significant difference between the initial sperm total motility (TM%) of (87.5±8.6) and samples stored for 24 h at 5曟 (55.0±8.0) and 25 曟(30.6±6.1) was recorded. The percentage live and normal sperm was 87.0% and 93.5% (P< 0.05) respectively. The TM% recorded was significantly different in samples supplemented with DMSO (46.0±8.3), ethylene glycol (EG) (45.0±12.2) and propanediol (PND) (21.8±10.4), following thawing. Detailed velocity values showed consistent differences between the raw and cryoprotectant-free semen samples. Conclusions: In conclusion, the Venda cock semen was subsequently found to have a higher TM% when stored in vitro at 5 曟. DMSO and EG were found to be suitable for the cryopreservation of Venda cock semen.
Document heading doi: 10.1016/j.apjr.2016.01.009
1. Introduction
The cryopreservation of cock semen has been extensively researched and sperm banking provides a possible effective method of maintaining superior male genetic material. However, cryopreserved cock semen has a limited on-farm use, due to its presumably low sperm motility with the primary role of the sperm being to fertilize the ovum [1]. An efficient method for chicken semen storage is thus necessary for future use. Generally, cold semen storage is used to reduce the metabolism of the sperm cell and to maintain sperm viability over an extended period of time [2]. These semen extenders and holding temperature play a significant role in maintaining cock sperm motility [3]. So it was found that in contrast to semen samples stored at 5, 15 or 25 曟 [4], sperm stored at 41 曟 showed a significant greater rate of sperm death [5]. Diluted semen stored at 2 to 5 曟 retained its fertilizing capacity, even after 24 h [6]. The fertility rate following artificial insemination with frozen sperm is however low, than in fresh cock semen. So for example, hens inseminated with frozen sperm produced fewer fertile eggs, than those inseminated with fresh raw semen [7,8].Methodologies for improved in vitro semen storage and thawing are thus required.
The Venda chicken has not been extensively researched on ex situ conservation. In the past, information on the characterization of the breed was collected at the Agricultural Research Council (ARC) and there was very limited research on the cryopreservation of sperm for use in the chicken breeding programmes. According to the ARC, the Venda chicken breed was first recognised in the Venda region of the South African province of Limpopo. The breed is dual purpose, moderately large and multi-coloured with white, black and red as the predominant colours [9]. These Venda chickens are then known to survive under harsh conditions [10] and to have a low reproductive potential [11]. This breed reaches sexual maturity at 20 weeks of age, the hens are broody with good mothering ability and lay tinted eggs of a medium size. There is however cause for concern as the fertility of the experimental flock of Venda chickens at the ARC has declined over time. These indigenous chickens are generally raised by small scale farmers, with few resources in African countries mainly because of their hardness [12]. At present chicken genetic resources can only be conserved by maintaining a living flock which might be costly [9]. As a result, the cryopreservation of cock semen could play an important role in chicken breeding and genetic resource conservation.
Glycerol was initially used as the main cryoprotectant for the preservation of the sperm in most animals, including the stallion [13], ram [14] and the fowl [15]. However, cryopreservation was found to hamper fertilization in poultry [16]. The cause of this lowered fertility in artificially inseminated, cryopreserved sperm using glycerol as cryoprotectant was not identified, but may possibly be related to the osmotic shock following the rapid loss of glycerol from the sperm cell in the hen’s reproductive tract [17].
Other cryoprotectants used for poultry semen have been dimethyl acetamide and DMSO [18,19]. These were then selected in terms of their low molecular weight and toxicity when used at low temperatures[20]. The addition of these cryoprotectants to cock semen resulted in an exposure to osmotic stress to sperm due to the osmotic efflux of extracellular water with a subsequent increase in cell volume as the cryoprotectant permeated and water concomitantly re-entered the sperm cell [21]. Further, ethylene glycol (EG) has a lower molecular weight (62.07 g/molar) and a greater membrane permeability than e.g. propanediol (PND) (76.10 g/molar) and DMSO (78.13 g/molar) [22]. It is suspected that EG permeates the sperm plasma membrane faster than PND and DMSO, hence causing damage to the sperm during equilibration and cryopreservation [23].
The semen analyses essential for the study of cock fertility generally includes the evaluation of sperm concentration, motility, morphology and semen volume [24]. The sperm motility analysis is repeatable and may then be linked to fertility [25]. This analysis generally makes use of manual and microscopic techniques. Alternatively a computer aided sperm analyser (CASA) system objectively analyses the characteristics of sperm, providing scientists and breeders with a fertility prediction of individual cocks [26]. The aims of the study were to characterize Venda cocks semen and find the most suitable short-term diluted semen storage temperature and find a suitable cryoprotectant. In addition, the study was designed to determine the effect of cryoprotectant toxicity on sperm motility and the cryotolerance of sperm in individual Venda cocks.
2. Materials and methods
2.1. Experimental cocks
The flock consisted of pure-bred Venda cocks hatched from parent stock and housed at the Poultry Breeding Section of the ARC at Irene, South Africa. The cocks were vaccinated with live vaccines against Marek’s disease, infectious bronchitis and Newcastle Disease at hatching. At week 24 of age, cocks were transferred to individual battery cages and fed a commercial diet ad libitum, until attaining a live body weight of 2.3 kg. All cocks were exposed to 16 h of light between 05: 00 and 21:00.
2.2. Semen collection and characterization
Semen collection was based on the method as described by Burrows and Quinn [27] from cocks at 26 weeks of age. This abdominal massage technique was used to collect semen three times per week from six Venda cocks [28]. Individual ejaculates were collected and placed in a thermos flask containing water at a temperature between 38 and 40 曟[29]. The semen being transported to the laboratory within 5 min following collection. All the experimental cocks were cared for, according to the guidelines of the ARC Animal Production Institute ethics committee (Ref: APIEC08/06).
Semen volume (mL) was measured visually using a graduated collection tube and the pH with the aid of a calibrated pH meter (Hanna instruments®, Portugal). Sperm concentration was determined using a JENWAY®6310 spectrophotometer. The wavelength being set at 650 nm. The addition of 3 mL of a 2.9% Sodium citrate solution (pH 7.0), together with 15 µL semen in a cuvette was utilized and the absorbance was then converted to sperm concentration. The formula used: (11.170 伊 Absorbance) – 90. This was recorded in sperm/mL (伊109/mL)[30]. Following the sperm swim-up preparation (10 µL of raw semen was mixed with 500 µL of Kobidil+ extender, at 38 曟), 5 µL of the semen diluted 1:50 with Kobidil+ extender, was placed on a warm microscope slide and covered with a warmed coverslip. This was then examined on a microscope fitted with a warm-plate (Omron®), at 37 曟. The TM% of the sperm was determined with clarity a Sperm Class Analyzer®(Microptic, Spain), at a magnification of 伊10 (Nikon®, China). The total sperm motility was further classified into rapid, medium or slow motility and progressive and non-progressive motility. The sperm velocity characteristics measured included the curvilinear, straight line and average path velocity, linearity, straightness, wobble, amplitude of lateral head displacement and beat cross frequency.
Sperm morphology was microscopically evaluated by assessing 100 sperm per ejaculate per replicate. The 7 µL semen was mixed with 20 µL of a eosin/nigrosin stain, in a 0.6 mL graduated micro-centrifuge tube (Simport, Canada) [31]. Thereafter, 5 µL of the stained, raw semen sample was placed at the end of a microscope slide, smeared andfixed by air drying at 25 曟 for 10 min, before evaluation [32]. Five µL of the stained, raw semen sample were then placed on the end of a microscope slide, smeared and fixed by air drying at 25 曟 for 10 min before evaluation [33]. The sperm morphology was evaluated under a fluorescent microscope (BX51 TF®, Olympus, Japan). Viable sperm remained unstained and dead cells were totally or partially pink to red/brown. Viable sperm were further classified as morphologically normal or abnormal, depending on the head, mid-piece, and tail morphology[32]. The procedure was repeated four times.
2.3. Effect of different temperatures on semen storage
In order to determine the effect of temperature on short-term semen storage, individual cock ejaculates were slowly diluted (1:2), with the Kobidil+ extender (Landata®, Groupe Cobiporc, France) supplemented with 20% Venda egg yolk. Diluted semen samples were compared necessary following equilibration at 5 曟 and 25 曟 for 0 (control), 4, 8, 12 and 24 h for sperm motility and sperm velocity characteristics. These observation were repeated six times.
2.4. Toxicity test of cryoprotectants
Individual semen samples were diluted (1:1) with the Kobidil+ extender, supplemented with 20% egg yolk and divided into a fraction A and B. Fraction A was cryoproctant free, while fractions B contained either 8% DMSO or 8% EG or 8% PND. The cryoprotectant free sample were equilibrated at 5 曟 for 0 (control), 1 or 2 h while the cryoprotectant containing samples were equilibrated at 5 曟 for 1 or 2 h. Sperm were then microscopically evaluated for changes in motility and velocity. This procedure was also repeated six times.
2.5. Cryopreservation of semen with DMSO, EG or PND cryoprotectant
Raw semen from the Venda cocks was pooled, stored at 5 曟 and the sperm motility and velocity characteristics determined. The pooled semen was diluted (1:1) with the Kobidil+ extender and supplemented with either 8% DMSO or 8% EG or 8% PND and equilibrated at 5 曟 for 2 h together with a cryoprotectant-free control (CPA-free) group. Straws containing 0.25 mL of the diluted semen were placed in a portable programmable freezer (Halikan®88 LX 2002, Taiwan). The semen was initially cooled to 5 曟 and then at a rate of -1 曟/ min until the target temperature of -20 曟 was reached [34]. Semen was equilibrated at -20 曟 for 5 min before suspending 5 cm above liquid nitrogen for 5 min and the straws then plunged directly into a Styrofoam®container containing liquid nitrogen (-196 曟), also for an additional 5 min before storage in a liquid nitrogen container. Straws were thawed at 5 曟 for 5 min, for determining the sperm motility and velocity characteristics. This was repeated six times.
2.6. Cryotolerance of individual ejaculates
The pre-freezing procedure of the conventional slow freezing method was used for individual ejaculates diluted with Kobidil+ extender supplemented with 8% DMSO. Semen straws were thawed at 5 曟and evaluated for sperm motility and velocity. The experiment was repeated six times.
2.7. Statistical analyses
Data were analysed using the statistical programme GenStat® at a significance level (P<0.05). Analysis of variance was used to establish differences in the effect of temperature on equilibration, liquid semen storage time, cryoprotectant, cryoprotectant toxicity and individual cryotolerance on the sperm motility and velocity characteristics. Treatment means were separated using the Fisher’s protected t-test least significant difference. The data are presented as mean ± standard deviation (S.D.).
3. Results
3.1. Semen collection and characterization
There were no significant differences in the semen volume, pH and sperm concentration of individual cocks, indicating homogeneity within the breed (Table 1). Individual semen volumes ranged from 0.2 to 0.4 mL, the semen pH from 6.7 to 7.0 and the sperm concentration from 6.4 to 7.7伊109/mL. The Pearson correlation coefficients between body weight and semen characteristics are set out in Table 2. The coefficients were generally very low to medium with positive correlation values ranging from r = 0.10 to 0.38. A positive correlation being recorded between body weight and semen volume (r = 0.38) and between sperm concentration and semen volume (r = 0.16).
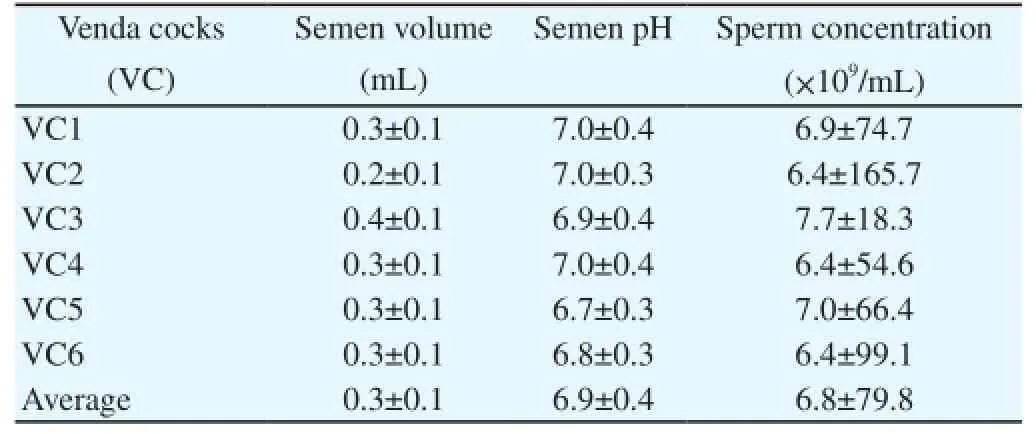
Table 1 Characterization of semen volume, pH and sperm concentration in Venda cock.
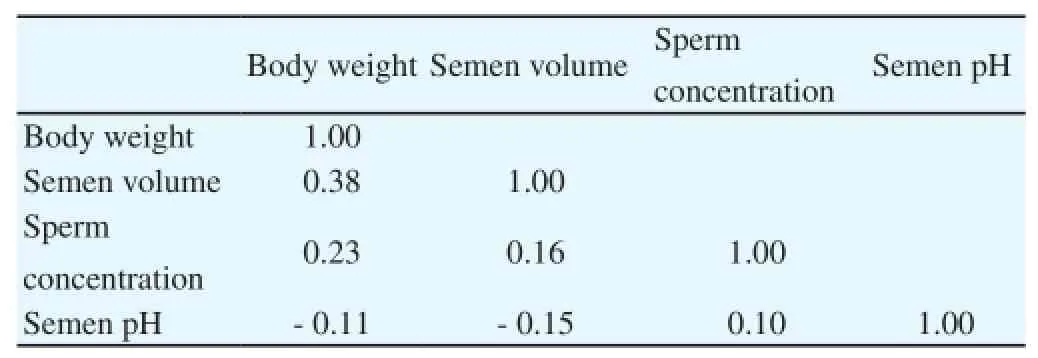
Table 2 Pearson correlation coefficients of body weight and semen characteristics in Venda cocks.
Sperm morphology results are set out in Table 3. More than 87% of the sperm were alive and normal. Venda cock number 3 (VC3) recorded a higher sperm viability (95.8%) than VC1 (89.8%), VC2 (87.0%) and VC4 (88.5%). However, for VC3 (95.8%) sperm viability did not differ with cock VC5 (92.5%) and VC6 (93.5%). The percentage of live sperm with head, mid-piece and tail abnormalities did not differ significantly between individuals.

Table 3 The sperm morphology of Venda cock (mean±SD).
The toxicity of the extender was evaluated by determining the TM% at 0, 1 and 2 h of equilibration (Table 6). The sperm TM% and semen pH were not significantly different for the equilibration periods. At the end of equilibration with the fraction A and B extenders, the sperm TM% was recorded above 82% in all the groups. The rapid sperm velocity, progressive and non-progressive motility at 0 h was significantly different after 2 h equilibration with the fraction B extender.
3.2. Effect of different temperature on semen storage
The effect of temperature on sperm motility during storage at 5曟 and 25 曟 is shown in Table 4. The TM% decreased significantly during in vitro storage and after 24 h at 25 曟, was 30.6%. Semen samples stored at 5 曟 showed an overall TM% of > 50% after 24 h. A slight linear decrease in the progressive motility percentage and rapid velocity was recorded as the storage period increased. The progressive motility was higher in raw semen and significantly lower following prolonged in vitro semen storage, irrespective of the storage temperature. Sperm velocity characteristics such as linearity and straightness percentage decreased slightly during in vitro semen storage (Table 5). The straight line and curvilinear velocities and the average path at 0 and 4 h of storage at 5 曟 were not statistically different. The average sperm linearity recorded was 53.6% within 4 h of storage at 5 曟.
3.3. Toxicity testing of cryoprotectants
3.4. Effect of cryoprotectants on sperm motility
Following the freezing and thawing procedure, the TM% was higher (P< 0.05) for the Kobidil+ extender, supplemented with either 8% DMSO (46.0%) or EG (45.0%), compared to Kobidil+plus PND (21.8%) and the control (5.6%) groups (Table 7). The cryopreservation process resulted in a noticeable decline in TM and rapid sperm motility rates, irrespective of the cryoprotectant used (Table 8). The curvilinear, straight-line and average path velocity rates of sperm was reduced after thawing. In addition, a drastic decrease (P<0.05) in sperm velocity was recorded in control group.
3.5. Individual cryotolerance
A variation in the TM% for the frozen and thawed semen samples between individuals was recorded (Table 9). However, there were no significant differences in the other sperm characteristics measured. A significant difference was recorded in the TM% between the VC1 and VC3 (53.8 and 38.8%, respectively). Frozen semen showed a sperm linearity above 50%, which was comparable to the raw semen (Table 10). Characteristics such as sperm velocity on the curve, straight line and average path were reduced following the freezing/thawing process in certain cocks, a variation in cryotolerance being evident.
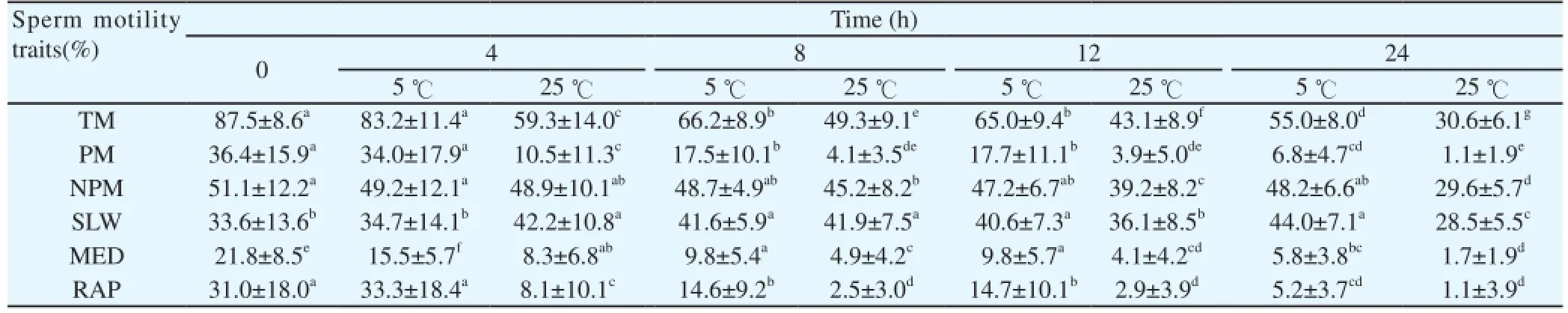
Table 4 Effect of temperature on sperm motility traits during storage at 5 and 25 曟 of Venda cocks.
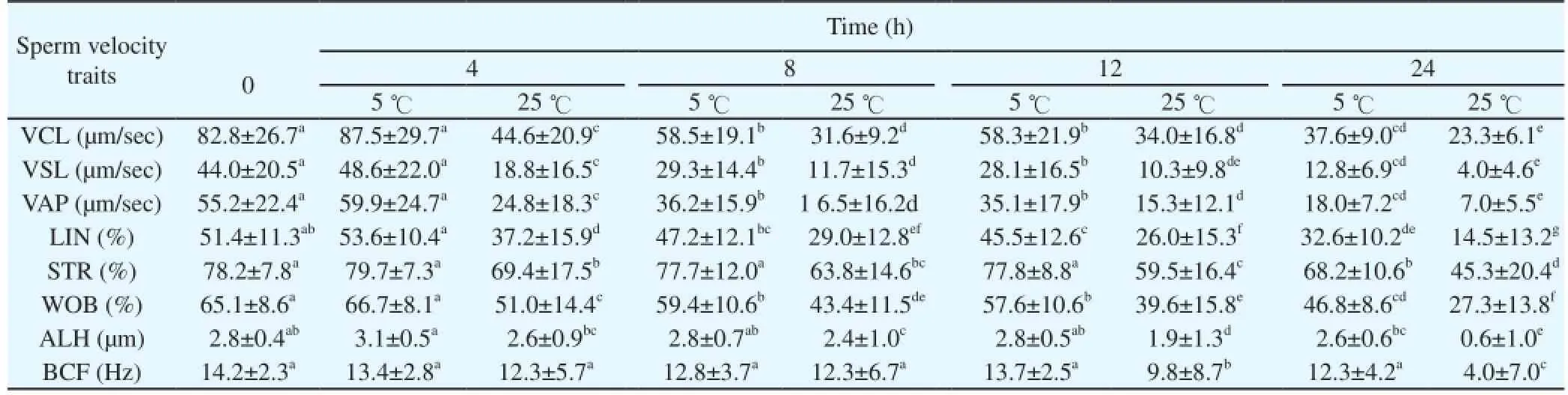
Table 5 The effect of temperature on sperm velocity traits during storage at 5 and 25 曟 in Venda cock.
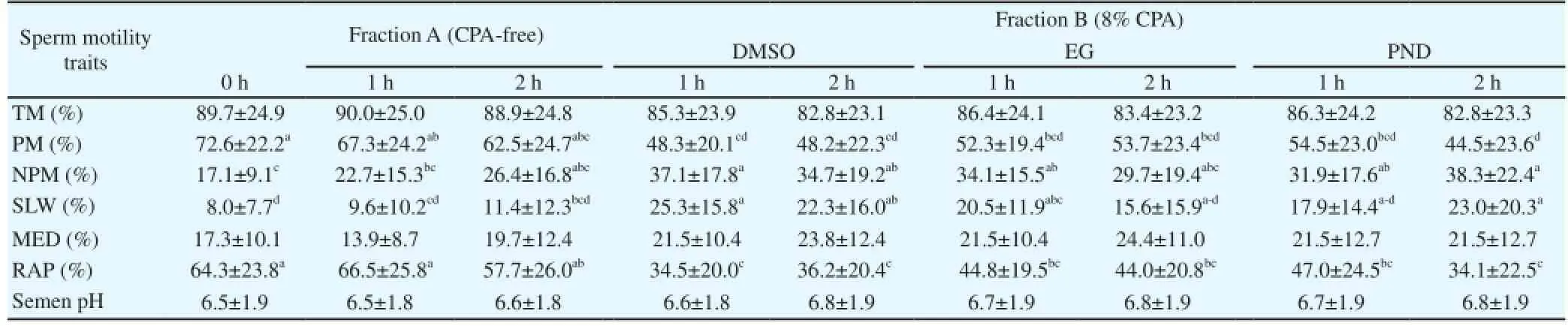
Table 6 Toxicity effect of fraction A and B extender(s) on Venda cock sperm motility during equilibration at 5 曟.
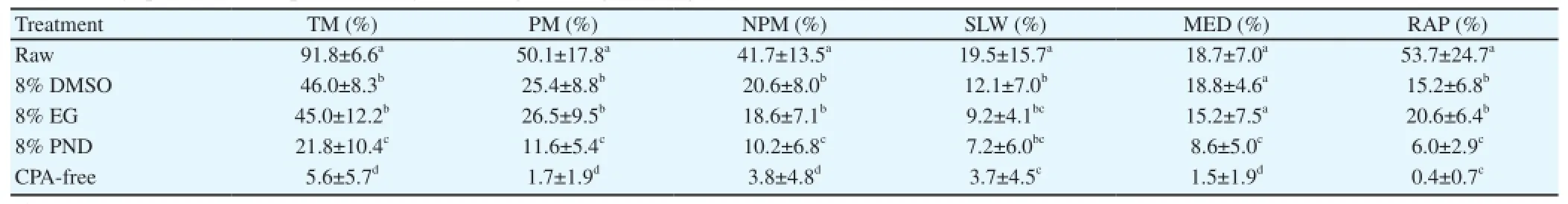
Table 7 Effect of cryoprotectant on sperm motility following freezing/thawing of semen in Venda cocks.
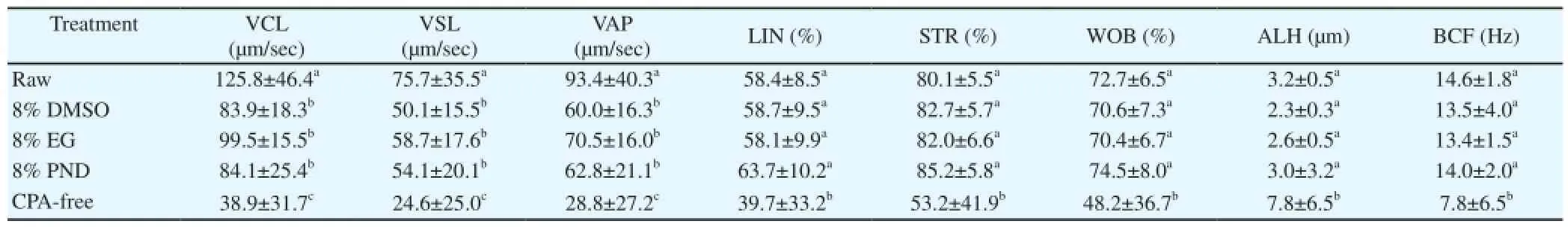
Table 8 The mean (±SD) effect of cryoprotectants on sperm velocity traits following freezing/thawing of Venda cock semen.
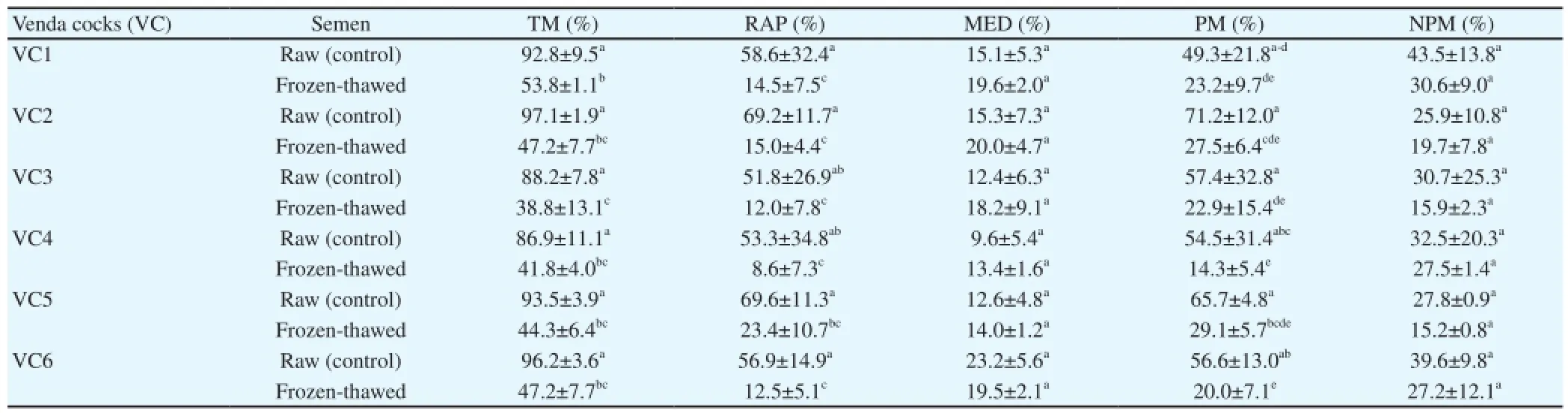
Table 9 Cryotolerance of raw and frozen sperm regarding motility in the individual Venda cocks traits (Mean±SD).
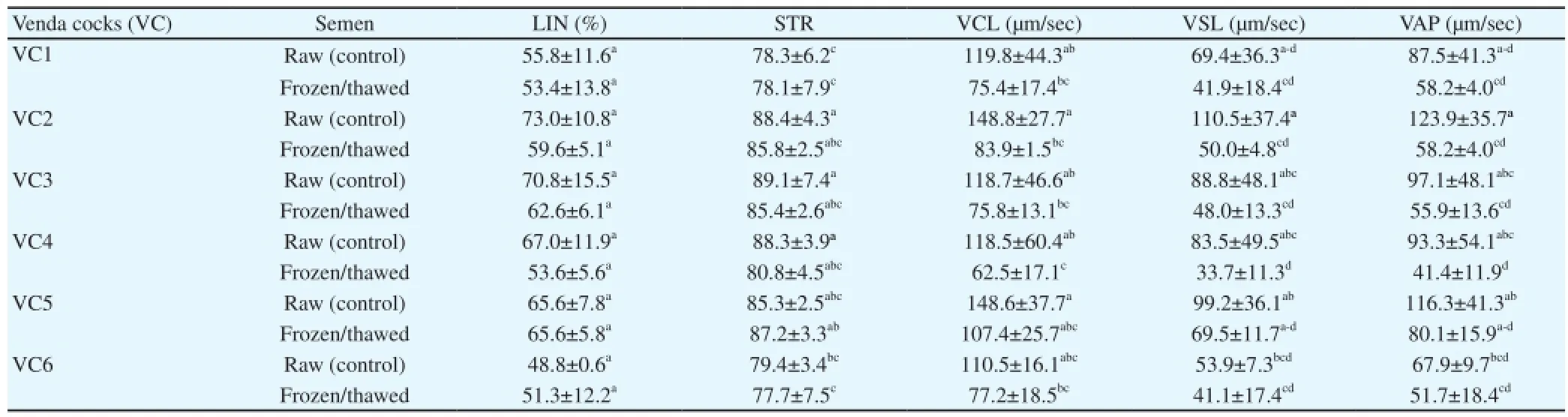
Table 10Cryotolerance of raw and frozen sperm regarding velocity traits in individual Venda cocks (Mean±SD).
4. Discussion
This study provided the first characteristics of Venda cock sperm using the computer-aided sperm analyser (CASA) system. Moreover, a positive correlation between body weight and semen volume (r= 0.38) and semen volume and sperm concentration (r=0.16) were recorded. An increase in semen volume may not necessarily translate to a higher sperm concentration in cocks. The average semen volume of Venda cockerels was 0.3 mL. These findings contradict with those of Molekwa and Umesiobi [35] who reported a lower semen volume of 0.2 mL in the same breed. Differences in semen volume may depend mainly on the relative influence of various reproductive glands, management and the extent to which the genetic potential is exploited[36].
The sperm concentration of 6.8伊109/mL reported in the present study, was higher than that reported by other researchers [32]. Siudzinska and Lukaszewicz[32] reported an average sperm concentration of 4.7伊109/mL in White Crested Black Polish cocks and 4.2 伊109/mL in the Black Minorcas breeds. Tuncer et al.[37] and Obidi et al. [38] reported sperm concentrations of 2.4伊109/mL in Gerze cocks and 3.6伊109/mL in Shikabrown cocks. Semen pH is generally correlated to sperm motility and the metabolic rate. Turkey and chicken sperm were reported to tolerate a semen pH of 6.0 to 8.0 [39] which is in line with the findings of this study (pH 6.9). Generally, semen pH of < 6.0 reduces sperm motility, lactic acid production and oxygen uptake. A higher pH again increases the metabolic rate during in vitro semen storage [39]. Tuncer et al.[37] reported a higher semen pH of 7.7 recorded in Gezere cocks.
The most commonly accepted method of semen preservation is cryopreservation in liquid nitrogen at -196 曟[40,41]. However, liquid nitrogen, semen extenders, reagents and a programmable freezer are available to relative few farmers, especially those in the small scale farming sector of South Africa. The primary aim of any semen preservation protocol is to maintain the sperm motility rate over an extended period of time[32]. A sperm TM of 87% was found in the control treatment assessed immediately after the swim-up test. Semen samples of the Venda breed recorded a TM of 55% after 24 h in vitro storage at 5 曟 while, semen samples stored at 25 曟, showed a drastic reduction to 30% after 24 h. There was also a steady decline in sperm motility rate during storage at 5 曟, compared to the more rapid decrease for those sperm stored at 25 曟. Sontakke et al. [42] reported time-dependant changes in sperm motility characteristics during in vitro liquid semen storage.
This study demonstrated that it was possible to preserve semen of Venda cocks at 5 曟 for 24 h. This was in agreement with the findings of Van Wambeke [43] who reported no loss in the fertilisation capacity of semen stored in vitro for 24 h, at 2 to 5 曟. The Hubbard broiler sperm motility rate was reported to be 58.6% after semen storage at 5 曟 for 24 h [44]. This result is comparable to the present study of 55.0% after storage for 24 h at 5 曟. Tabatabaei & Aghaei[45] reported a lower motility rate of 45.6% in semen from Ross-308 broilers stored 24 h at 4 曟. Karunakaran et al. [2] observed a decrease in progressive sperm motility during storage. In the present study, progressive sperm motility rate recorded was an average 6.8% after 48 h of in vitro semen storage at 5 曟.
Previous reports indicated that cock semen stored at 41 曟drastically decreases the sperm motility compared to that stored at 5, 15 or 25 曟[46]. Dumpala et al. [28] reported that the number of dead sperm in cock semen stored at 41 曟 was significantly higher when compared to those stored at 4 or 21 曟. In vitro sperm storage at a temperature of 5 曟 reduced the cell metabolism and maintained sperm motility over a prolonged period[2].
The preservation of endangered poultry male gametes is necessary for the maintenance of genetic variability in domestic farm chicken breeds [47]. In domesticated avian species, sperm number, type of cock (broiler or layer type) and age may affect the in vitro storage and fertility rate [48]. The current study presents the first attempt to cryopreserve semen from indigenous Venda cocks. It focused on semen cryotolerance using DMSO or EG or PND as cryoprotectants. The success of semen cryopreservation generally is measured by assessing sperm survival and motility rates. Moreover, this study found that sperm motility rates were lower than 50% following thawing, compared to 91.8% before freezing. The TM recorded was 46.0%, 45.0%, 21.8% and 5.6% in the DMSO, EG, PND and cryoprotectant-free groups, respectively.
The cellular activity of the sperm that ceases at cryopreservation, resumes after thawing [48,49] and freezing and thawing have been found to damage or kill poultry sperm[32]. DMSO was found to be the cryoprotectant of choice for the cryopreservation of cock semen[18,19]. Moce et al.[50] used dimethyle acetamide as a cryoprotectant and recorded a cock sperm motility rate of 38.4% after thawing. In the absence of the cryoprotectant, a lower sperm motility of 19% was recorded. The results of the present study indicated that DMSO and EG are better cryoprotectants for cryopreserving Venda cock sperm. Similar results were recorded in thawed ram semen [51], although cock sperm and bull are significant different in morphology [52].
The mechanisms of cryoprotectant permeation of sperm are not known [53]. According to Gilmore et al.[54], the optimal cryoprotectant is one that can permeate the cell in the shortest period of time thus causing the least amount of volume excursion during its addition and removal. As a result, the action of a cryoprotectant depends on its permeability coefficient [53]. For decades, poultry egg yolk, a natural complex mixture of cholesterol, phospholipids and antioxidants, has been used to try to reduce the negative effects of osmotic shock following semen cryopreservation. Chicken egg yolk was widely used in most extenders during the short term storage or cryopreservation of semen, in order to protect sperm from cold shock[55,56]. In the present study, cooling semen slowly in the presence of a protective agent using Venda egg yolk, resulted in a recovery of sperm motility after thawing.
The major source of variation in semen characteristics between cocks is unknown. Waterhouse et al. [57] reported that variation in frozen boar semen is longstanding and seems to be a trait of the individual boar, rather than a trait of individual ejaculates. Pukazhenthi et al. [58] also reported a population specific difference in the sperm cryotolerance of cats. Generally, post-thaw sperm survival is consistently poor [59]. It has also been observed that the choice of individual cocks with superior sperm motility, both before and after the cryopreservation cycle, is crucial. In the current study, cocks were the same age, originated from one flock and were maintained under the same management procedures. A variation in the cryotolerance of post-thaw sperm of individual cocks was recorded and there was a significant difference in the sperm motilityof raw and frozen/thawed semen, such as straightness percentage.
The proportion of distinct, morphologically different subpopulations of raw sperm varies in different species and has been linked to sperm quality following the freezing/thawing process[60]. This was also reported as an additional source of variation in cryotolerance between boars [59]. The proportion of motile sperm following cryopreservation varied between species [56,57] and was possibly influenced by the choice of semen extender used for cryopreservation [61].
In conclusion, it can be said that this study provides the first characterization information of Venda cock semen. A positive correlation between body weight and semen volume and between body weight and sperm concentration was recorded. The proportion of live, normal sperm was higher than the abnormal sperm. Venda cock sperm was also found to have a higher motility rate when the semen was stored in vitro at 5 曟compared to 25 曟. Sperm characteristics such as TM%, progressive motility and straightness varied between individual cocks after cryopreservation. The use of DMSO and EG resulted in higher sperm motility rates than the PND treated group. Compared to the control group, the cryopreservation process reduced sperm motility and velocity rate, regardless of the cryoprotectant used.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
The study was supported by grants from the Agricultural Research Council Parliamentary Grant, the Department of Agriculture Forestry and Fishery and the National Research Foundation -Grant No RPPP12 & 15, RT21 and 24000 (NRF). The Germplasm Conservation and Reproductive Biotechnologies (GCRB) group is thanked for their support.
References
[1] Hou ML, Huang SY, Lai YK, Lee WC. Geldanamycin augments nitric oxide production and promotes capacitation in boar spermatozoa. Anim Reprod Sci 2008;104 :56–68.
[2] Karunakaran M, Dhali A, Mech A, Khate K, Rajkhowa C, Mishra DP. Preservation of mithun (Bos frontalis) semen at refrigeration temperature. Anim Reprod Sci 2007;101:257–264.
[3] Dumpala PR, Parker HM, McDaniel CD. The effect of semen storage temperature and diluent type on the sperm quality index of broiler breeder semen. Int J Poult Sci 2006a;5: 838–845.
[4] Clarke RN, Sexton TJ, Ottinger MA. Effect of holding temperature and storage time on respiratory rate, motility and fertility of chicken and turkey spermatozoa incubated under various conditions. Poult Sci 1982;61:1912–1917.
[5] Clarke RN, Bakst MR, Ottiger MA. Morphological changes in chicken and turkey spermatozoa incubated under various conditions. Poult Sci 1984;63:801–805.
[6] Van Wambeke F. Fertility and hatchability results with fowl spermatozoa stored in fresh and freeze-dried diluent. Br Poult Sci 1972; 13:179–183.
[7] Blanco M, Donoghue AN, Gee G, Wildt DE. Species variation in osmotic, cryoprotectant and cooling rate tolerance in poultry, eagle and peregrine falcon spermatozoa. Biol Reprod 2000; 63:1164–1171.
[8] Besnard J, Bleisbos E, Boichard MT, Coquerelle G, Gourichon D, Grasseau I, et al. Semen cryopreservation for ex situ management of genetic diversity in chicken: creation of the raw avian cryobank. Poult Sci 2007; 86: 555–564.
[9] Fulton JE. Avian genetic preservation: an industry perspective. Poult Sci 2006; 85 :227–231.
[10] Van Marle-Köeste E, Hefer CA, Nel LH, Groenen MAM. Genetic diversity and population structure of locally adapted South African chicken lines: implications for conservation. S Afr J Anim Sci 2008;38:271–281.
[11] Norris D, Ngambi JW, Benyi K, Makgahlela ML, Shimelis HA, Nesamvuni EA. Analysis of growth curves of indigenous male Venda and Naked neck chickens. S Afr J Anim Sci 2007;37:21–26.
[12] Mtileni BJ, Muchadeyi FC, Maiwashe A, Groeneveld E, Groeneveld LF, Dzama K, et al. Genetic diversity and conservation of South African indigenous chicken populations. J Anim Breed Genet 2011;128:209–218. [13] Scerzer J, Fayres-Hosken RA, Aceves M, Hurley DJ. Freezing equine semen: the effect of combinations of semen extenders and glycerol on post-thaw motility. Aust Vet J 2009;87:275–279.
[14] Colas G. Effect of initial freezing temperature, addition of glycerol and dilution on the survival and fertilizing ability of deep-frozen ram semen. J Reprod Fertil 1975;42:277–285.
[15] Polge C, Smith AU, Parkes AS. Revival of spermatozoa after vitrification and dehydration at low temperature. Nature 1949;164:666–676.
[16] Herrera JA, Quintana JA, Lopez MA, Betancourt M, Fierro R. Individual cryopreservation with dimethyl sulfoxide and polyvinylpyrrolidone of ejaculates and pooled semen of three avian species. Arch Androl 2005;51:353–360.
[17] Lake PE, Buckland RB, Ravie O. Effect of glycerol on the viability of fowl spermatozoa, implications for its use when freezing semen. Cryo-Lett 1980;1:299–304.
[18] Tselutin K, Narubina L, Mavrodina T, Tur B. Cryopreservation of poultry semen. Brit Poult Sci 1995;36:805–811.
[19] Purdy Y, Song FG, Silversides HD, Blackburn HD. Evaluation of glycerol removal techniques, cryoprotectants and insemination methods for cryopreserving rooster sperm with implications of regeneration of breed or line or both. Poult Sci 2009;88: 2184–2191.
[20] Lake PE, Ravie O. An exploration of cryoprotective compounds for fowl spermatozoa. Br Poul Sci 1984;25:145–150.
[21] Blanco JM, Long JA, Gee G, Donoghue AM, Wildt DE. Osmotic tolerance of avian spermatozoa: influence of time, temperature, cryoprotectant and membrane ion pump function on sperm viability. Cryobiology 2008;56: 8–14.
[22] Awad MM. Effect of some permeating cryoprotectants on CASA motility results in cryopreserved bull spermatozoa. Anim Reprod Sci 2011; 123:157–162.
[23] Gillmore JA, Liu J, Woods EJ, Peter AT, Crister JK. Cryoprotective agent and temperature effects on human sperm membrane permeabilities: convergence of theoretical and empirical approaches for optimal cryopreservation methods. Hum Reprod 2000;15:335–343.
[24] Rijsselaere T, Van Soom A, Tanghe S, Coryn M, Maes D, de Kruif A. New techniques for the assessment of canine semen quality: A review. Theriogenology 2005;64:706–719.
[25] Broekhuijse ML, Sontaric E, Feitsman H, Gadella BM. Additional valueof computer assisted semen analysis (CASA) compared to conventional motility assessments in pig artificial insemination. Theriogenology 2011;76:1473–1486.
[26] Parker HM, Yeatman JB, Schultz CD, Zumwalt CD, Mcdaniel CD. Use of sperm analyser for evaluating broiler breeder males: selection of young broiler breeder roosters for the sperm quality index increases fertile egg production. Poult Sci 2000;79:771–777.
[27] Burrows HW, Quinn JP. The collection of spermatozoa from the domestic fowl and turkey. Poult Sci 1937;16:19–24.
[28] Dumpala PR, Parker HM, McDaniel CD. Similarities and differences between the sperm quality index and sperm mobility index of Broiler Breeder semen. Poult Sci 2006b;85:2231–2240.
[29] Makhafola MB, Lehloenya KC, Mphaphathi ML, Dinnyes A, Nedambale TL. The effect of breed on the survivability and motility rate of cryopreserved cock semen. S Afr J Anim Sci 2009; 39:242–245.
[30] Makhafola MB, Umesiobi DO, Mphaphathi ML, Masenya MB, Nedambale TL. Characterisation of sperm cell motility rate of southern african indigenous cockerel semen following analysis by sperm class analyser. Anim Sci Adv 2012;2:416–424.
[31] Love CC. Relationship between sperm motility, morphology and the fertility of stallions. Theriogenology 2011;76:547–557.
[32] Siudzinska A, Lukaszewick E. The effect of breed on freezability of semen of fancy fowl. Anim Sci Pap and Rep 2008;4:331–340.
[33] Bjorndahl L, Soderlund I, Kvist U. Evaluation of the one-step eosinnigrosin staining techniques for human sperm viability assessment. Hum Reprod 2003;18:813–816.
[34] Williamson RG, Etches RJ, Reinhart BS, Macpherson JW. The effect of cooling rate before freezing and the temperature of the semen upon addition of DMSO on the fertilizing capacity of chicken semen stored at -196 曟. Reprod Nutr Dev 1981; 21:1033–1042.
[35] Molekwa JT, Umesiobi DO. Relationship between cock semen viability and the fertility of artificially inseminated South African indigenous chicken breeds. S Afr J Anim Sci 2009;39:24–28.
[36] George FG, Bertschinger H, Donoghue AM, Blanco J, Soley J. Reproduction in nondomestic birds: physiology, semen collection, artificial insemination and cryopreservation. Avian Poult Biol Rev 2004; 15:47–101.
[37] Tuncer PB, Kinet H, Ozdogan N. Evaluation of some spermatological characteristics in Gerze cocks. Ankara Univ Vet Derg 2008;55:99–102.
[38] Obidi JA, Onyeanusi BI, Rekwot PI, Ayo JO, Dzenda T. Seasonal variations in seminal characteristics of characteristics of Shikabrown breeder cocks. Int J Poult Sci 2008; 7:1219–1223.
[39] Donoghue AM, Wishart GJ. Storage of poultry semen. Anim Reprod 2000;62:213–232.
[40] Tselutin K, Seigneurin F, Blesbois E. Comparison of cryoprotectants and methods of cryopreservation of fowl spermatozoa. Poult Sci 1999;78:586–590.
[41] Van Thuan N, Wakayama S, Kishigami S, Wakayama T. New preservation method for mouse spermatozoa without freezing. Biol Reprod 2005;2:44–45.
[42] Sontakke SD, Umaphathy G, Sivaram V, Khokutel SD, Shivaji S. Semen characteristics, cryopreservation and successful artificial insemination in the Blue rock pigeon (Columba livia). Theriogenology 2004;62:139–153.
[43] Van Wambake F. Fertility and hatchability results with fowl spermatozoa stored in raw and freeze-dried diluent. Br Poult Sci 1972;13:179–183.
[44] Latif A, Ijaz A, Aleem M, Mahmud A. Effect of osmotic pressure and pH on the short-term storage and fertility of broiler breeder sperm. Pak Vet J 2005;25:179–182.
[45] Tabatabaei S, Aghaei A. Effect of L-carnitine on sperm quality during liquid storage of chicken semen. Comp Clin Path 2010; 30:163–169.
[46] Clarke RN, Sexton TJ, Ottinger MA. Effect of holding temperature and storage time on respiratory rate, motility and fertility of chicken and turkey semen. Poult Sci 1982;61:1912–1917.
[47] Lukaszewicz E, Kruszynski W, Fujihara N. Effect of age on quality of raw and frozen-thawed semen in White Italian ganders. Asian J Andro 2003;5:89-93.
[48] Tabatabaei S, Batavani RA, Talebi AR. Comparison of oviducttal sperm age on fertility, hatchability and embryonic death rates in Iranian indigenous and Ross-308 broiler breeder chickens. J Anim Vet Advanc 2009;8:85–89.
[49] Madeddu M, Berlinguera F, Pasciu V, Succu S, Satta V, Leoni GG, et al. Differences in semen freezability and intracellular ATP content between the rooster (Gallus gallus domesticus) and the Barbary partridge (Alectoris barbara). Theriogenology 2010;74:1010–1018.
[50] Moce E, Grasseau I, Blesbois E. Cryoprotectant and freezing-process alter the ability of chicken sperm to acrosome react. Anim Reprod Sci 2010;122:359–366.
[51] De Leeuw FE, De Leeuw AM, Den Daas JHD, Colenbrander B, Verkleu AJ. Effects of various cryoprotective agents and membrane-stabilizing compounds on bull sperm membrane intergrity after cooling and freezing. Cryobiology 1993;30:32–44.
[52] Salamon S, Maxwell WMC. Frozen storage of ram semen, processing, freezing, thawing and fertility after cervical insemination. Anim Reprod Sci 1995; 37:185–249.
[53] Fernandez-Santos MR, Esteso MC, Montoro V, Soler AJ, Garde JJ. Influence of various permeating cryoprotectants on freezability of Iberian red deer (Cervus elaphus hispanicus) epididymal spermatozoa: Effects of concentration and temperature of asddition. J Androl 2006;27:734–745.
[54] Gilmore JA, Liu J, Gao DY, Crister JK. Determination of optimal cryoprotectants and procedures for their addition and removal from human spermatozoa. Hum Reprod 1997;12:112–118.
[55] Umaphathy G, Sontake S, Reddy A, Ahmed S, Shivaji S. Semen characteristics of the captive Indian White-backed vulture (Gyps bengalensis). Biol Reprod 2005; 73:1039–1045.
[56] Pena FJ, Saravia F, Martinnez IN, Johannisson A, Wallgren M, Martinez HR. Do different portions of the boar ejaculate vary in their ability to sustain cryopreservation? Anim Reprod Sci 2006;93:101–113.
[57] Waterhouse KE, Hofmo PO, Tverdal A, Miller RR. Within and between breed differences in freezing tolerance and plasma membrane fatty acid composition of boar sperm. J Soc Reprod Fertil 2006;131:887–894.
[58] Pukazhenthi B, Pelican K, Wildt D, Howard J. Sensitivity of domestic cat (felis catus) sperm from normospermic versus teratospermic donors to cold-induced acrosomal damage. Biol Reprod 1999;61:135–141.
[59] Curry MR. Cryopreservation of semen from domestic livestock. J Reprod Fertil 2000;5:46-52.
[60] Thurston LM, Watson PF, Mileham AJ, Holt WV. Morphologically distinct sperm subpopulations defined by Fourier shape descriptors in raw ejaculates correlate with variation in boar semen quality following cryopreservation. J Androl 2001;22:382–394.
[61] Celeghini ECC, Paes De Arruda RP, Cesar De Andrade AF, Nascimento J, Raphael CF, Rodrigues PHM. Effects that bovine sperm cryopreservation using two different extenders has on sperm membranes and chromatin. Anim Reprod Sci 2008;104:119–131.
9 December 2015
Tshimangadzo L. Nendambale, Tshwane University of Technology, Faculty of Science, Department of Animal Sciences, Private Bag X 680, Pretoria, 0001, Republic of South Africa.
E-mail: NendambaleTL@TUT.ac.za; masindim@arc.agric.za
Foundation project: This study was supported by grants from the Agricultural Research Council Parliamentary Grant, the Department of Agriculture Forestry and Fishery and the National Research Foundation - GUN No RPPP12 & RT 24000 (NRF).
Received in revised form 12 January 2016 Accepted 20 January 2016
Available online 1 March 2016
杂志排行
Asian Pacific Journal of Reproduction的其它文章
- Effect of growth regulators on rapid micropropagation and antioxidant acitivity of Canscora decussata (Roxb.) Roem. & Schult.-A threatened medicinal plant
- A new nucleotide variant G1358A potentially change growth differentiation factor 9 profile that may affect the reproduction performance of Friesian Holstein cattle
- Pregnancy rate in Bulgarian White milk goats with natural and synchronized estrus after artificial insemination by frozen semen during breeding season
- Effect of heparin, caffeine and calcium ionophore A 23187 on in vitro induction of the acrosome reaction of fresh ram spermatozoa
- Pregnancy outcomes of using ICSI with frozen-thawed spermatozoa in Riyadh, Saudi Arabia
- Effects of intramuscular injections of vitamin E-selenium and a gonadotropin releasing hormone analogue (GnRHa) on reproductive performance and blood metabolites of post-molt male broiler breeders
