Rapid and Sensitive Chemiluminescent Enzyme Immunoassay for the Determination of Neomycin Residues in Milk*
2016-08-15LUOPengJieZHANGJianBoWANGHuaLiCHENXiaWUNanZHAOYunFengWANGXiaoMeiZHANGHongZHANGJiYueZHULeiandJIANGWenXiao
LUO Peng Jie, ZHANG Jian Bo, WANG Hua Li, CHEN Xia, WU Nan, ZHAO Yun Feng,WANG Xiao Mei, ZHANG Hong, ZHANG Ji Yue, ZHU Lei, and JIANG Wen Xiao,#
Letter to the Editor
Rapid and Sensitive Chemiluminescent Enzyme Immunoassay for the Determination of Neomycin Residues in Milk*
LUO Peng Jie1, ZHANG Jian Bo1, WANG Hua Li1, CHEN Xia2, WU Nan3, ZHAO Yun Feng1,WANG Xiao Mei3, ZHANG Hong1, ZHANG Ji Yue1, ZHU Lei1, and JIANG Wen Xiao3,#
Immunoassays greatly contribute to veterinary drug residue analysis. However, there are few reports on detecting neomycin residues by immunoassay. Here, a rapid and sensitive chemiluminescent enzyme immunoassay (CLIEA)was successfully developed for neomycin residue analysis. CLIEA demonstrated good cross-reactivity for neomycin, and the IC50value was 2.4 ng/mL in buffer. The average recovery range was 88.5%-105.4% for spiked samples (10, 50, and 100 μg/kg), and the coefficient of variation was in the range of 7.5%-14.5%. The limit of detection of CLEIA was 9.4 μg/kg, and this method was compared with the liquid chromatography-tandem mass spectrometry method using naturally contaminated samples, producing a correlation coefficient of >0.95. We demonstrate a reliable CLIEA for the rapid screening of neomycin in milk.
Neomycin (Figure S1) is an aminoglycoside antibiotic produced by Streptomyces fradiae. Neomycin can disturb protein synthesis in bacteria by binding to the 30S subunit of ribosomal RNA,which causes misreading of the genetic code and inhibiting translation[1]. Due to its growth inhibition of Gram-negative bacteria, neomycin is widely used in veterinary medicine to treat gastrointestinal infections by the oral route and mastitis by intramammary administration. However,intravenous or intramuscular injections of neomycin normally produce residues in milk, particularly when the withdrawal time is respected. Neomycin can present potentially ototoxic and nephrotoxic activity to humans and animals despite its impressive clinical successes. In addition, the excessive use of neomycin has been associated with the drastically increasing prevalence of multidrug-resistant pathogens. Therefore, the European Union, the United States,and China have set maximum residue limits (MRLs)for neomycin to be 1500 μg/kg for milk[2]
.
Various analytical techniques have been established to detect neomycin residues[3]. The reference analysis methods are instrumental methods, such as high-performance liquid chromatography with UV, fluorescence, or liquid chromatography-tandem mass spectrometry (LC-MS/MS) detection. These methods are sensitive and highly specific but require expensive equipment,large volumes of solvents, and a time-consuming sample cleanup process[4]. Therefore, they are unsuitable for use in routing screens or field detection.
Immunoassays are widely used methods for routine screening analysis of veterinary drugs in a large number of samples. The antibody-based analytical methods, primarily enzyme-linked immunosorbent assay (ELISA), are useful as simple,fast, and sensitive tools for screening veterinary drug residues[5]. ELISA, immunochromatographic assay,and immunosensors are described for neomycin residue analysis[4,6-8]. To improve the detection ability, we have developed a more sensitive chemiluminescent enzyme immunoassay (CLEIA) for the trace determination of neomycin in milk. Compared with conventional colorimetric detection,CLEIA offers the possibility of improving sensitivity by at least 2-3 orders of magnitude.
The artificial antigen was constituted by coupling neomycin to ovalbumin (OVA) as per previous research[7]. Next, 10 mg OVA was dissolved in 2 mL 0.01 mol/L PBS (pH 7.4), which contained 20 mg neomycin. Then, 31 mg of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride was added drop-wise to this solution. The reaction mixture was gently stirred for 6 h at 4 °C. The synthesized coating antigen NOE-OVA was dialyzed against PBS for 3 days before use.
Three commercially available antibodies were used to develop an ELISA to assess their analytical performance. Antibody 1, which was purchased from Abcam, has the best sensitivity, and was optimized for the following experiments (Figure S2).
Here,our aim was to develop a sensitive CLEIA method for the detection of neomycin in milk. CLEIA can be described as follows: microtiter plates were coated with coating antigen (100 µL/well), and then the plates were incubated at 4 °C overnight. After blocking, 50 µL of neomycin standard solutions and 50 µL of diluted antibody solutions were added to each well, and the plates were incubated for 30 min at 37 °C. After washing, 100 µL of goatanti-rabbit IgG-HRP solutions were added, and the plates were incubated for 30 min at 37 °C. After washing another three times, 100 µL of super signal substrate solutions were pipetted, the intensity of light emission was measured, and the results were expressed in relative light units (RLU).
CLEIA optimization was performed for the most sensitive assay using neomycin as a competitor analyst. Several physicochemical factors influencing immunoassay performance were studied in CLEIA. Modifications of RLUmaxand IC>50>parameters of the standard curves were evaluated under different conditions. The experimental conditions for the concentrations of coating antigen and antibody, the immunoreaction time, and pH and ionic strength of assay buffer were optimized in order to improve the performance of the immunoassay and the results are represented in Table S1. Competition experiments were carried out by checkerboard titration to select the suitable concentrations of immunoreagents. The optimal dilutions of the coating antigen NEO-OVA and the antibody were 1:16,000 and 1:8000,respectively.
The effect of the competition time (15-60 min)was tested. In general, the RLUmaxvalues decreased gradually and the IC50values increased gradually as competition time decreased. The best competition time was 30 min, because the RLUmaxvalue was high and the sensitivity was improved. The incubation time for the goatanti-rabbit IgG-HRP was tested at 15-60 min. The decrease in RLUmaxvalues and increase insensitivity (IC50) were observed as the incubation time decreased. Based on the results of Table S1, 30 min was selected as a compromise between RLUmaxand IC50.
When the pH effect was tested (6.0-8.0), a gradual increase in the RLUmaxvalue was observed. However, since the RLUmaxvalues obtained at pH 6.0 and 7.0 were <0.8×106, pH 9.0 was selected as the optimum to keep an acceptable RLUmaxvalue and a minimum IC50value. The effect of ionic strength, from NaCl concentrations of 10-500 mmol/L, was evaluated. The optimum concentration of NaCl, selected as a compromise between the RLUmaxvalue and sensitivity (IC50), was 200 mmol/L (Table S1).
Standards or samples were assayed in triplicate wells, and the chemiluminescence intensity values were divided by RLUmax(chemiluminescence intensity in the assay buffer). The RLU value of the wells containing only assay buffer was referred to as B0. The RLU values of the standards were normalized against the RLU value of the assay buffer (B/B0). The standard concentration at the midpoint of the standard curve was the concentration of competitor that inhibited the binding of the antibody by 50% (IC50)[9]. Competition curves were fitted to a four-parameter logistic equation, from which IC50values were calculated. Representative CLEIA and ELISA standard curves for neomycin obtained in this study are demonstrated (Figure 1). The IC50values were 2.4 ng/mL and 26.2 ng/mL for CLEIA and ELISA for detecting neomycin in buffer, respectively.
In addition to sensitivity, specificity is an important parameter. The specificity of CLEIA was evaluated by determining the cross-reactivity with a set of structurally related analogues using the following equation[10]: cross-reactivity (%) = (IC50of neomycin)/(IC50of analogues) × 100%. The IC50and cross-reactivity values are summarized (Table S2). CLEIA was highly specific for neomycin, and demonstrated negligible cross-reactivity with the other analytes.
Milk samples were purchased from local supermarkets and stored in a refrigerator (4 °C)before use. Each sample was verified to be neomycin-free by LC-MS/MS before the spike and recovery tests. One milliliter of each milk sample was transferred to 50 mL polypropylene centrifuge tubes. After addition of 4 mL of 0.1 mol/L citrate buffer solution (pH 4.3), the mixtures were vigorously vortexed. After centrifugation at 4000 ×g for 5 min,the supernatants were diluted 4-fold in the assay buffer before analysis.
The matrix effect may reduce the sensitivity and reliability of the immunoassay, and cause false positives by lowering the RLU values. To obtain the basic information on matrix effects, standard curves generated in PBS were compared with curves obtained using diluted matrix. A dilution of 20 times with PBS could significantly reduce matrix effects.
Assay validation of CLEIA was conducted according to the related content of the Commission Decision 2002/657/EC. The LOD was the lowest amount of analyte in a sample that could be detected but not necessarily quantified exactly. Based on the determination of 20 different blank samples, the LOD of the developed CLEIA was 9.4 µg/kg. To evaluate the accuracy and precision of CLEIA, a spike-and-recovery test was conducted. Blank milk samples were spiked with known amounts of neomycin and then assayed using the proposed CLEIA. The results of the accuracy and precision tests are represented in Table 1. When the control samples were spiked at levels of 10, 50, and 100 µg/kg, the average recovery was in the range of 88.5%-105.4%, with the intra- and inter-assay coefficients of variation (CVs) in the range of 7.5%-14.5%. According to the standard of the European Commission (2002), the neomycin mass fractions are >100 µg/kg but <1000 µg/kg, the average recovery should be in the range of 80%-110%, and the intra- and interassay CVs should be ≤15%. Therefore, the accuracy and precision are acceptable, and CLEIA has good repeatability and reproducibility.
经过治疗,观察组的疾病知晓率是90.00%(45/50),治疗依从性是92.00%(46/50),对照组的疾病知晓率是78.00%(39/50),治疗依从性是82.00%(41/50),结果存在统计学差异性(P<0.05)。两组的阳性、阴性症状经过治疗均有所改善,观察组的改善情况比对照组突出,结果存在统计学差异性(P<0.05)。
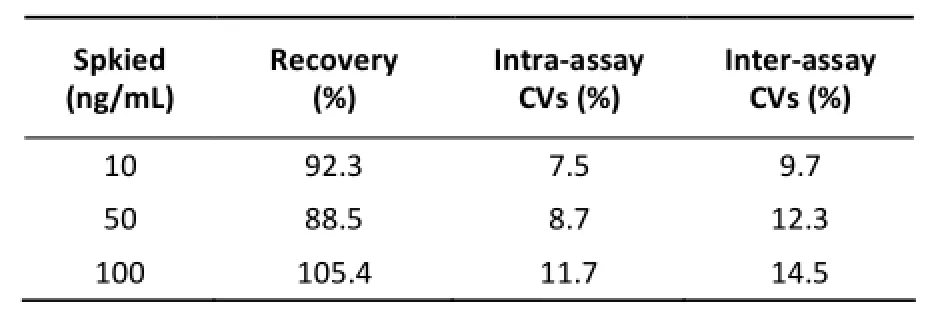
Table 1. Average Recovery and Coefficients of Variation (CVs) for Detecting Neomycin in Milk (n=4)
To demonstrate the reliability of CLEIA in the present study, 5 field samples were analyzed using the developed CLEIA and LC-MS/MS methods. The CLEIA results were compared with those of LC-MS/MS analysis using a correlation test. The results obtained by CLEIA were compatible with those obtained by the instrumental method, and the coefficient of correlation R2was 0.95 (Figure 2).
Immunoassays are widely used methods for routine screening analysis of veterinary drugs in a large number of samples. During the last 10 years,few researchers have reported the production of antineomycin antibodies. ELISA,immunochromatographic assay, amperometric immunosensors, and several other sensors have been previously described for the residue analysis of neomycin in different food matrices[2,6-8]. The LODs of these methodological approaches are varied, and most of these reports resulted in lower LODs in the range of 6.76-20 µg/kg. Till date, no CLIEAs have been established for the detection of neomycin residues in milk. In contrast to these reports, a sensitive CLEIA was established to estimate neomycin in milk with a high sample throughput. Moreover, performance characteristics (specificity,accuracy, and detection capability) were validated according to the provisions of Council Decision 2002/657. The LODs of our developed CLEIA are below said values and satisfy the MRLs set by the European Commission, the USA, and China. This method would be a cost-effective tool for rapid and semi-quantitative detection of neomycin residues in milk before confirmation by instrumental methods.
#Correspondence should be addressed to JIANG Wen Xiao, Tel: 86-755-86671936, Fax: 86-755-86671906, E-mail:jiangwenxiao@szu.edu.cn
^These author contributed equally to this work.
Biographical notes of the authors: LUO Peng Jie, male,1980, PhD, majoring in food safety; ZHANG Jian Bo, male,1975, PhD, majoring in food safety.
Accepted: May 5, 2016
REFERENCES
1. Ahmed AM, Maruyama A, Khalifa HO, et al. Seafood as a reservoir of gram-negative bacteria carrying integrons and antimicrobial resistance genes in japan. Biomed Environ Sci,2015; 28, 924-7.
3. McGlinchey TA, Rafter PA, Regan F, et al. A review of analytical methods for the determination of aminoglycoside and macrolide residues in food matrices. Anal Chim Acta, 2008; 624,1-15.
4. Jiang W, Beloglazova NV, Wang Z, et al. Development of a multiplex flow-through immunoaffinity chromatography test for the on-site screening of 14 sulfonamide and 13 quinolone residues in milk. Biosens Bioelectron, 2015; 66, 124-8.
5. Jiang W, Beier RC, Luo P, et al. Analysis of pirlimycin residues in beef muscle, milk, and honey by a biotin-streptavidin-amplified enzyme-linked immunosorbent assay. J Agric Food Chem, 2016;64, 364-70.
6. Gaudin V, Cadieu N, Sanders P. Results of a European proficiency test for the detection of streptomycin/dihydrostreptomycin, gentamicin and neomycin in milk by ELISA and biosensor methods. Anal Chim Acta, 2005;529, 273-83.
7. Wang S, Xu B, Zhang Y, et al. Development of enzyme-linked immunosorbent assay (ELISA) for the detection of neomycin residues in pig muscle, chicken muscle, egg, fish, milk and kidney. Meat Sci, 2009; 82, 53-8.
8. Zhu Y, Son JI, Shim Y-B. Amplification strategy based on gold nanoparticle-decorated carbon nanotubes for neomycin immunosensors. Biosens Bioelectron, 2010; 26, 1002-8.
9. Wang X, Luo P, Chen J, et al. Development of a quantitative immuno-affinity test column assay for on-site screening of clindamycin residues in milk. Int Dairy J, 2016; 55, 59-63.
10. Jiang W, Wang Z, Beier RC, et al. Simultaneous determination of 13 fluoroquinolone and 22 sulfonamide residues in milk by a dual-colorimetric enzyme-linked immunosorbent assay. Anal Chem, 2013; 85, 1995-9.
10.3967/bes2016.048
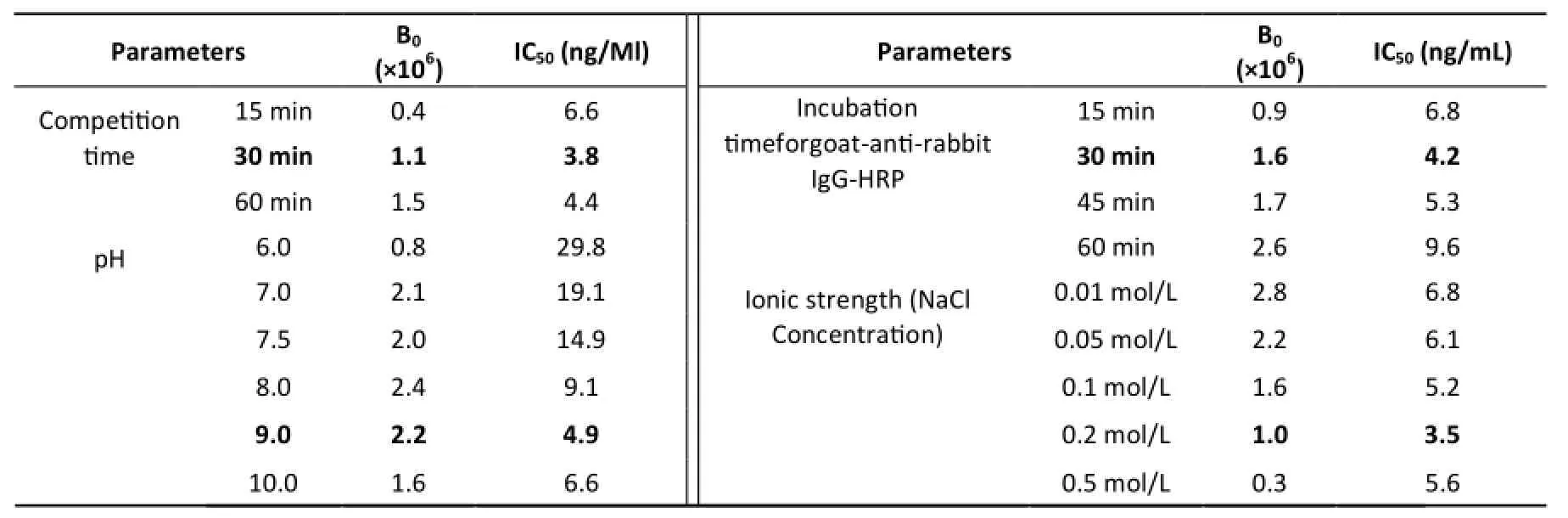
Table S1. Refined Parameters on the Indirect Competitive CLEIA Performance
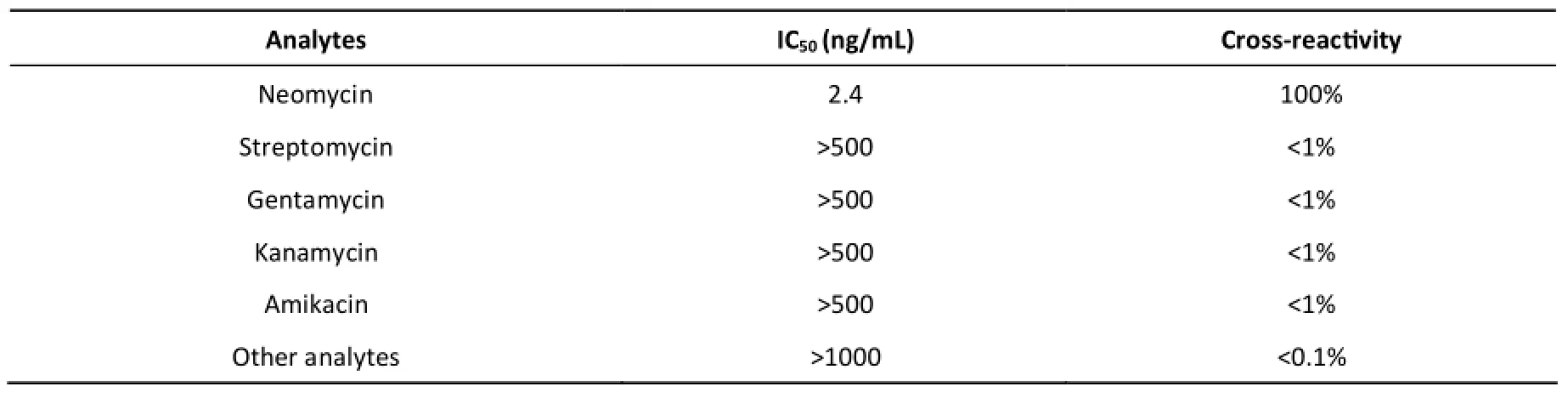
Table S2. Cross-reactivity Results of the Neomycin Antibody
*This work was supported by the project for talent training and development of the China National Center for Food Safety Risk Assessment (523 plan); Natural Science Foundation of Guangdong Province (No. 2014A030310289 and No. 2016A020210055); Natural Science Foundation of SZU (No. 201576); and National Natural Science Foundation of China (No. 21107104).
1. Key Laboratory of Food Safety Risk Assessment, Ministry of Health, China National Center for Food Safety Risk Assessment, Beijing 100022, China; 2. State Key Laboratory for infectious Disease Prevention and Control, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing 102206, China; 3. Department of Physiology,School of Basic Medical Sciences, Shenzhen University Health Sciences Center, Shenzhen 518060, Guangdong, China
October 14, 2015;
猜你喜欢
杂志排行
Biomedical and Environmental Sciences的其它文章
- Development of a Novel PmpD-N ELISA for Chlamydia psittaci Infection*
- Evaluation of Six Recombinant Proteins for Serological Diagnosis of Lyme Borreliosis in China*
- Viral Etiology Relationship between Human Papillomavirus and Human Breast Cancer and Target of Gene Therapy
- Whole Genome Sequencing and Comparisons of Different Chinese Rabies Virus Lineages Including the First Complete Genome of an Arctic-like Strain in China*
- The Status and Associated Factors of Successful Aging among Older Adults Residing in Longevity Areas in China*
- Cognitive Training in Older Adults with Mild Cognitive Impairment
