Interspecific variation of thermoregulation between small migratory and resident passerines in Wenzhou
2016-07-25QingGangQIAOHongJiLIANGMinLanBAIWeiHongZHENGJinSongLIUSchoolofLifeandEnvironmentalSciencesWenzhouUniversityWenzhou35035ChinaZhejiangProvincialKeyLabforSubtropicalWaterEnvironmentandMarineBiologicalResourcesProtectionW
Qing-Gang QIAO, Hong-Ji LIANG, Min-Lan BAI, Wei-Hong ZHENG,, Jin-Song LIU,,*School of Life and Environmental Sciences, Wenzhou University, Wenzhou 35035, ChinaZhejiang Provincial Key Lab for Subtropical Water Environment and Marine Biological Resources Protection, Wenzhou 35035, China
Interspecific variation of thermoregulation between small migratory and resident passerines in Wenzhou
Qing-Gang QIAO1, Hong-Ji LIANG1, Min-Lan BAI1, Wei-Hong ZHENG1,2, Jin-Song LIU1,2,*1School of Life and Environmental Sciences, Wenzhou University, Wenzhou 325035, China
2Zhejiang Provincial Key Lab for Subtropical Water Environment and Marine Biological Resources Protection, Wenzhou 325035, China
ABSTRACT
Physiological adaptation arises from several fundamental sources of phenotypic variation.Most analyses of metabolic adaptation in birds have focused on the basal metabolic rate (BMR), the lower limit of avian metabolic heat production.In this study, we investigated thermoregulation in three passerine species; the yellow-billed grosbeak Eophona migratoria, white-rumped munia Lonchura striata and black-throated bushtit Aegithalos concinnus, in Wenzhou, China.Metabolic rate was measured using the closed-circuit respirometer containing 3.5 L animal chambers.Body temperature (Tb) was measured during metabolic measurements using a lubricated thermocouple.The minimum thermal conductance of these species was calculated by measuring their Tband metabolic rates.The yellow-billed grosbeak remained largely normothermic, and the white-rumped munia and black-throated bushtit exhibited variable Tbat ambient temperatures (Ta).Mean metabolic rates within thermal neutral zone were 2.48±0.09 O2(mL)/g/h for yellow-billed grosbeaks, 3.44±0.16 O2(mL)/g/h for white-rumped munias, and 3.55±0.20 O2(mL)/g/h for black-throated bushtits, respectively.Minimum thermal conductance of yellow-billed grosbeak,white-rumped munia and black-throated bushtit were 0.13±0.00, 0.36±0.01, and 0.37±0.01 O2(mL)/g/h/˚C,respectively.The ecophysiological characteristics of these species were: (1) the yellowbilled grosbeak had relatively high Tband BMR, a low lower critical temperature and thermal conductance, and a metabolic rate that was relatively insensitive to variation in Ta; all of which are typical of cold adapted species and explain its broader geographic distribution; (2) the white-rumped munia and blackthroated bushtit had high thermal conductance,lower critical temperature, and relatively low BMR,all which are adapted to warm environments where there is little selection pressure for metabolic
thermogenesis.Taken together, these data illustrate small migratory and resident passerinesthat exhibit the different characteristics of thermoregulation.
Keywords:Basal metabolic rate; Body temperature;Thermal conductance; Eophona migratoria; Lonchura striata; Aegithalos concinnus
lNTRODUCTlON1
Heat production in response to ambient temperature is commonly referred to as facultative, or adaptive, thermogenesis (Angilletta et al., 2010; Silva, 2006; Zhou et al., 2016).Endotherms primarily use mechanisms that equalize rates of heat production and loss to maintain a high and constant body temperature (Corp et al., 1997; Liu et al., 2004a).The necessity of maintaining an optimal body temperature is one of the major factors influencing the abundance and distribution of birds (Liu et al., 2005; Weathers, 1979), and birds have evolved many morphological and physiological adaptations to achieve this (Swanson & Merkord, 2013; Wiersma et al., 2007).Physiological adaptation arises from several fundamental sources of phenotypic variation.Most analyses of metabolic adaptation in birds have focused on the basal metabolic rate (BMR), the lower limit of avian metabolic heat production (McKechnie et al., 2006; McNab, 2009).BMR is a standardized baseline metabolic parameter that reflects a species' resting energy requirements in the absence of the increased metabolic demands associated with thermoregulation, digestion, activity or circadian rhythms (McKechnie, 2008; McNab, 2009; Zhou et al., 2016).BMR is a widely-accepted benchmark of metabolic expenditure for birds that is commonly used as a measure of
Received: 05 Feburary 2016; Accepted: 20 May 2016
Ambient temperature (Ta) is considered one of the most important environmental factors affecting birds because it causes marked changes in their energy expenditure (Nzama et al., 2010) and has driven the evolution of a suite of morphological and physiological adaptations (Swanson et al.,2014).A considerable body of research has been conducted to examine the effects of temperature on animal adaptation,survival, and reproductive success (Sgueo et al., 2012; Zhou et al., 2016).In a comparative study of small birds with different habitats and habits, Rezende et al.(2002) recently emphasized the ecological significance of BMR.For example, tropical birds typically have a lower BMR than cold temperate birds, which is thought to be an adaptation to avoid heat stress and conserve water (Weathers, 1997; Wiersma et al., 2007).Conversely, the higher BMR of cold temperate and Arctic birds is thought to be an adaptation to colder temperatures and shorter breeding seasons (Klaassen, 1995; Zheng et al., 2014b).It has been suggested that the BMR of a number of long-distance migratory birds is lower in their tropical overwintering range than at their temperate breeding grounds (Lindström & Klaassen, 2003;Zheng et al., 2013).The higher metabolic capacities of high latitude species may involve a combination of genetic responses to climatic factors (Liknes & Swanson, 2011;Swanson, 2010; Wikelski et al., 2003).These findings indicate that environmental conditions are very important in shaping the thermoregulatory features of a species. There is now considerable evidence to show that the metabolic characteristics of birds are part of a network of physiological mechanisms that mediate major life-history trade-offs (Wikelski et al., 2003).
The yellow-billed grosbeak Eophona migratoria is a migratory bird that inhabits vast areas of northeast Asia.The whiterumped munia Lonchura striata is a common resident breeder in southern China and South Asia.The distribution of the blackthroated bushtit Aegithalos concinnus ranges from the foothills of the Himalayas, across northern India through Nepal, to northern Vietnam and Taiwan (MacKinnon & Phillipps, 2000).Although all three species experience the same temperature and photoperiod in autumn and winter in Wenzhou, the yellowbilled grosbeak is a Palearctic bird that migrates to Wenzhou in winter whereas the white-rumped munia and black-throated bushtit are Indomalayan species that are resident in Wenzhou,and their thermoregulatory responses could differ (e.g., climatic differences across the ranges).To test the hypothesis that characteristics of thermoregulation in these three species are consistent with their respective biogeographic distributions, we compared body temperature, metabolic rate and thermal conductance among three small birds at different ambient temperatures. These results could make us better understand how these species adapt their environments. In addition,through the comparison with other small birds, it will help identify sources of variation in thermoregulatory characteristics in these small birds.
MATERlALS AND METHODS
Animals
Seven yellow-billed grosbeaks (six male, one female), ten white-rumped munias (six male, four female) and nine blackthroated bushtits (seven male, two female) were captured by mist nets in Wenzhou city (N27°29', E120°51'), Zhejiang Province, China.The yellow-billed grosbeak Eophona migratoria is a granivorous and insectivorous migratory bird.The white-rumped munia Lonchura striata is a gregarious bird that feeds mainly on seeds.The black-throated bushtit Aegithalos concinnus mainly feeds on small insects and spiders,as well as small seeds, fruits and berries (particularly raspberries).The climate in Wenzhou is warm-temperate with the mean annual temperature is 18 ˚C.There are seven months of the year (March through September) in which the maximum temperature is above 37 ˚C (Zheng et al., 2008a; 2014a).All experiments were carried out from October to December 2012.Animals were kept in individual cages (50 cm×30 cm×20 cm)under natural photoperiod (14L:10D) with lights on at 0600h and temperature (25 ˚C).Food and water were supplied ad libitum.Yellow-billed grosbeaks and white-rumped munias were fed millet seeds, and black-throated bushtits were fed bird cake and mealworm Tenebriomolitor larvae. The mean body mass of yellow-billed grosbeaks, white-rumped munias and blackthroated bushtits was 50.5±0.6 g (47.9-52.4 g), 12.6±0.3 g (11.1-14.5 g) and 6.8±0.1 g (6.4-7.4 g), respectively.All experimental procedures were approved by the Wenzhou City Animal Care and Use Committee, Zhejiang Province, China (Wu et al., 2015).
Measurement of metabolic rate
Oxygen consumption was measured using a closed-circuit respirometer according to the methods described by Górecki (1975) and Liu et al.(2004a; 2005).The volume of the metabolic chamber was 3.5 L and its temperature was controlled by a water bath in Artificial Climatic Engine (BIC-300,Shanghai) and maintained to ±0.5 ˚C. Every bird was tested only one Ta per day with at least two days between tests (Xia et al., 2013).Oxygen consumption rates were measured over a temperature range of 5 ˚C to 35 ˚C.Food was withheld four hours before animals were placed in the metabolic chamber to minimize the heat increment associated with feeding before each test.Birds were weighed to the nearest 0.1 g before being put in the chamber.Water and CO2were absorbed from the air in the chamber by silica gel and KOH.All measurements were made between 2000h and 2400h.Each trial lasted for one hour and commenced after animals had been inside the metabolic chamber for about one hour to acclimate.The reading interval for O2consumption was 10 min.Two or three consecutive,stable, minimum, recordings were used to calculate metabolic rates (Zheng et al., 2008b).Records of oxygen consumption when birds were active within the chamber were not used to compute the metabolic rate of each individual.Metabolic rates were expressed as O2(mL)/g/h, and corrected to standardtemperature and pressure conditions (Schmidt-Nielsen, 1997).Body mass was measured to the nearest 0.1 g before and after experiments.Mean body mass was used in calculations (Liu et al., 2005; Wu et al., 2015).
Measurement of body temperature
Body temperature (Tb) was measured during metabolic measurements using a lubricated thermocouple.This was inserted into the cloaca of each bird to a depth at which a slight withdrawal did not result in a change in the reading (1-2 cm).Thermocouple outputs were digitized using a thermocouple meter (Beijing Normal University Instruments Co., China) (Wu et al., 2015).
Calculation of thermal conductance

Total wet thermal conductance (C, O2(mL)/g/h/˚C) at any given Tawas calculated using the formula: Where MR is metabolic rate (O2(mL)/g/h/˚C), Tbthe body temperature (˚C), and Tathe ambient temperature (˚C).This formula was suggested by Aschoff (1981) for calculating conductance at any given Ta.
Statistics
The data were analyzed using the SPSS statistical package (version 12.0 for windows).The effect of Taon body temperature, metabolic rate and thermal conductance were analyzed using repeated measures ANOVA.Where appropriate,multiple post hoc comparisons were performed using the least significant difference method (LSD).The relationships between metabolic rate and Tband Tawere modeled by fitting linear regression models.The relationships between thermal conductance and Tawere modeled by fitting exponential equation models, to the data, as appropriate.All results were expressed as mean±SE and P<0.05 was taken to be statistically significant.
RESULTS
Males and females of each species did not differ significantly in any measured variable (P>0.05 in all cases), so we pooled data for each species for subsequent analyses.
Yellow-billed grosbeak
The mean Tbof this species was 39.9±0.1 ˚C.Although there was no significant difference in Tbover a range of Tafrom 5 ˚C to 32.5 ˚C (F7,41=1.059, P>0.05, Figure 1A), there was, however,significant differences in metabolic rate (MR) over this temperature range (F7,41=21.231, P<0.001, Figure 1B).We were unsuccessful in identifying a join-point using a two-phase regression procedure (Nickerson et al., 1989), so we instead fit a linear regression model to data below 25 ˚C.Below 25 ˚C,MR increased with decreasing temperature as per the following equation:

At 25 ˚C, MR appeared independent of Ta, averaging 2.48± 0.09 O2(mL)/g/h.The line described by the above equation intersected MR at 23.5 ˚C, the lower critical temperature.Thermal conductance increased from 15 °C to 32.5°C (F7,41=49.802, P<0.001, Figure 1C), as it was stable within the range of 5˚ C-15 °C.Minimum thermal conductance was 0.13± 0.00 O2(mL)/g/h/˚C (Table 1).Thermal conductance increased exponentially from 25 ˚C to 32.5 ˚C as per the equation: Maximum thermal conductance was 0.41±0.02 O2(mL)/g/h/˚C at 32.5 ˚C.


Figure 1 Mean body temperature (A), metabolic rate (B) and thermal conductance (C) of wild caught yellow-billed grosbeaks Eophona migratoria measured in an experimental facility in Wenzhou, China at ambient temperatures of approximately 5 ˚C-32.5 ˚C
White-rumped munia
The Tbof the white-rumped munia fluctuated significantly over ambient temperatures between 5 ˚C and 37.5 ˚C (F9,90= 11.190, P<0.001, Figure 2A).Mean Tbwas 39.9±0.1 ˚C (Table 1)and ranged from 39.1±0.2 ˚C at 5 ˚C to 40.6±0.2 ˚C at 37.5 ˚C.There was a positive, linear relationship between Tband Taover this temperature range as per the following equation:

The MR of this species varied significantly between 5 ˚C and 37.5 ˚C (F9,90=98.310, P<0.001, Figure 2B).Between 30 ˚C and 35 ˚C, MR appeared to be independent of Ta and averaged 3.44±0.16 O2(mL)/g/h (n=36).For Tas below 30 ˚C,

The line intersected MR at 34.5 ˚C, so the lower critical temperature was 34.5 ˚C.Thermal conductance varied significantly within a temperature range from 5 ˚C to 37.5 ˚C (F9,90=51.434, P<0.001, Figure 2C), but was stable within a temperature range of 5 ˚C to 20˚C.Minimum thermal conductance was 0.36±0.01 O2(mL)/g/h/˚C. There was asignificant, linear relationship between thermal conductance and Tabetween 32.5 ˚C and 37.5˚C described by the equation:

Maximum thermal conductance attained 1.52±0.13 O2(mL)/g/h/˚C at 37.5 ˚C.

Table 1 Energetic parameters of wild-caught yellow-billed grosbeaks, white-rumped munias and black-throated bushtits measured in an experimental facility at Wenzhou, China

Figure 2 Mean body temperature (A), metabolic rate (B) and thermal conductance (C) of wild caught white-rumped munias Lonchura striata measured in an experimental facility in Wenzhou,China at ambient temperatures of approximately 5 ˚C-37.5 ˚C
Black-throated bushtit
Tbvaried significantly in this species between 5 ˚C and 34 ˚C (F7,64=8.173, P<0.001, Figure3A).Mean Tbwas 38.9±0.1 ˚C (Table 1) and tended to increase with Ta, ranging from 37.8±0.4 ˚C at 5 ˚C to 40.4±0.3 ˚C at 34 ˚C.The relationship between Tband Taover this temperature range can be described by the following equation:

There were also significant differences in MR from 5 ˚C to 34˚C (F7,64=58.547, P<0.001, Figure3B).At 28 ˚C, MR appeared to be independent of Taand averaged 3.55±0.20 O2(mL)/g/h.For Tas below 28˚C,
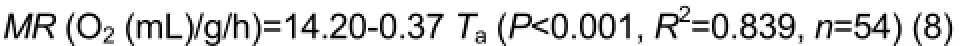
The line intersected MR at 28.8 ˚C, so the lower critical temperature was 28.8 ˚C. Thermal conductance increased significantly with Ta(F7,64=20.001, P<0.001, Figure3C).Minimum thermal conductance was 0.37±0.00 O2(mL)/g/h/˚C from 5 ˚C to 28 ˚C. The relationship between thermal conductance and Tacan be described by the equation:

Maximum thermal conductance averaged 0.80±0.08 O2(mL)/g/h/˚C at 34 ˚C.
DlSCUSSlON
lnterspecific variation in metabolic rate, thermal neutral zone (TNZ) and thermoregulation
Londoño et al.(2015) used allometric equations to calculate the expected the BMR of a range of bird species from their published body mass. According to Londoño et al.(2015), therelationship between BMR and Mbfor tropical species, BMR (watts)=0.449 Mb0.589(BMR=Watt, Mb=g); for temperate species,BMR (watts)=0.023 Mb0.729.The BMRs of the yellow-billed grosbeak, white-rumped munia and black-throated bushtit were 172%, 124% and 99%, respectively, of the values predicted from body mass (Londoño et al., 2015).At lower Tavalues, the BMRs of the yellow-billed grosbeak, white-rumped munia and black-throated bushtit generally decreased with increasing temperature, a pattern typical of endotherms (Schmidt-Nielsen,1997; Willmer et al., 2005).Consequently we consider the minimum MR recorded in these species to be their true BMR.Many factors, such as body size, phylogeny, climate conditions,activity, and feeding habits, are thought to affect the metabolic levels of birds (McNab, 2000; 2009).Rezende et al.(2002) and McNab (2009) suggested that avian BMR is generally correlated with climate.A reduced level of endogenous heat production may thus be adaptive in low-latitude species(Wiersma et al., 2007; Wikelski et al., 2003), and conversely,higher metabolic rates may be adaptive in mid-latitude and high-latitude species (Swanson, 2010; Zheng et al., 2008b;2014b).The low BMR of tropical species may arise directly from living in warm environments, with modest demands for metabolic thermogenesis and activity reflected in low rates of baseline energy expenditure (Jetz et al., 2008; White et al.,2007), and linked to their generally slow pace of life and lower investment in reproduction (Londoño et al., 2015; Ricklefs & Wikelski, 2002; Wiersma et al., 2007; Williams et al.2010).Conversely, the higher BMR of temperate and arctic birds is thought to be an adaptation to colder temperatures and higher investment in reproduction (Klaassen, 1995; Londoño et al.,2015).Previously published data on the BMR of temperate and tropical birds, together with metabolic rates predicted from Londoño et al.(2015) equation are presented (Table 2).The BMR of temperate species is generally higher tropical species.

Table 2 Comparison of observed and predicted basal metabolic rates (BMR) of bird species from cold, temperate, and tropical regions

Continued
Feeding habits are also an important factor affecting both the metabolic rates of animals and their geographic distribution.Birds feeding on seeds and fruits tend to have high metabolic rates that thought to be related to the consistency and abundance of food in their environment.In contrast,insectivorous birds tend to have lower metabolic rates (McNab,1988).The yellow-billed grosbeak and white-rumped munia mainly feed on seeds with the addition of some insects and fruit in summer and autumn, whereas the black-throated bushtit is predominantly insectivorous.It is possible that the different dietary preferences of these species may affect their respective metabolic rates.Our data show that the BMR of the yellowbilled grosbeak and white-rumped munia were higher than the predicted BMR using allometric equations, whereas that of the black-throated bushtit was similar to predicted values, and that this difference could be due to the grosbeak and munia being predominantly granivorous, whereas the bushtit is more insectivorous. These results are consistent with the previous studies (McNab, 1988).
TNZ is defined as the range of Taat which temperature regulation is achieved only by control of sensible heat loss,without regulatory changes in metabolic heat production or evaporative heat loss (IUPS Thermal Commission, 1987;Willmer et al., 2005).A lower critical temperature and broader TNZ are typical of species that are adapted to cold (Schmidt-Nielsen, 1997; Willmer et al., 2005).For example, the arctic ptarmigan Lagopus spp.does not increase its metabolic rate unless the external temperature falls to-5 °C, and even then its metabolic rate is not greatly affected by ambient temperature (Mortensen & Blix, 1986).Conversely, a higher upper criticaltemperature is typical of adaptation to hot climates, especially with regard to water conservation (Williams & Tieleman, 2000).For example, the Chinese hwamei (Garrulaxcanorus) may increase its metabolic rate if the ambient temperature drops to just 31 °C, and its metabolic rate is much more sensitive to change in ambient temperature (Wu et al., 2015; Xia et al.,2013).The lower critical temperature of the yellow-billed grosbeak was 23.5 ˚C, and the slope of the regression equation describing the relationship between metabolic rate and ambient temperature for this species was 0.14 (Table 1), results typical of a cold tolerant species. However, the white-rumped munia and black-throated bushtit increased their metabolic rates when the ambient temperature dropped to 30 °C and 28 °C,respectively, and the slopes of the regression equations describing the relationship between metabolic rate and temperature in these species were steeper; 0.24 O2(mL)/g/h/˚C for the white-rumped munia and 0.37 O2(mL)/g/h/˚C for blackthroated bushtit (Table 1). These data indicate that these species are relatively intolerant to cold.

Figure 3 Mean body temperature (A), metabolic rate (B) and thermal conductance (C) of wild-caught black-throated bushtits Aegithalos concinnus measured in an experimental facility in Wenzhou, China at ambient temperatures of approximately 5 ˚C-34 ˚C
lnterspecific variation in body temperature and thermal conductance
Considerable research has been devoted to the study of avian energetics, including body temperature (Clarke & Rothery, 2008;Prinzinger et al., 1991; Xia et al., 2013).The body temperature of birds depends upon their metabolic rate and heat loss. Small birds have higher body temperatures than larger ones because they have higher mass-specific rates of heat production.For example, the rest-phase Tbof most birds is 38.4 ˚C or less, but that of passerines is 39.0 ˚C (Prinzinger et al., 1991). We found that the yellow-billed grosbeak had the least variable Tbamong the three species studied at lower Tavalues.The high and constant Tbof the yellow-billed grosbeak would be advantageous in boreal latitudes, whereas the lower Tbof the white-rumped munia and black-throated bushtit may be the result of an optimization process through which these species attempt to minimize their energy expenditure (McKechnie & Lovegrove, 2001).
According to Aschoff (1981) formula, minimal thermal conductance depends on body temperature, metabolic rate and body mass.Small birds have a relatively large surface to volume ratio, less insulation, and higher heat loss, resulting in higher thermal conductance (Schmidt-Nielsen, 1997).Our results show that the minimum thermal conductance of the yellow-billed grosbeak, white-rumped munia and black-throated bushtit were 137%, 200% and 155%, respectively, of the values predicted from Aschoff (1981) allometric equation, indicating that these species are poorly insulated for their body size.Birds that live at low latitudes generally have higher thermal conductance than expected based on their body mass, a feature that may facilitate heat loss (Schleucher & Withers,2001).In Wenzhou, the mean temperature in October is about 23 °C, so birds inhabiting this region would be expected to have low metabolic heat production and high thermal conductance as a means of avoiding hyperthermia (Liu et al., 2006; Xia et al.,2013; Wu et al., 2015).
Metabolic properties and distribution
The metabolic properties of these species may also be an important factor affecting their distribution in China.In the present study, the high BMR, low lower critical temperature, and relatively stable metabolic rate, of the yellow-billed grosbeak are typical of a species adapted to cold.These features, in conjunction with its relatively lower thermal conductance and granivorous diet, may explain its broad geographic distribution.Conversely, the relatively low BMR, high thermal conductance,and temperature-sensitive metabolic rate, of the black-throated bushtit are typical of a tropical species.These features, together with its predominantly insectivorous diet, may explain why it is confined to relatively warm regions where insects and other invertebrates are more abundant.The white-rumped munia shares characteristics of both the yellow-billed grosbeak and black-throated bushtit.It has a relatively high BMR, thermal conductance, lower critical temperature, and its metabolic rate is relatively sensitive to changes in ambient temperature.These features, coupled with its food habit, may explain its relatively warm geographic distribution.In the present study, our data illustrate variation in the thermoregulatory characteristics of small passerine species that differ in their biogeographic distributions.
ACKNOWLEDGEMENTS
We thank Dr.Ron Moorhouse for revising the English and for some constructive suggestions.We also thank the anonymous reviewers for their helpful comments and suggestions.
REFERENCES
Angilletta MJ Jr, Cooper BS, Schuler MS, Boyles JG.2010.The evolution ofthermal physiology in endotherms.Frontiers in Bioscience (Elite Edition),2(3): 861-881.
Aschoff J.1981.Thermal conductance in mammals and birds: its dependence on body size and circadian phase.Comparative Biochemistry and Physiology Part A: Physiology, 69(4): 611-619.
Bartholomew GA, Vleck CM, Bucher TL.1983.Energy metabolism and nocturnal hypothermia in two tropical passerine frugivores, Manacus vitellinus and Pipra mentalis.Physiological Zoology, 56(3): 370-379.
Burton CT, Weathers WW.2003.Energetics and thermoregulation of the gouldian finch (Erythrura gouldiae).Emu, 103(1): 1-10.
Chaplin SB.1974.Daily energetics of the Black-capped Chickadee, Parus atricapillus, in winter.Journal of Comparative Physiology B, 89(4): 321-330.Clarke A, Rothery P.2008.Scaling of body temperature in mammals and birds.Functional Ecology, 22(1): 58-67.
Corp N, Gorman ML, Speakman JR.1997.Seasonal variation in the resting metabolic rate of male wood mice Apodemus sylvaticus from two contrasting habitats 15 km apart.Journal of Comparative Physiology B,167(3): 229-239.
Deng HL, Zhang XA.1990.Standard metabolic rate in several species of passerine birds in alpine meadow.Acta Zoologica Sinica, 36(4): 377-384.(in Chinese)
Górecki A.1975.Kalabukhov-Skvortsov respirometer and resting metabolic rate measurement.In: Grodziński W.IBP Handbook No.24.Methods for Ecological Bioenergetics.London: Oxford Press, 309-313.
IUPS Thermal Commission.1987.Glossary of terms for thermal physiology.Pflügers Archiv, 410(4): 567-587.
Jetz W, Freckleton RP, McKechnie AE.2008.Environment, migratory tendency, phylogeny and basal metabolic rate in birds.PLoS One, 3(9):e3261.
Klaassen M.1995.Moult and basal metabolic costs in males of two subspecies of stonechats: the European Saxicola torquata rubicula and the East African S.t.axillaris.Oecologia, 104(4): 424-432.
Li M, Liu JS, Han HL, Zhang HJ, Fang H.2005.Metabolism and thermoregulation in waxwings (Bombycilla garrulous) and black-faced buntings (Emberiza spodocephala).Zoological Research, 26(3): 287-293.(in Chinese)
Liknes ET, Swanson DL.2011.Phenotypic flexibility in passerine birds:Seasonal variation of aerobic enzyme activities in skeletal muscle.Journal of Thermal Biology, 36(7): 430-436.
Lin L, Wang LH, Liu JS.2010.Metabolism and thermoregulation in crested mynas (Acridotheres cristatellus).Chinese Journal of Zoology, 45(5): 47-53.(in Chinese)
Lindström Å, Klaassen M.2003.High basal metabolic rates in shorebirds: a circumpolar view.The Condor, 105(3): 420-427.
Liu JS, Zhang ZY, Ma H, Hou ZS.2001a.Characteristics of resting metabolic rate in little bunting (Emberiza pusilla) and chestnut bunting (E.rutila).Acta Zoologica Sinica, 47(3): 347-350.(in Chinese)
Liu JS, Wang Y, Li HR.2001b.Preliminary study of standard metabolic rate in scarlet grosbeak (Carpodacus erythrinus).Chinese Journal of Zoology,36(3): 16-19.(in Chinese)
Liu JS, Wang DH, Wang Y, Chen MH, Song CG, Sun RY.2004a.Energetics and thermoregulation of the Carpodacus roseus, Fringilla montifringilla and Acanthis flammea.Acta Zoologica Sinica, 50(3): 357-363.
Liu JS, Chen MH, Wang Y, Wang XH, Song CG.2004b.Metabolic thermogenesis of Siberian accentor (Prunella montanella).Zoological Research, 25(2): 117-121.(in Chinese)
Liu JS, Wang DH, Sun RY.2005.Climatic adaptations in metabolism of four species of small birds in China.Acta Zoologica Sinica, 51(1): 24-30.
Liu JS, Li M.2006.Phenotypic flexibility of metabolic rate and organ masses among tree sparrows Passer montanus in seasonal acclimatization.Acta Zoologica Sinica, 52(3): 469-477.
Londoño GA, Chappell MA, Castañeda MR, Jankowski JE, Robinson SK.2015.Basal metabolism in tropical birds: latitude, altitude, and the ‘pace of life'.Functional Ecology, 29(3): 338-346.
MacKinnon J, Phillipps K.2000.A Field Guide to the Birds of China.London: Oxford University Press.
Marschall U, Prinzinger R.1991.Verleichende ökophysiologie von fünf prachtfinkenarten (Estrildidae).Journal of Ornithology, 132(3): 319-323.
McKechnie AE.2008.Phenotypic flexibility in basal metabolic rate and the changing view of avian physiological diversity: a review.Journal of Comparative Physiology B, 178(3): 235-247.
McKechnie AE, Lovegrove BG.2001.Heterothermic responses in the speckled mousebird (Colius striatus).Journal of Comparative Physiology B,171(6): 507-518.
McKechnie AE, Wolf BO.2004.The allometry of avian basal metabolic rate:good predictions need good data.Physiological and Biochemical Zoology,77(3): 502-521.
McKechnie AE, Freckleton RP, Jetz W.2006.Phenotypic plasticity in the scaling of avian basal metabolic rate.Proceedings of the Royal Society B,273(1589): 931-937.
McNab BK.1988.Food habits and the basal rate of metabolism in birds.Oecologia, 77(3): 343-349.
McNab BK.2000.The influence of body mass, climate, and distribution on the energetics of South Pacific pigeons.Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 127(3): 309-329.
McNab BK.2009.Ecological factors affect the level and scaling of avian BMR.Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 152(1): 22-45.
Mortensen A, Blix AS.1986.Seasonal changes in resting metabolic rate and mass-specific conductance in Svalbard ptarmigan, Norwegian rock ptarmigan and Norwegian willow ptarmigan.Ornis Scandinavica, 17(1): 8-13.
Nickerson DM, Facey DE, Grossman GD.1989.Estimating physiological thresholds with continuous two-phase regression.Physiological Zoology,62(4): 866-887.
Nzama SN, Downs CT, Brown M.2010.Seasonal variation in the metabolism-temperature relation of House Sparrow (Passer domesticus) in KwaZulu-Natal, South Africa.Journal of Thermal Biology, 35(2): 100-104.
Prinzinger R, Preßmar A, Schleucher E.1991.Body temperature in Birds.Comparative Biochemistry and Physiology Part A: Physiology, 99(5): 499-506.
Rezende EL, Swanson DL, Novoa FF, Bozinovic F.2002.Passerines versus nonpasserines: so far, no statistical differences in the scaling of avian energetics.The Journal of Experimental Biology, 205(1): 101-107.
Ricklefs RE, Wikelski M.2002.The physiology/life-history nexus.Trends in Ecology and Evolution, 17(10): 462-468.
Schleucher E, Withers PC.2001.Re-evaluation of the allometry of wet thermal conductance for birds.Comparative Biochemistry and PhysiologyPart A: Molecular & Integrative Physiology, 129(4): 821-827.
Schmidt-Nielsen K.1997.Animal Physiology: Adaptation and Environment.Cambridge: Cambridge University Press.
Sgueo C, Wells ME, Russell DE, Schaeffer PJ.2012.Acclimatization of seasonal energetics in northern cardinals (Cardinalis cardinalis) through plasticity of metabolic rates and ceilings.The Journal of Experimental Biology, 215(14): 2418-2424.
Silva JE.2006.Thermogenic mechanisms and their hormonal regulation.Physiological Reviews, 86(2): 435-464.
Swanson DL.2010.Seasonal metabolic variation in birds: functional and mechanistic correlates.In: Thompson CF.Current Ornithology.New York:Springer, 17: 75-129.
Swanson DL, Merkord C.2013.Seasonal phenotypic flexibility of flight muscle size in small birds: a comparison of ultrasonography and tissue mass measurements.Journal of Ornithology, 154(1): 119-127.
Swanson DL, Zhang YF, Liu JS, Merkord CL, King MO.2014.Relative roles of temperature and photoperiod as drivers of metabolic flexibility in darkeyed juncos.The Journal of Experimental Biology, 217(6): 866-875.
Tieleman BI, Williams JB, Buschur ME.2002.Physiological Adjustments to Arid and Mesic Environments in Larks (Alaudidae).Physiological and Biochemical Zoology, 75(3): 305-311.
Weathers WW.1977.Temperature regulation in the Dusky munia,Lonchura fuscans (Cassin) (Estrildidae).Australian Journal of Zoology,25(2): 193-199.
Weathers WW.1979.Climatic adaptation in avian standard metabolic rate.Oecologia, 42(1): 81-89.
Weathers WW.1997.Energetics and thermoregulation by small passerines of the humid, lowland tropics.The Auk, 114(3): 341-353.
White CR, Blackburn TM, Martin GR, Butler PJ.2007.Basal metabolic rate of birds is associated with habitat temperature and precipitation and not primary productivity.Proceedings of the Royal Society of London Series B,274(1607): 287-293.
Wiersma P, Muñoz-Garcia A, Walker A, Williams JB.2007.Tropical birds have a slow pace of life.Proceedings of the National Academy of Sciences of the United States of America, 104(22): 9340-9345.
Wikelski M, Spinney L, Schelsky W, Scheuerlein A, Gwinner E.2003.Slow pace of life in tropical sedentary birds: a common-garden experiment on four stonechat populations from different latitudes.Proceedings of the Royal Society B, 270(1531): 2383-2388.
Williams JB, Tieleman BI.2000.Flexibility in basal metabolic rate and evaporative water loss among hoopoe larks exposed to different environmental temperatures.The Journal of Experimental Biology, 203(20):3153-3159.
Williams JB, Miller RA, Harper JM, Wiersma P.2010.Functional linkages for the pace of life, life-history, and environment in birds.Integrative and Comparative Biology, 50(5): 855-868.
Willmer P, Stone G, Johnston I.2005.Environmental Physiology of Animals.Oxford: Blackwell Publishing Company.
Wu MX, Zhou LM, Zhao LD, Zhao ZJ, Zheng WH, Liu JS.2015.Seasonal variation in body mass, body temperature and thermogenesis in the Hwamei, Garrulax canorus.Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 179: 113-119.
Xia SS, Yu AW, Zhao LD, Zhang HY, Zheng WH, Liu JS.2013.Metabolic thermogenesis and evaporative water loss in the Hwamei Garrulax canorus.Journal of Thermal Biology, 38(8): 576-581.
Zheng WH, Liu JS, Jang XH, Fang YY, Zhang GK.2008a.Seasonal variation on metabolism and thermoregulation in Chinese bulbul.Journal of Thermal Biology, 33(6): 315-319.
Zheng WH, Li M, Liu JS, Shao SL.2008b.Seasonal acclimatization of metabolism in Eurasian tree sparrows (Passer montanus).Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology,151(4): 519-525.
Zheng WH, Lin L, Liu JS, Xu XJ, Li M.2013.Geographic variation in basal thermogenesis in little buntings: Relationship to cellular thermogenesis and thyroid hormone concentrations.Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 164(3): 483-490.
Zheng WH, Liu JS, Swanson DL.2014a.Seasonal phenotypic flexibility of body mass, organ masses, and tissue oxidative capacity and their relationship to RMR in Chinese bulbuls.Physiological and Biochemical Zoology, 87(3): 432-444.
Zheng WH, Li M, Liu JS, Shao SL, Xu XJ.2014b.Seasonal variation of metabolic thermogenesis in Eurasian tree sparrows (Passer montanus)over a latitudinal gradient.Physiological and Biochemical Zoology, 87(5):704-718.
Zhou LM, Xia SS, Chen Q, Wang RM, Zheng WH, Liu JS.2016.Phenotypic flexibility of thermogenesis in the Hwamei (Garrulax canorus):responses to cold acclimation.American Journal of Physiology: Regulatory,Integrative & Comparative Physiology, 310(4): R330-?R336.
Foundation items: This study was financially supported by grants from the National Natural Science Foundation of China (No.31470472), the National Undergraduate “Innovation” Project and the Zhejiang Province “Xinmiao” Project
*Corresponding author, E-mail: ljs@wzu.edu.cn
DOI:10.13918/j.issn.2095-8137.2016.3.167the energetic cost of thermoregulation.It has consequently been the focus of considerable research interest from environmental physiologists and comparative physiologists (e.g.,Liu et al., 2005; Zheng et al., 2014a).
杂志排行
Zoological Research的其它文章
- Who is innocent in authorship misconduct?
- Systematics of the Artiodactyla of China in the 21stcentury
- Comparative transcriptome analysis on the alteration of gene expression in ayu (Plecoglossus altivelis) larvae associated with salinity change
- Expression levels of GSTA2 and APOD genes might be associated with carotenoid coloration in goldenpheasant (Chrysolophus pictus) plumage
- Effects of forest fragmentation on nocturnal Asian birds:A case study from Xishuangbanna, China
- Conservation education and habitat restoration for the endangered Sagalla caecilian (Boulengerula niedeni)in Sagalla Hill, Kenya
