Synthesis of La-Co-O Mixed Oxides via Polyethylene Glycol-Assisted Co-Precipitation Method for Total Oxidation of Benzene
2016-07-12WEIXiaofengLIDalinXIAOYihongCAIGuohuiDAIWuXIEZenghongWEIKemei
WEI Xiao-feng, LI Da-lin, XIAO Yi-hong, CAI Guo-hui,DAI Wu, XIE Zeng-hong, WEI Ke-mei
1.National Engineering Research Center of Chemical Fertilizer Catalyst, Fuzhou University, Fuzhou 350002, China 2.Faculty of Chemistry, Fuzhou University, Fuzhou 350108, China
Synthesis of La-Co-O Mixed Oxides via Polyethylene Glycol-Assisted Co-Precipitation Method for Total Oxidation of Benzene
WEI Xiao-feng1,2, LI Da-lin1, XIAO Yi-hong1, CAI Guo-hui1,DAI Wu1, XIE Zeng-hong2*, WEI Ke-mei1
1.National Engineering Research Center of Chemical Fertilizer Catalyst, Fuzhou University, Fuzhou 350002, China 2.Faculty of Chemistry, Fuzhou University, Fuzhou 350108, China
La-Co-O mixed oxides (LCO) were prepared by co-precipitation method with the presence of polyethylene glycol (PEG) as dispersant.The influence of adding different molecular weight of PEG (0, 2 000, 6 000, 20 000 g·mol-1) on the physicochemical and catalytic properties of La-Co-O mixed oxides for total oxidation of benzene was investigated.The samples were characterized by means of N2physical adsorption, X-ray diffraction (XRD), scanning electron microscopy (SEM), temperature-programmed reduction by H2(H2-TPR), temperature-programmed desorption of O2(O2-TPD), and X-ray photoelectron spectroscopy (XPS).The order of catalytic activity was found to be LCO-PEG6000>LCO>LCO-PG20000>LCO-PG2000.Particularly, LCO-PEG6000 exhibited benzene conversion of 99% at temperature as low as 383 ℃, which was 126 ℃ lower than that of LCO.The characterization result reveals that all samples had a BET surface area of about 9~10 m2·g-1.The XRD result shows that on all samples LaCoO3perovskite was mainly formed together with a small amount of La2O3and Co3O4.The addition of PEG was favorable for the formation of LaCoO3perovskite.Particularly, the addition of PEG-6000 effectively suppressed the agglomeration of LaCoO3perovskite, giving rise to small and uniform particles as observed by SEM.Moreover, the results of H2-TPR and O2-TPD indicate that the obtained La-Co-O mixed oxides showed higher reducibility and lattice oxygen mobility, and the Co 2p XPS analysis suggests that more surface Co3+active species were presented by the addition of PEG-6000.These properties are thought to contribute to the high activity in benzene total oxidation.
Mixed oxides; LaCoO3; Polyethylene glycol; Co-precipitation; Catalytic combustion; Benzene total oxidation
Introduction
Volatile organic compounds (VOCs) emitted from industrial processes are considered as the significant atmospheric pollutants due to their toxicity and malodorous nature as well as their contribution in the formation of suspended particulate matter and photochemical smog[1].Thus environmental legislation has imposed increasingly stringent targets for permitted levels of VOCs emission.From an economical point of view, catalytic combustion is one of the most interesting technologies for the destruction of VOCs emissions as compared to the incineration process[2].VOCs catalytic oxidation occurs at much lower temperatures than that required for thermal oxidation.There is no associated pollution by dioxins and nitrogen oxides (NOx), which are exclusively formed under high temperature conditions.
Various catalysts have been investigated for the catalytic oxidation of VOCs, including noble metals such as Ag, Au, Pt, Pd, etc, and metal oxides.Although noble metals usually exhibit high performance for VOCs catalytic oxidation, the high-cost and limited availability of noble metals restrict their application.Therefore, non-precious metal oxides have attracted much attention.Among the metal oxides, perovskite-type catalysts with a general formula of ABO3(A represents a lanthanide and/or alkaline earth metal ion and B a transition metal ion) have been found to be effective for the total oxidation of VOCs.In particular, the cobalt- and manganese-based perovskites were found to be the most active, and were the most studied for catalytic oxidation[3-6].Typical methods for the preparation of perovskite include co-precipitation[7], citrate complexation[8], solid-state reaction[9], and combustion synthesis[10].In recent years, some new methods have been developed to prepare perovskite-based catalyst with large surface area to increase the number of active sites.For instance, Sadakane et al[11]and Liu et al[12]reported the preparation of three-dimensionally ordered macroporous (3-DOM) perovskite by using colloidal crystal templating method, and Szabo et al[13]used reactive grinding at room temperature for the synthesis of LaCo1-xFexO3.Among these methods, co-precipitation is one of the most convenient methods for the perovskite preparation.Nevertheless, the agglomeration of as-synthesized precursor may occur during the subsequent heat treatment, leading to large particle size of the product.Recently, Wei et al[14]investigated the effect of additive-polyethylene glycol (PEG-6000) in the preparation of LaCoO3by co-precipitation and found that the addition of suitable amount of PEG-6000 was favorable for the formation of well-dispersed LaCoO3ultrafine powder.It was suggested that the agglomeration of precursor was reduced by the steric effect of PEG-6000 adsorbing on the precursor.They focused on the study of function and mechanism of PEG-6000 in the co-preparation process, however, the catalytic property of the LaCoO3ultrafine powder and its relationship with catalyst structure has not been reported.
In the present work, various La-Co-O mixed oxides were prepared by co-precipitation method in the presence of PEG and tested for the VOCs total oxidation.The influence of addition of different molecular weight of PEG (0, 2 000, 6 000, 20 000) in the co-precipitation process on the structural and catalytic properties of La-Co-O mixed oxides was investigated.The obtained solids were characterized by N2physical adsorption, X-ray diffraction (XRD), scanning electron microscopy (SEM), temperature-programmed reduction by H2(H2-TPR), temperature-programmed desorption of O2(O2-TPD), and X-ray photoelectron spectroscopy (XPS).Their activities were tested in benzene total oxidation.Based on the results of characterization and catalytic test, the relationship between the structure and catalytic performance was discussed.
1 Experimental
1.1 Catalyst preparation
All of the chemicals used for the catalyst preparation were analytic grade and purchased from Sinopharm Chemical Reagent Co., Ltd, Shanghai.Stoichiometric amounts of cobalt nitrate and lanthanum nitrate (molar ratio of La∶Co=1∶1) were dissolved with de-ionized water and heated to 70 ℃ with a water bath, then 3% PEG (molecular weight of 2 000, 6 000 and 20 000) was added under stirring, followed by dropwise addition of (NH4)2CO3solution to the mixed solution until pH 9.After filtration, washing with de-ionized water, and drying at 100 ℃, the precipitate was calcined in air at 800 ℃ for 4 h.The resulting La-Co-O mixed oxides were denoted as LCO-PEG2000, LCO-PEG6000, and LCO-PEG20000, respectively.For a comparison, no PEG-assisted La-Co-O mixed oxide (LCO) was also prepared by the co-precipitation method with a similar procedure, followed by calcination at 800 ℃ for 4 h.
1.2 Characterization of the catalysts
The X-ray powder diffraction (XRD) patterns of samples were recorded on a Panalytical X’Pert Pro diffractometer at 40 kV and 40 mA with a step size of 0.016 7° using Co-Kαradiation.The XRD patterns were displayed based on Cu-Kαinstead of Co-Kα.
N2adsorption/desorption measurements were carried out at -196 ℃ on a Micromeritics ASAP 2020.Prior to analysis, sample was heated at 250 ℃ for 3 h under vacuum.Surface areas were calculated using the BET method.The pore size was calculated using the BJH model.
The morphologies of samples were observed by scanning electron microscopy (SEM) on Hitachi S-4800, which was operated at an acceleration voltage of 5.0~10 kV.
Temperature-programmed reduction by H2(H2-TPR) and temperature-programmed desorption of O2(O2-TPD) were conducted using an Autochem 2920 instrument equipped with a TCD.About 100 mg of sample was used in each measurement.For TPR experiments, the sample was first treated in a He stream at 550 ℃ for 0.5 h.After being cooled down to room temperature in the same atmosphere, the sample was swept with 10 vol%H2/Ar until the baseline on the recorder remained unchanged.Then the sample was heated in 10 vol% H2/Ar from room temperature to 900 ℃ at a rate of 10 ℃ min-1.For O2-TPD, the sample was heated in pure O2from room temperature to 550 ℃ and held for 0.5 h, subsequently cooled to room temperature in He atmosphere.Then the sample was heated from room temperature to 900 ℃ in He at a heating rate of 10 ℃·min-1.
The X-ray photoelectron spectroscopy (XPS) measurements were performed on a Physical Electronics Quantum 2000 spectrometer.A monochromatic Al-Kαsource (Kα=1 486.6 eV) and a charge neutralizer were equipped in the instrument.The surface charging effect was corrected by setting the binding energy of adventitious carbon (C 1s) at 284.6 eV.
1.3 Catalytic activity measurement
Benzene total oxidation was carried out in a U-type quartz tubular reactor packed with 0.1 g of catalyst under atmospheric pressure.The temperature of the catalyst bed was measured as reaction temperature by inserting a thermal couple into the catalyst bed.Then, the reactant gas containing 500 ppm C6H6, 20 vol% O2, and balanced N2was fed into the reactor with a space velocity of 108 000 mL·g-1·h-1.The content of C6H6in the effluent gas was analyzed by an on-line GC equipped with a FID.Catalytic activity was measured in the range of 200~600 ℃.Conversion of C6H6was calculated as follows
Conversion of C6H6(%)=
(1)
where [C6H6]inand [C6H6]outwere the C6H6concentrations in the feed gas and product, respectively.
2 Results and discussion
2.1 Catalytic performance of La-Co-O mixed oxides for benzene total oxidation
Figure 1 shows the conversion of benzene as a function of temperature over the La-Co-O mixed oxides prepared by co-precipitation method with or without the addition of PEG.No other products than CO2and H2O were detected during the reaction.In all cases, the benzene conversion increased significantly with increasing the reaction temperature from 200 to 450 ℃ and reached a high conversion above 500 ℃.For LCO without the PEG addition, the temperatures for 30%, 50%, and 99% benzene conversion (T30,T50,T99) were 264 ℃, 294 ℃, and 509 ℃, respectively.Compared to LCO, the LCO-PEG6000 sample exhibited higher catalytic activity for benzene total oxidation.TheT30,T50, andT99for LCO-PEG6000 were 251, 270 and 383 ℃, respectively.Particularly, theT99was 126 ℃ lower for LCO-PEG6000 than LCO, indicating the significant promoting effect of PEG-6000 addition.However, LCO-PEG2000 and LCO-PEG20000 showed lower activity as compared to LCO.This strongly suggests that the addition of different molecular weight of PEG during the synthesis had a remarkable effect on the catalytic activity.
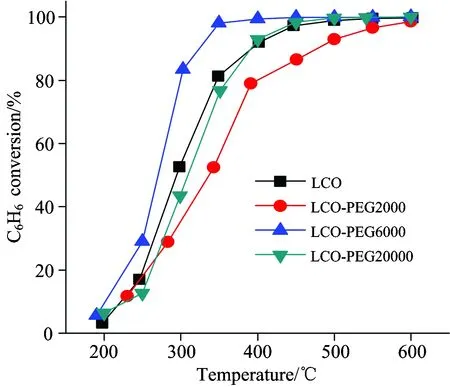
Fig.1 Temperature dependence of activity in benzene total oxidation over La-Co-O mixed oxides
Reaction conditions: 500 ppm C6H6, 20% O2, balanced by N2, GHSV = 108 000 mL·g-1·h-1
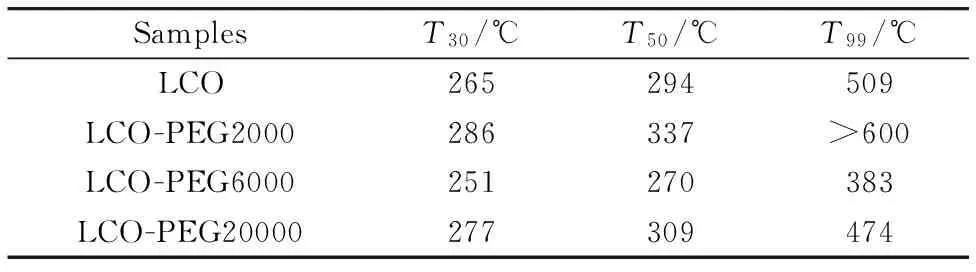
Table 1 T30, T50, and T99 in benzene total oxidation
2.2 Textural and structural properties of samples
The BET surface area, pore diameter and pore volume of La-Co-O mixed oxides are listed in Table 2.The BET surface area of LCO-PEG samples was slightly lower than that of LCO, and tended to increase with increasing the molecular weight of PEG.On the other hand, the pore diameter and pore volume were enlarged by the presence of PEG and became larger as the molecular weight of PEG increased.
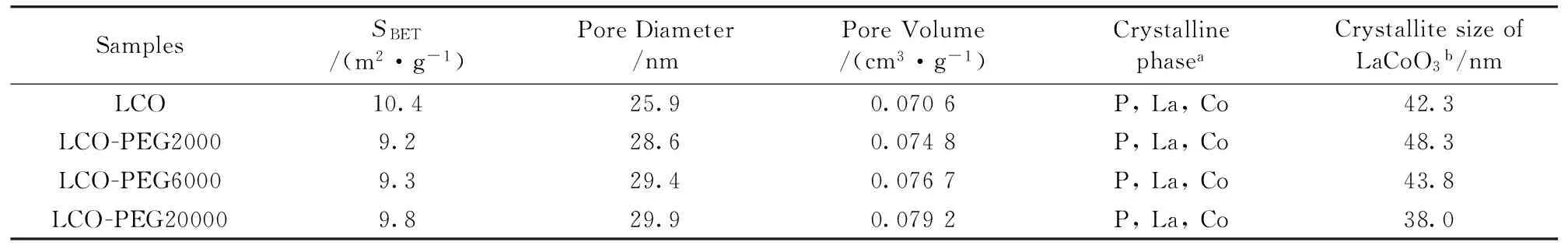
Table 2 Surface area, porous property, and LaCoO3 crystallite size of samples
a:P, perovskite; La, lanthanum oxide; Co, cobalt oxide; b:Calculated from the diffraction peak at 2θ=47.5 ° using the Scherrer equation
Figure 2 shows the XRD patterns of the samples.The diffraction peaks at 2θ=23.2°, 32.9°, 33.3°, 47.5°, and 59.0° were characteristic of LaCoO3perovskite with rhombohedral structure and a space group R-3c (JCPDS 01-084-0848)[15].In addition, a small amount of La2O3(JCPDS 00-054-0213) and Co3O4(JCPDS 01-078-1969) phases were also identified on all of the samples.As compared to LCO, the peak intensity of LaCoO3perovskite on LCO-PEG samples was stronger and the peak intensity of Co3O4and La2O3phases was weaker, suggesting that the addition of PEG was favorable for the formation of LaCoO3perovskite.The crystallite size of LaCoO3perovskite calculated by the Scherrer equation is listed in Table 2.As compared to LCO, the addition of PEG-2000 slightly enlarged the LaCoO3crystallite size, while the crystallite size gradually decreased with increasing the molecular weight of PEG.
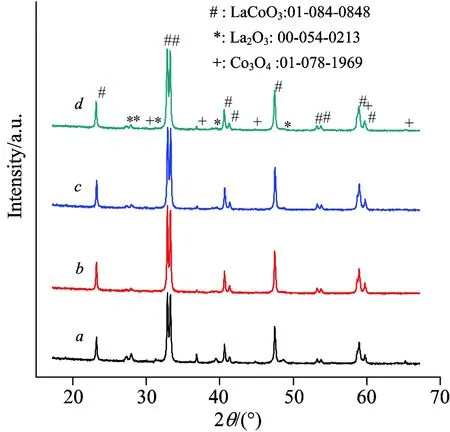
Fig.2 XRD patterns of (a) LCO, (b) LCO-PEG2000, (c) LCO-PEG6000, and (d) LCO-PEG20000
2.3 Morphology observation by SEM
Figure 3 shows the SEM images of the samples.For LCO [Fig.3(a)], large particles (100~150 nm) were presented together with small particles (<50 nm).The particle size observed by SEM was larger than the LaCoO3crystallite size estimated from XRD (Table 2), which might imply that the observed particles were polycrystalline ones.This was properly caused by the agglomeration of mixed oxides during the high-temperature calcination.The addition of PEG2000 did not change the morphology remarkably [Fig.3(b)].Interestingly, by the addition of PEG6000 [Fig.3(c)], small particles (<50 nm) were mainly observed and the particle size was relatively uniform, approximately equal to the LaCoO3crystallite size estimated from XRD (Table 2).This result suggests that the addition of PEG6000 effectively suppressed the agglomeration of LaCoO3perovskite.Nevertheless, by the addition of PEG2000 [Fig.3(d)], the particles became larger as compared to the case of PEG6000 addition, although the particle size was still uniform.
2.4 H2-TPR
The reducibility of the La-Co-O mixed oxides was investigated by H2-TPR.Figure 4 shows the obtained H2-TPR profiles and the amount of H2consumption is listed in Table 3.Two major H2consumption peaks at temperatures around 300~420 ℃ and 500~700 ℃ were observed on each sample.Crespin and Hall[16]reported two reduction peaks at 420 and 550 ℃ for LaCoO3, attributable to the reduction of Co3+to Co2+and Co2+to Co0, respectively.On the other hand, Arnoldy and Moulijn[17]observed a reduction peak for Co3O4at 320 ℃, which was ascribed to the reduction of Co3+to Co2+and finally Co0.Based on these reports combined with the XRD result, we assigned the low-temperature shoulder peak at 390 ℃ to the reduction of Co3O4to Co0, the peak at 420 ℃ to the reduction of Co3+to Co2+on LaCoO3, and the high-temperature peak to the second reduction step of LaCoO3(Co2+to Co0).It can be seen from Table 3 that the H2consumption ratio of high-temperature peak to low-temperature peak was much lower on LCO than on LCO-PEG samples.The large difference in the peak-area ratio between LCO and LCO-PEG suggests that LCO had higher content of Co3O4, which was directly reduced to metallic Co0at low temperature, in agreement with the XRD result.It is noted that the high-temperature reduction peak on LCO-PEG6000 was slightly shifted toward low temperature as compared to LCO.It seems that LaCoO3on LCO-PEG6000 had higher reducibility.
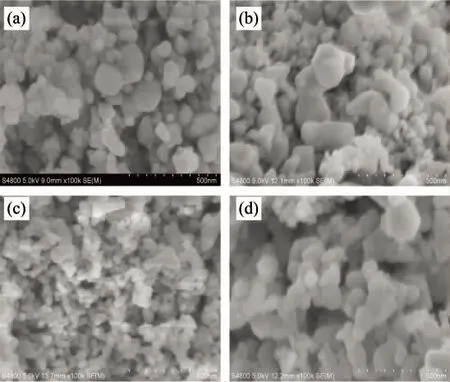
Fig.3 SEM images of (a) LCO, (b) LCO-PEG2000, (c) LCO-PEG6000, and (d) LCO- PEG20000
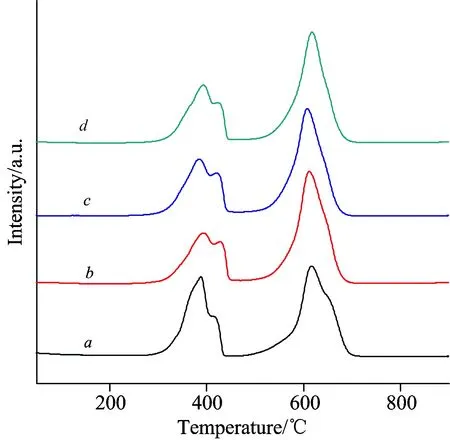
Fig.4 H2-TPR profiles of (a) LCO, (b) LCO-PEG2000, (c) LCO-PEG6000, and (d) LCO-PEG20000
Table 3 Results of H2-TPR on the samples
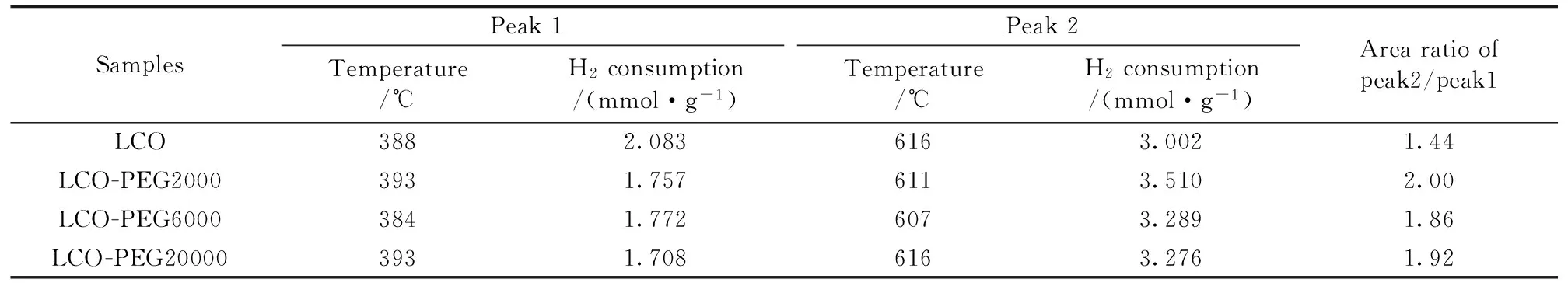
SamplesPeak1Peak2Temperature/℃H2consumption/(mmol·g-1)Temperature/℃H2consumption/(mmol·g-1)Arearatioofpeak2/peak1LCO3882.0836163.0021.44LCO-PEG20003931.7576113.5102.00LCO-PEG60003841.7726073.2891.86LCO-PEG200003931.7086163.2761.92
2.5 O2-TPD
To investigate the redox properties of the La-Co-O mixed oxides, O2-TPD was carried out.As shown in Fig.5, the samples displayed three desorption peaks.According to the literatures[18-19], the small desorption peak below 500 ℃ was assumed to the oxygen chemisorbed on the surface of the solid (α1-oxygen), and the desorption peak at 500~740 ℃ was described as the oxygen chemisorbed on the vacancies (α2-oxygen), whereas the desorption peak above 740 ℃ was attributed to the lattice oxygen (β-oxygen).Particularly, β-oxygen was reported to originate from the bulk of the perovskite and is generally considered as a measure of the bulk oxygen mobility[20-22].It is found that both the intensities of α- and β-oxygen species on LCO-PEG decreased as compared to those of LCO.The larger amount of α-oxygen on LCO might be explained by its slightly higher specific surface area[12]and/or the presence of larger amounts of lanthanum oxide and cobalt oxide, which led to more defects in the surface structure of the perovskite, resulting in increased oxygen vacancies[13].Nevertheless, the peak of β-oxygen on LCO-PEG slightly shifted to lower temperature (794 ℃) as compared to that of LCO (811 ℃), suggesting that the LCO-PEG samples had higher lattice oxygen mobility.Among the LCO-PEG samples, the LCO-PEG6000 exhibited the highest amount of mobile lattice oxygen judging from the peak intensity.
2.6 XPS
2.4 多导睡眠记录仪 是通过对病人的脑电、肌电、眼动、心电、呼吸气流、胸腹运动等多导生理信号的记录,对个体睡眠时各种生理状况进行记录,以反应其睡眠质量,这是睡眠障碍诊断的金标准。但是这一检测技术应用于儿童,对场地以及技术的要求较成人睡眠检查的要求更高。因为小年龄儿童的睡眠分期、睡眠期间各生理参数的判断以及如何让儿童适应并接受大量电极接在头面部及躯干等都是很具有挑战的问题。多导睡眠记录仪主要可以用于睡眠呼吸障碍、周期性腿动、不明原因嗜睡、睡眠中发作性行为的诊断等领域[6]。
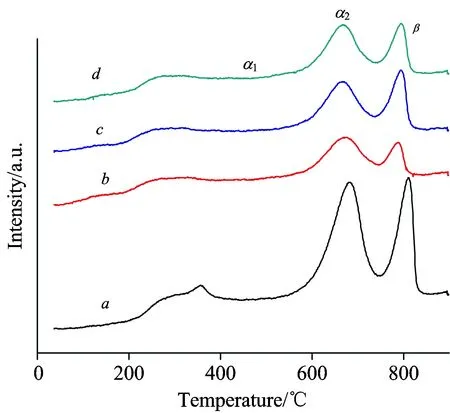
Fig.5 O2-TPD profiles of (a) LCO, (b) LCO-PEG2000, (c) LCO-PEG6000, and (d) LCO- PEG20000
XPS was employed to get information about the state of surface Co species on the La-Co-O mixed oxides.Figure 6 shows the Co 2p XPS of LCO and LCO-PEG6000.As can be seen, two main peaks at 779.8 and 794.8 eV together with two small peaks at approximately 790.0 and 805.0 eV were observed.According to the literatures , the peaks at 779.8 and 790.0 eV might be assigned to Co 2p3/2, while the peaks at 794.8 and 805.0 eV could be ascribed to Co 2p1/2.It is noted that the peak intensities of Co 2p3/2and Co 2p1/2on the LCO-PEG6000 sample were much higher than those on the LCO sample.A similar phenomenon was reported for Sr substituted LaCoO3[16]and it was interpreted as due to the rise of Co3+concentration.Therefore, we thought that the LCO-PEG6000 sample had a higher concentration of surface Co3+species, which are known as the active sites for VOCs total oxidation.
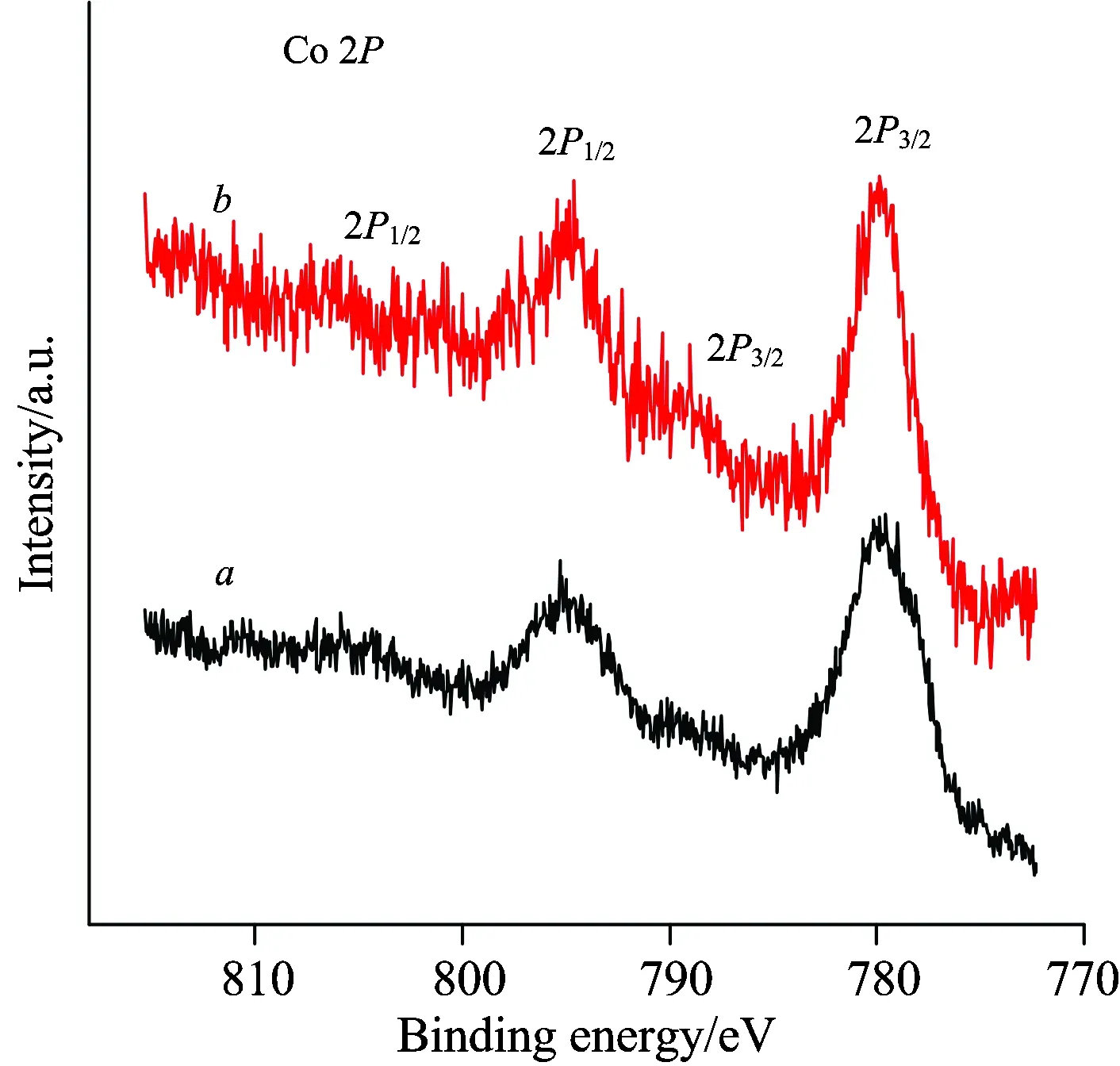
Fig.6 Co 2p XPS of (a) LCO and (b) LCO-PEG6000
3 Conclusion
The La-Co-O mixed oxide prepared by the co-precipitation method with the addition of polyethylene glycol (PEG-6000) showed improved activity for benzene total oxidation, while the addition of PEG-2000 and PEG-20000 decreased the activity.The characterization results revealed that the addition of PEG was favorable for the formation of LaCoO3perovskite.Particularly, the addition of PEG-6000 effectively suppressed the agglomeration of LaCoO3perovskite, giving rise to small and uniform particles as observed by SEM.Moreover, the obtained La-Co-O mixed oxides showed higher reducibility and lattice oxygen mobility as well as more surface Co3+active species.These properties are thought to be responsible for the high activity in benzene total oxidation.
[1] Mølhave L, Clausen G, Berglund B, et al.Indoor Air, 1997, 7: 225.
[2] Hu C Q.Chemical Engineering Journal, 2011, 168: 1185.
[3] Spinicci R, Faticanti M, Marini P, et al.Journal of Molecular Catalysis A: Chemical, 2003, 197: 147.
[4] Spinicci R, Delmastro A, Ronchetti S, et al.Materials Chemistry and Physics, 2002, 78: 393.
[5] Yang W D, Chang Y H, Huang S H.Journal of the European Ceramic Society, 2005, 25(16): 3611.
[6] Esmaeilnejad-Ahranjani P, Khodadadi A, Ziaei-Azad H, et al.Chemical Engineering Journal, 2011, 169: 282.
[8] Pecchi G, Reyes P, Zamora R, et al.Journal of Solid State Chemistry, 2008, 181: 905.
[9] Kuhn J N, Ozkan U S.Journal of Catalysis, 2008, 253: 200.
[10] Xue J, Shen Y, Zhou Q, et al.International Journal of Hydrogen Energy, 2010, 35: 294.
[11] Sadakane M, Asanuma T, Kubo J, et al.Chemistry of Materials, 2005, 17: 3546.
[12] Liu Y X, Dai H X, Du Y C, et al.Journal of Catalysis, 2012, 287: 149.
[13] Szabo V, Bassir M, Van Neste A, et al.Applied Catalysis B: Environmental, 2002, 37: 175.
[14] Wei Zhixian, Ou Haifeng, Li Yanbin, et al.Journal of the Chinese Rare Earth Society, 2006, 24: 41.
[15] Predoana L, Malic B, Kosec M, et al.Journal of the European Ceramic Society, 2007, 27: 4407.
[16] Crespin M, Hall W K.Journal of Catalysis, 1981, 69: 359.
[17] Arnoldy P, Moulijn J A.Journal of Catalysis, 1985, 93: 38.
[18] Royer S, Bérubé F, Kaliaguine S.Applied Catalysis A: General, 2005, 282: 273.
[19] Zhou Ying, Lu Hanfeng, Zhang Honghua, et al.China Environmental Science, 2012, 32: 1772.
[20] Levasseur B, Kaliaguine S.Applied Catalysis B: Environmental, 2009, 88: 305.
[21] Xiao Xiuzhen, Lu Guanzhong, Mao Dongsen.Journal of Shanghai Institute of Technology, 2014, 14(1): 1.
[22] Zhang H M, Shimizu Y, Teraoka Y, et al.Journal of Catalysis, 1990, 121: 432.
[23] Niu J, Deng J, Liu W, et al.Catalysis Today, 2007, 126: 420.
[24] Kaliaguine S, Van Neste A, Szabo V, et al.Applied Catalysis A: General, 2001, 209: 345.
O657.9
A
聚乙二醇辅助下的La-Co-O 复合氧化物合成及其对苯的完全氧化性能研究
魏笑峰1, 2, 李达林1,肖益鸿1, 蔡国辉1, 戴 武1,谢增鸿2*,魏可镁1
1.福州大学化肥催化剂国家工程研究中心, 福建 福州 350002 2.福州大学化学学院,福建 福州 350108
以聚乙二醇(PEG)作为分散剂,采用共沉淀法合成La-Co-O复合氧化物,考察添加不同分子量的PEG (0, 2 000, 6 000, 20 000 g·mol-1) 对复合氧化物的物化性质及苯完全氧化性能的影响。采用N2物理吸附、XRD、SEM、H2-TPR、O2-TPD和XPS进行催化剂表征。苯完全氧化反应结果显示催化剂活性顺序为LCO-PEG6000>LCO>LCO-PG20000>LCO-PG2000,LCO-PEG6000催化剂在383 ℃时对苯的转化率达到99%,比LCO低126 ℃。N2物理吸附实验表明所制备的样品的SBET均为9~10 m2·g-1。XRD分析显示合成的催化剂均为LaCoO3钙钛矿主相伴生少量La2O3和Co3O4杂相,但添加PEG有利于钙钛矿主相的形成。尤其是添加PEG6000有效地抑制了催化剂颗粒的团聚,合成的样品颗粒均匀且尺寸最小。H2-TPR和O2-TPD结果表明该催化剂具有更高的还原性能和晶格氧迁移能力,同时XPS分析显示表面活性Co3+含量最高,这些性质使其具有最高的催化氧化活性。
复合氧化物;LaCoO3;聚乙二醇;共沉淀法;催化燃烧;苯完全氧化
2015-04-17,
2015-08-20)
Foundation item:National Science Foundation (40976071 and 21377024), Key S&T Project of Fujian Province (2014H6015), and the Program from the Fujian Education Department (JA12023)
10.3964/j.issn.1000-0593(2016)09-3062-06
Received:2015-04-17; accepted:2015-08-20
Biography:WEI Xiao-feng,(1976—),female,PhD student of Fuzhou University e-mail: xfwei316@fzu.edu.cn *Corresponding author e-mail: zhxie@fzu.edu.cn
*通讯联系人
