In situ synthesis ofhydrophobic magnesium hydroxide nanoparticles in a novelimpinging stream-rotating packed bed reactor☆
2016-05-30HongyanShenYouzhiLiu
Hongyan Shen,Youzhi Liu*
ShanxiProvince Key Laboratory of Higee-Oriented ChemicalEngineering,College ofChemicalEngineering and Environment,North University ofChina,Taiyuan 030051,China
1.Introduction
Magnesium hydroxide[Mg(OH)2],as an environmentally benign flame retardant,has recently attracted increased attention because of its high decomposition temperature,smoke suppressibility,flame retardancy,nontoxicity,and neutralization with acid gas from polymer combustion[1–10].However,the hydrophilic surfaces of Mg(OH)2are not favorable for their dispersion in hydrophobic polymer matrices,requiring addition levels ofup to 60 wt%to achieve high flame-retardant ef ficiency.Such high addition could negatively affect the mechanical properties of the resulting flame retardant polymeric composites[11–14].Therefore,improving the dispersibility and compatibility between Mg(OH)2and the polymer matrix is necessary[15–17].
Surface modi fication has been proposed to solve the problems associated with Mg(OH)2use.Zhang et al.successfully surface modi fied micro Mg(OH)2by means ofan ultrasonic method with stearic acid as a modi fier[12].Yan etal.prepared hydrophobic Mg(OH)2nanoparticles via PMMA grafting by dispersion polymerization after oleic acid(OA)surface modi fication[18].However,these methods often require several reaction steps and are too complex to synthesize hydrophobic Mg(OH)2in large scale.
Higee equipmenthas been successfully applied to prepare Mg(OH)2nanoparticles.There are two types of Higee equipment,e.g.,the rotating packed bed(RPB)and the spinning disk reactor(SDR).They were able to generate a high gravity environment that can intensify micromixing and mass transfer,and this is very helpfulfor achieving uniform supersaturation in a precipitation process.According to the above mentioned advantages,Song etal.successfully prepared lamellar Mg(OH)2nanoparticles with 20 nm to 80 nm size in an RPB at temperatures between 60 °C and 160 °C[19].Tai et al.successfully synthesized lamellar Mg(OH)2nanoparticles with 50 nm to 80 nm in length and 10 nm in thickness using SDR[8].However,the preparation of hydrophobic Mg(OH)2nanoparticles by one step precipitation through higee equipment has not been reported.
A novelhigee equipment,impinging stream-rotating packed bed(IS-RPB)reactor was proposed by Liu(2009)[20].In the IS-RPB,the two reactant solutions flow along the same axis in the opposite direction into collision to change the liquid non-uniform distribution of RPB,which primarily intensi fies micromixing and mass transfer.As the result ofsuch a collision,the fringe of the impinging surface region is then dispersed into the rotating packing,which intensi fies the micromixing of the two liquid reactants secondly.Therefore,uniform supersaturation and homogeneous nucleation occur,yielding relatively smallparticles with a narrow size distribution with limited agglomeration.
In this work,we have successfully prepared hydrophobic Mg(OH)2nanoparticles with good dispersibility and compatibility in organic phase by one step precipitation in IS-RPB using OA as a modi fier.The obtained hydrophobic Mg(OH)2nanoparticles were characterized and the surface properties of Mg(OH)2nanoparticles were investigated.The results indicated that the proposed method had the potentialapplicability to produce hydrophobic Mg(OH)2nanoparticles in large scale.
2.Experimental
2.1.Materials
Magnesium chloride hexahydrate(MgCl2·6H2O),OA,absolute ethanol,and liquid paraf fin were purchased from Tianjin Guangfu Chemical Reagent Factory(China).Sodium hydroxide(NaOH)was purchased from Tianjin Dalu Chemical Reagent Factory(China).All of the above chemicalreagents were analytically pure and used as received without further puri fication.High-purity water was prepared by a water puri fication system(GWA-UN,China)and used in all procedures.
2.2.Synthesis ofhydrophobic Mg(OH)2 nanoparticles
Fig.1(a)shows a photograph of the IS-RPB reactor applied to prepare Mg(OH)2nanoparticles.The IS-RPB reactor included an impinging stream liquid distributor containing a straight tube with a 1.5 mm hole on one end and an L-type tube with a 1.5 mm hole on the inside of its front end.The packed bed in the IS-RPB reactor had an inner radius of 50 mm,an outer radius of 125 mm,and an axial height of 210 mm.The reactor was packed with stainless steelwire mesh 0.3 mmin diameter and with 0.96 porosity.
Fig.1(b)schematically depicts the experimentalsetup for preparing Mg(OH)2nanoparticles.Predetermined masses of OA and MgCl2were dissolved in 2 L of a deionized water/absolute ethanolmixture(10:23,v/v);here,the concentration of Mg2+was fixed to 0.75 mol·L-1.Tank A contained this as-prepared solution.NaOH was dissolved in 2 L ofdeionized water to achieve a fixed OH-concentration of1.5 mol·L-1.This solution was then placed in tank B.The reaction temperature,rotation speed,and liquid flow rate were maintained at 60°C,800 rpm,and 40 L·h-1,respectively.The MgCl2and NaOH solutions were simultaneously and continuously pumped from tanks A and B into the IS-RPB reactor,where both solutions were mixed and reacted with each other and the obtained suspension was separated in a centrifuge.The precipitate was washed three times with deionized water and then three times with absolute ethanolto remove residualimpurities.The wet particles were dried in a vacuum oven at 60°C for 6 h.Finally,the ethanolin the mother liquor was recovered by distillto reduce operation costs.
2.3.Characterization
A field-emission scanning electron microscope(FESEM;JSM-6700F,JEOL,Tokyo,Japan)was used to observe the morphologies of the Mg(OH)2nanoparticles.The phase purity and crystallographic structures of the samples were examined by X-ray diffraction(XRD,Bruker D8 Advance diffractometer)using CuKα(λ =0.15406 nm)radiation at an operation voltage and currentof40 kV and 40 mA,respectively.Fourier transform infrared spectroscopy(FTIR)was performed by a Spectrum Two spectrometer employing the KBr pellet method.The thermalbehavior of the Mg(OH)2nanoparticles was determined on a STA 449F3 simultaneous thermal analyzer(Netzsch,Germany)undera nitrogen atmosphere from 35 °C to 600 °C ata heating rate of10℃·min-1.
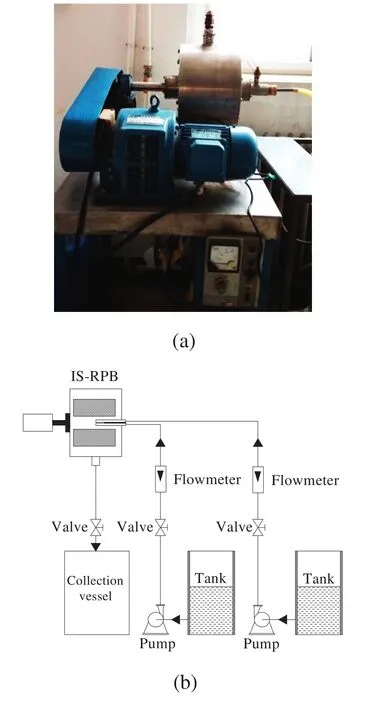
Fig.1.Preparation of Mg(OH)2 nanoparticles:(a)photograph of IS-RPB and(b)the experimentalsetup.
2.4.Surface property of Mg(OH)2 nanoparticles
2.4.1.Surface wettability property
The surface wettability of the nanoparticles was characterized in terms of water contact angle.Water contact angle tests were carried outusing a contactangle analyzer(JC2000D1,China).The powder samples were pressed into thin pellets under 10 MPa and contact angles were measured by the sessile drop method.The probe fluid used was reverse osmosis-puri fied water.
2.4.2.Dispersion characteristics of Mg(OH)2in liquid paraf fin
Exactly 0.1 g ofpowder and 10 mlofliquid paraf fin were placed into a testtube and dispersed by ultrasonication for 3 min.The obtained suspension was dropped onto a crystal flake and observed using a bioopticalmicroscope(OPTEC BK 300).
2.4.3.Sedimentation characteristics of Mg(OH)2in water and liquid paraf fin
Exactly 0.1 g ofpowder was mixed into 10 mlofwater or liquid paraf fin and the sedimentation of the nanoparticles was observed.
十队是在60年代末由两个生产队合并而成,所以,在日常劳作中,社员总会习惯性地分成两组。为了公正记录工分,两边各推选一名记分员记录对方的工分,同时还另选一名总记工员,两位记分员每天都要把各个社员的工分汇总到总记工员处。由于社员在一天内经常做不同的工种,如果自己记了本组社员的工分,另一个记分员则要把他们的工分、工种抄回去,所以我们在工分簿上看到很多“√”。当时出工就画一个圈,不出工则打个叉,并作相关说明,以免社员日后翻旧账。
3.Results and Discussion
3.1.FTIR analysis
The FTIR spectra of the modi fier OA,blank Mg(OH)2,and hydrophobic Mg(OH)2are shown in Fig.2(a)–(c).In blank Mg(OH)2,the peak appearing at 3699 cm-1is matched to–OH stretching vibrations.The broad peak appearing at 3440 cm-1is attributed to the stretching vibrations of O–H bonds,while the peak appearing in the range of 1450–1650 cm-1is associated with the bending vibrations of O–H bond.These peaks are attributed to the water absorbed by Mg(OH)2nanoparticles.
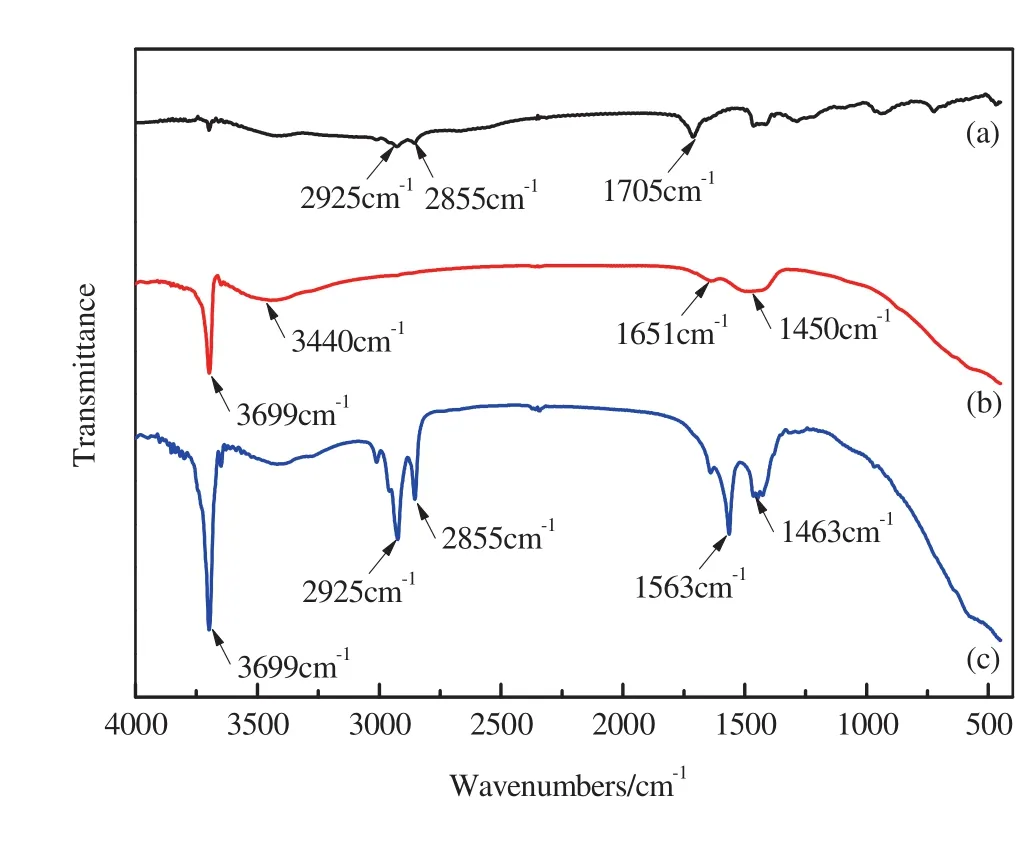
Fig.2.FTIR spectra of(a)the modi fier OA,(b)blank Mg(OH)2,and(c)hydrophobic Mg(OH)2.
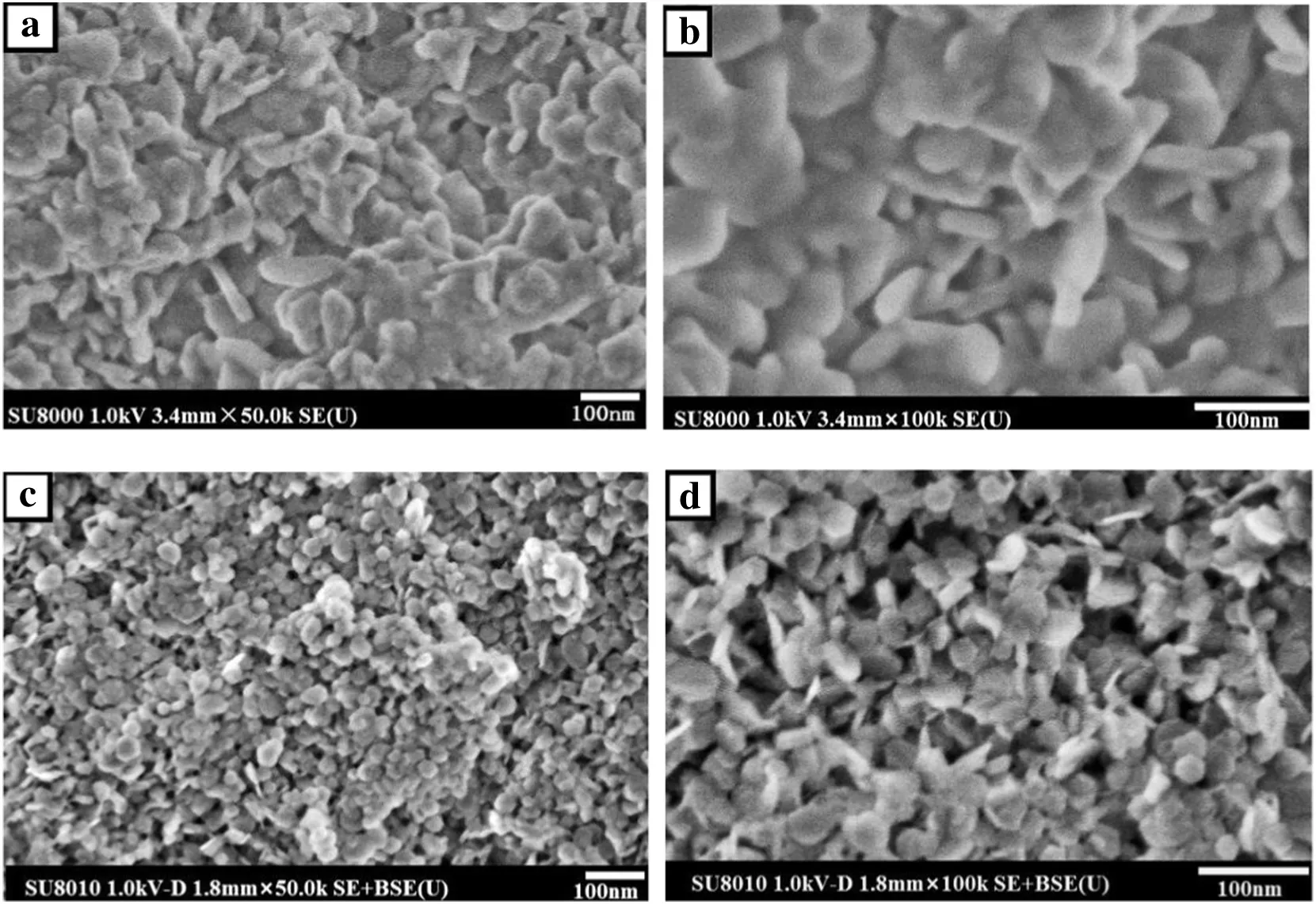
Fig.3.FESEMimages of MH(a),(b)blank Mg(OH)2 and(c),(d)hydrophobic Mg(OH)2.
In agent OA,peaks at2925 and 2855 cm-1are ascribed to the vibrations of–CH2– and –CH3groups.The sharp peak at 1705 cm-1corresponds to–COOH vibrations.In hydrophobic Mg(OH)2,peaks at 2925 and 2855 cm-1,which are attributed to –CH2– and CH3groups,clearly suggest the presence of OA on the surface of Mg(OH)2nanoparticles.Peaks corresponding to the–COOH groups of OA and magnesium carboxylate are absent from the spectrum ofhydrophobic Mg(OH)2.This result indicates that OA molecules were notabsorbed on the surface of Mg(OH)2nanocrystals;instead,OA substituted the surface-absorbed–OH groups of Mg(OH)2.The two peaks at 1576 and 1465 cm-1in Fig.2(c)are due to the symmetric and asymmetric COO stretching vibrations of carboxylate bonds.These observations indicate that OA was bonded on the surface ofMg(OH)2through the interaction between the–COOH group of OA and the–OH group on the surface of Mg(OH)2.
3.2.SEM analysis
The morphology ofblank and hydrophobic Mg(OH)2samples were examined by FESEM.Fig.3 showsthe FESEMimages ofblank and hydrophobic Mg(OH)2.Compared with the blank Mg(OH)2nanoparticles,hydrophobic Mg(OH)2nanoparticles exhibit a more regular morphology(hexagonallamella),smaller mean diameter ofabout30 nm,and better dispersibility without obvious agglomeration.This contrast demonstrates that OA can effectively modify the morphology of Mg(OH)2nanoparticles.Thus,addition of OA to the synthesis system favors the production ofsmaller-sized Mg(OH)2nanoparticles with even crystal growth.These observations are consistent with the XRD results.
3.3.TEManalysis
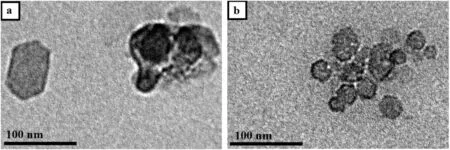
Fig.4.TEMimages of MH(a)blank Mg(OH)2 and(b)hydrophobic Mg(OH)2.
The morphology of blank and hydrophobic Mg(OH)2samples was further analyzed by TEM(shown in Fig.4).As can be observed,the hydrophobic Mg(OH)2nanoparticles had a more regular shape(hexagonallamella)and uniform particle size distribution with particle size ofaround 30 nmin comparison with irregularly shaped large particles of the blank Mg(OH)2sample.This contrast demonstrates that the addition of OA is favourable to obtain smaller sized Mg(OH)2nanoparticles with regular hexagonal lamella and uniform particle size distribution.
3.4.XRD analysis
The crystal phases and crystallinity of blank and hydrophobic Mg(OH)2nanoparticles were determined by XRD.As shown in Fig.5,the interaction between Mg(OH)2and OA may also be observed from the XRD patterns.The positions of diffraction peaks in Fig.5(a)and 5(b)showed no changes and were in accordance with the standard data(JCPDS Card No.7-239),thereby illustrating that the structure of the Mg(OH)2was hexagonalin nature.However,between the XRDpatterns ofblank Mg(OH)2and hydrophobic Mg(OH)2,allof the peaks,except that of(001)lattice plan,have no obvious difference in intensity.Comparing I001/I101ratios,hydrophobic Mg(OH)2(I001/I101ratio,0.86)exhibited obviously higher crystallinity than blank Mg(OH)2(I001/I101ratio,0.63),which indicates that OA was grafted onto the(001)lattice plane of Mg(OH)2.The dispersion properties ofhydrophobic Mg(OH)2also improved because of the increase in its I001/I101.The XRD findings were in accordance with the FESEMresults.
The crystallite size(D)of the Mg(OH)2samples could be calculated according to their XRDpatterns by the using the Debye–Scherrerformula[21],which is given in Eq.(1)

The D derived from the(001),(101),and(100)crystalfaces of the Mg(OH)2samples are listed in Table 1.The crystallite size ofhydrophobic Mg(OH)2crystals is smaller than that ofblank Mg(OH)2crystals.
The XRDdata were processed for Rietveld re finementofstructure by Re flex program.Fig.6 shows the Rietveld re finement of XRD data,and Table 2 lists the Rietveld re finement parameters,and crystallite size.The quality of the re finementwas quanti fied by weighted pro file residual Rwp.As can be seen from Fig.6 and Table 2,the blank Mg(OH)2and hydrophobic Mg(OH)2with space group P_-3_m_1,and the crystallite size of hydrophobic Mg(OH)2crystals is smaller than that of blank Mg(OH)2crystals,this result may be attributed to the large number of OA molecules present on the surface of Mg(OH)2nanoparticles,which can effectively avoid the agglomeration of the particles,thereby the addition of OA is favourable to obtain smaller sized Mg(OH)2nanoparticles.
3.5.TG-DSC analysis
Fig.7 shows the TG and DTG curves of blank and hydrophobic Mg(OH)2.The results obtained con firm the signi ficant improvement of the thermal stability of hydrophobic Mg(OH)2in comparison with that of the blank sample.Blank Mg(OH)2exhibits two mass loss steps in the temperature range of 89–376.2 °C.The first step of 2.31%may be attributed to desorption of water physisorbed on Mg(OH)2.The second-step mass loss(28.51%)begins at 340.2°C and ends at 376.2°C;this step can be attributed to the thermaldecomposition of Mg(OH)2.The theoreticalmass loss of the Mg(OH)2→MgO transformation is 30.8%,slightly larger than the observed 28.51%.This discrepancy in mass loss is ascribed to the incomplete decomposition of the sample in the temperature range studied.
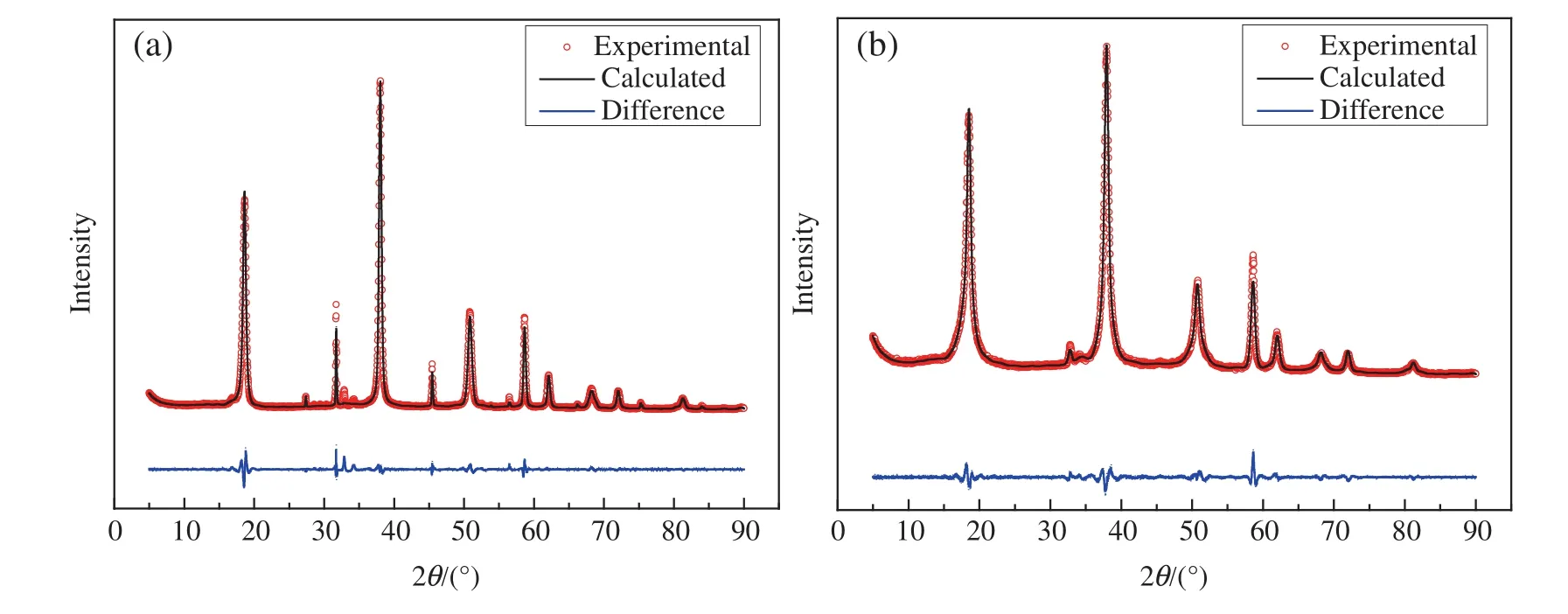
Fig.6.Finalplots of the Rietveld re finement,showing the experimental(circles)and calculated(line)intensities of(a)blank Mg(OH)2 and(b)hydrophobic Mg(OH)2.The lower curve is the difference diagram.

Table 2 Crystallite size and Rietveld re finement parameters of blank Mg(OH)2 and hydrophobic Mg(OH)2.
In the hydrophobic Mg(OH)2sample,decomposition occurred in three steps from 89 °C to 485.1 °C.The mass loss from 89 °C to 250 °C(1.59%)is due to desorption of adsorbed water.The mass loss from 320.8 °Cto 346.7 °C(26.29%)corresponds to the thermaldecomposition of magnesium hydroxide.The mass loss from 425.9 °C to 485.1 °C(11.14%)corresponds to the decomposition of OA.The totalpercentage ofmass loss ofhydrophobic Mg(OH)2was 40.88%,higher than that of blank Mg(OH)2(33.18%).This result suggests that a certain amount of OA is bonded to the surface of the Mg(OH)2nanoparticles,which could decrease the endothermic decomposition temperature of the Mg(OH)2nanoparticles,and enhance their thermalstability.
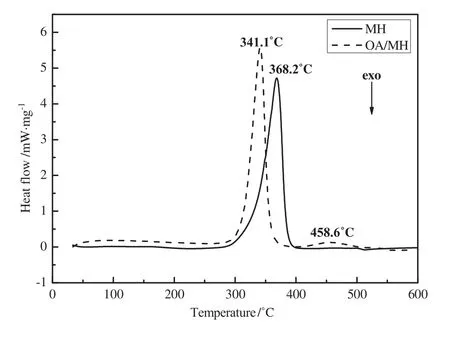
Fig.8.DSC curves of blank and hydrophobic Mg(OH)2.

Fig.7.TG and DTG curves ofblank and hydrophobic Mg(OH)2.
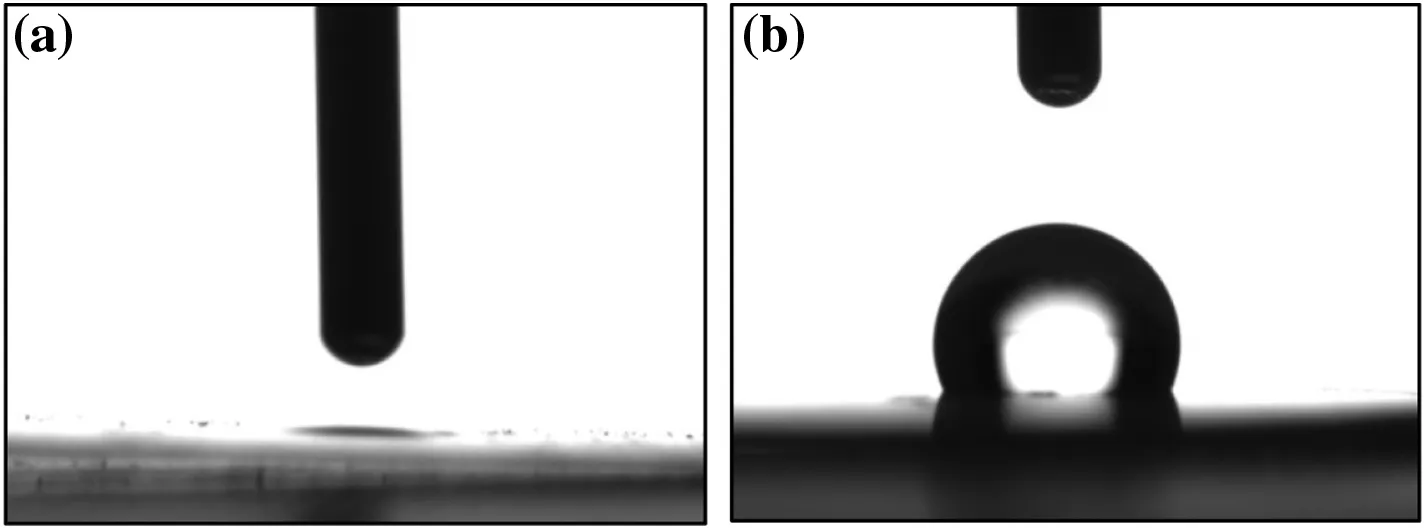
Fig.9.Behavior of water droplets on the surface ofthin Mg(OH)2 nanoparticle pellets:(a)blank Mg(OH)2 and(b)hydrophobic Mg(OH)2.
The DSC curves of blank and hydrophobic Mg(OH)2are shown in Fig.8.Because the thermal decomposition of Mg(OH)2is an endothermic reaction,the DSC curve ofhydrophobic Mg(OH)2shows an endothermic peak at 341.1°C that is sharper than that of blank Mg(OH)2(368.2°C).There is another small endothermic peak at 458.6°C for hydrophobic Mg(OH)2,which may be caused by the presence of OA on the surface of Mg(OH)2nanoparticles.This conclusion is in accordance to the TG result.
3.6.Surface property of Mg(OH)2 nanoparticles
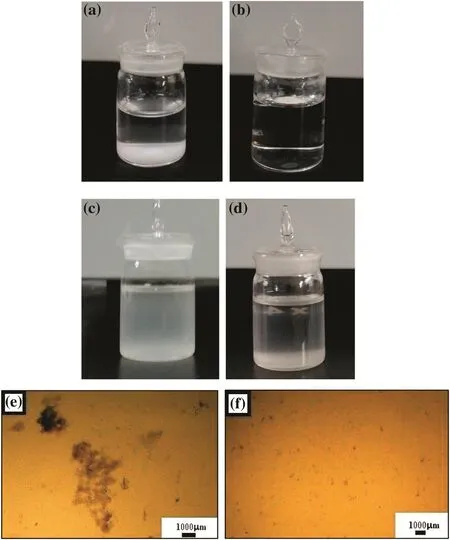
Fig.10.Photographs ofblank Mg(OH)2 in(a)water and(d,e)in liquid paraf fin and hydrophobic Mg(OH)2 in(b)water and(c,f)liquid paraf fin.
The surface wettability of blank and hydrophobic Mg(OH)2was characterized in terms of water contact angles,as shown in Fig.9.When a water droplet is applied to the surface of the blank Mg(OH)2sample,as shown in Fig.9(a),no stable drop shape was formed;this result indicates that blank Mg(OH)2nanoparticles are hydrophilic and easily wetted by water.The water contact angle of hydrophobic Mg(OH)2was 110.4°;this result demonstrates that the surface polarity of the sample was weak and its surface energy was low.These observations may be attributed to the large number of OA molecules presenton the surface of the hydrophobic Mg(OH)2nanoparticles with alkylchains predominantly exposed to the air–water interface.
To evaluate the hydrophobic nature of Mg(OH)2nanoparticles,the sedimentation behavior of the nanoparticles was observed in water.Fig.10(a)and 10(b)respectively illustrate the sedimentation of blank and hydrophobic Mg(OH)2nanoparticles in water.Blank Mg(OH)2nanoparticles sank into the water after 10 min,while hydrophobic Mg(OH)2nanoparticles floated on the water for 30 d.Fig.10(c)and 10(d)respectively show the sedimentation of blank and hydrophobic Mg(OH)2nanoparticles in liquid paraf fin.The blank Mg(OH)2showed rapid sedimentation because its hydrophilic surface was poorly wetted by the liquid paraf fin and its suspension in the liquid was achieved by agglomeration into large particles as shown in Fig.10(e).By contrast,hydrophobic Mg(OH)2dispersed very well through the liquid paraf fin,as shown in Fig.10(f).These results indicate that OA modi fication of Mg(OH)2surfaces can yield hydrophobic Mg(OH)2with signi ficantly improved compatibility with various organic phases.
4.Conclusions
In this study,we proposed an in situ surface modi fication method to synthesize hydrophobic Mg(OH)2nanoparticles in IS-RPB using OA as a surface modi fier.SEM images showed that the obtained nanoparticles present regular hexagonal lamellae with an average diameter of 30 nm.XRD revealed that the high-purity Mg(OH)2product presents a brucite structure and the I001/I101ofhydrophobic Mg(OH)2(0.86)was higher than that ofblank Mg(OH)2(0.63).FTIR analysis showed that the OA interacted with Mg(OH)2via chemical bonding.Compared with the blank Mg(OH)2product,the productobtained via the proposed method exhibited a higher water contactangle(101.4°)and better dispersion in liquid paraf fin.The improved hydrophobicity and surface dispersibility ofMg(OH)2nanoparticles were also veri fied by sedimentation tests.TG/DSC analysis indicated that the total percentage of weight loss of hydrophobic Mg(OH)2(40.88%)was higher than that of blank Mg(OH)2(33.18%),which indicates improved flame-retardant effectiveness.Considering these results,the proposed in situ surface modi fication method in IS-RPB presents potentialapplicability to the large-scale production of Mg(OH)2.
[1]X.L.Chen,J.Yu,S.Y.Guo,Surface modi fication of magnesium hydroxide and its application in flame retardant polypropylene composites,J.Mater.Sci.44(2009)1324–1332.
[2]H.Hu,X.R.Deng,Preparation and properties ofsuper fine Mg(OH)2flame retardant,Trans.Nonferrous Metals Soc.China 16(2006)488–492.
[3]H.Ma,Z.X.Chen,Z.P.Mao,Controlled growth ofmagnesium hydroxide crystals and its effect on the high-temperature properties ofcotton/magnesium hydroxide composites,Vacuum 95(2013)1–5.
[4]Y.H.Hu,S.F.Li,The effects of magnesium hydroxide on flash pyrolysis of polystyrene,J.Anal.Appl.Pyrolysis 78(2007)32–39.
[5]G.W.Beall,E.-S.M.Duraia,F.El-Tantawy,Rapid fabrication of nanostructured magnesium hydroxide and hydromagnesite via microwave-assisted technique,Powder Technol.234(2013)26–31.
[6]X.Li,T.X.Shi,P.Chang,Preparation of magnesium hydroxide flame retardant from light calcined powder by ammonia circulation method,Powder Technol.260(2014)98–104.
[7]J.P.Hsu,A.Nacu,Preparation ofsubmicron-sized Mg(OH)2particles through precipitation,Colloids Surf.A Physicochem.Eng.Asp.262(220–231)(2005).
[8]C.Y.Tai,C.T.Tai,M.H.Chang,Synthesis ofmagnesium hydroxide and oxide nanoparticles using a spinning disk reactor,Ind.Eng.Chem.Res.46(2007)5536–5541.
[9]C.Henrist,J.P.Mathieu,C.Vogels,Morphological study of magnesium hydroxide nanoparticles precipitated in dilute aqueous solution,J.Cryst.Growth 249(2003)321–330.
[10]X.Li,C.Ma,J.Y.Zhao,Preparation ofmagnesium hydroxide nanoplates using a bubbling setup,Powder Technol.198(2010)292–297.
[11]C.M.Liauw,G.C.Lees,S.J.Hurst,Effectofsilane-based filler surface treatmentformulation on the interfacial properties of impact modi fied polypropylene/magnesium hydroxide composites,Compos.A:Appl.Sci.Manuf.29(1998)1313–1318.
[12]F.Z.Zhang,H.Zhang,Z.X.Su,Surface treatmentofmagnesium hydroxide to improve its dispersion in organic phase by the ultrasonic technique,Appl.Surf.Sci.253(2007)7393–7397.
[13]D.M.An,L.L.Wang,Y.H.Zheng,In situ preparation and surface modi fication ofmagnesium hydroxide nanoparticles,Colloids Surf.A Physicochem.Eng.Asp.348(9–13)(2009).
[14]P.P.Wang,C.H.Li,H.Y.Gong,Morphology controland growth mechanism ofmagnesium hydroxide nanoparticles via a simple wet precipitation method,Ceram.Int.37(2011)3365–3370.
[15]X.T.Lv,H.Bala,M.G.Li,In situ synthesis ofnanolamellas ofhydrophobic magnesium hydroxide,Colloids Surf.A Physicochem.Eng.Asp.296(97–103)(2007).
[16]Y.F.Yang,X.F.Wu,G.S.Hua,Effects of stearic acid on synthesis of magnesium hydroxide via direct precipitation,J.Cryst.Growth 310(2008)3557–3560.
[17]G.L.Song,S.D.Ma,G.Y.Tang,Ultrasonic-assisted synthesis of hydrophobic magnesium hydroxide nanoparticles,Colloids Surf.A Physicochem.Eng.Asp.364(99–104)(2010).
[18]H.Yan,X.H.Zhang,L.Q.Wei,Hydrophobic magnesium hydroxide nanoparticles via oleic acid and poly(methyl methacrylate)-grafting surface modi fication,Powder Technol.193(2009)125–129.
[19]Song,Y.H.,Chen,C.M.,Chen,C.F.,A new technology of preparation of nanosized magnesium hydroxide flame retardant materials,China Pat,CN 1128199C(2003).
[20]Y.Z.Liu,ChemicalEngineering Process and Technology in High Gravity,NationalDefense Industry Press,Beijing,2009 172–253.
[21]C.C.Lin,J.M.Ho,Structural analysis and catalytic cactivity of Fe3O4nanoparticles prepared by a facile co-precipitation method in a rotating packed bed,Ceram.Int.40(2014)10275–10282.
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Heat transfer ofnano fluidics in hydrophilic pores:Insights from molecular dynamics simulations☆
- Numericalsimulation ofstirred tanks using a hybrid immersed-boundary method☆
- Numericalsimulation ofmicromixing effect on the reactive flow in a co-rotating twin screw extruder☆
- Coalescence behaviour ofwater droplets in water-oilinterface under pulsatile electric fields
- Effects of Sn residue on the high temperature stability of the H2-permeable palladium membranes prepared by electroless plating on Al2O3 substrate after SnCl2–PdCl2 process:A case study☆
- Application ofdiffusive transport modelfor better insight into retardation mechanisms involved in ion-imprinted membrane transport
