Experimental Study of UDS Solvents for Purifying Highly Sour Natural Gas at Industrial Side-stream Plant
2016-03-22
(State Key Laboratory of Chemical Engineering, East China University of Science and Technology, Shanghai 200237)
Experimental Study of UDS Solvents for Purifying Highly Sour Natural Gas at Industrial Side-stream Plant
Ke Yuan; Shen Benxian; Sun Hui; Liu Jichang; Liu Lu; Xu Shenyan
(State Key Laboratory of Chemical Engineering, East China University of Science and Technology, Shanghai 200237)
The desulfurization performance of the UDS solvents was investigated at an industrial side-stream plant and was compared with that of MDEA solvent. A mass transfer performance model was employed for explaining the COS absorption into different solvents. Meanwhile, the regeneration performance of the UDS solvents was evaluated in side-stream tests. Results indicate that under the conditions covering an absorption temperature of 40 ℃, a pressure of 8.0 MPa, and a gas to liquid volume ratio (V/L) of around 230, the H2S content in puri fi ed gas can be reduced to 4.2 mg/m3and 0 by using solvents UDS-II and UDS-III, respectively. Moreover, the total sulfur content in both puri fi ed gases is less than 80 mg/m3. As a result, the UDS-III solvent shows by 30 percentage points higher in COS removal ef fi ciency than MDEA. In addition, the total volume mass transfer coef fi cient of UDS solvent is found to be twice higher than that of MDEA. Furthermore, the UDS solvents exhibit satisfactory thermal stability and regeneration performance.
highly sour natural gas; UDS solvent; COS; desulfurization
1 Introduction
As a clean and efficient energy, the rapid development of natural gas has become a trend and requisite nowadays. With its increasing domestic demand, the proven reserve of Puguang gas fi eld in Northeastern Sichuan of China is up to 381 billion m3. The Puguang natural gas has a high content of sour components, especially a high carbonyl sulfide (COS) content[1-3]. As the requirements for natural gas puri fi cation are increasingly stringent, the academic and industrial investigation on ef fi cient puri fication technology and high-performance desulfurization solvents have been attracting increasingly wide interests[4-6]. Thanks to a lot of advantages including the large processing capacity, a wide range of adaptability to sulfi de concentration and the cheap equipment requirement, the absorption processes involving various aqueous alkanolamine solutions are considered as the most suitable technologies for removal of impurities from acid natural gas. However, the commonly used alkanolamines including monoethanolamine (MEA), diethanolamine (DEA), di-2-propanolamine (DIPA), and methyldiethanolamine (MDEA) feature a low ef fi ciency for removal of organosulfurs, such as COS and mercaptans[7-9]. Therefore, it is really a challenge to reducing the total sulfur content in puri fi ed gas to a required low level, since the sour natural gas has a high organosulfur concentration.
Based on the organosulfur removal mechanism in relation to different solvent components, the ef fi cient formulated solvents called UDS (unitedly developed desulfurization solvent) have been developed in this lab and proven to have excellent performance for both H2S and organosulfurs removal[10]. The UDS solvents are composed of UDS formula component (UDS-F) and MDEA solvent. Furthermore, their formulas can be varied according to the compositions of raw natural gas in compliance with the puri fi cation requirements. The UDS formula component is mainly composed of alkanolamine compound, heterocyclic amine compound, and sulfur-containing heterocyclic compound. Previous investigations have indicated that the UDS solvents show excellent performance for removing sour components including organosulfur compounds from simulated highly sour natural gas[11-13].
In present work, we studied the desulfurization perfor-mance of UDS solvents in an industrial side-stream unit by using the actual natural gas feed under the operation conditions that were similar to typical industrial process. The results can provide some guidance for industrial application of the UDS solvents.
2 Experimental
2.1 Feed and reagents
The actual natural gas from an industrial plant was used as the feed gas. Its average composition is given in Table 1. It had a high content of acid components, and the concentration of H2S and organosulfur compounds was 15.9% and 390.9 mg/m3, respectively. The UDS solvent, which was prepared in this laboratory, contained more than 99% of active components, with its properties (on an anhydrous basis) shown in Table 2. UDS-I, UDS-II and UDSIII solvents contained 5%, 10%, and 15% of UDS formula component (UDS-F), respectively. The mass fractions of the UDS aqueous solutions were fi xed at 50%. Deionized water was used in all cases.

Table 1 Composition of sour natural gas feed

Table 2 Properties of anhydrous UDS solvent
2.2 Experimental apparatus and procedure
The industrial side-stream experimental unit with a processing capacity of 80 Nm3of feed natural gas per h is shown in Figure 1. The highly sour natural gas feedstock enters the absorption tower at the bottom and contacts with the lean solution introduced from the top of the tower on the packing. The puri fi ed gas was sampled from the top and the content of sulfur compounds was analyzed by a gas chromatograph. The rich solution is heated at the regeneration tower for stripping the absorbed sour components. The regenerated lean solution is then discharged from the bottom of the regeneration tower followed by cooling prior to being recycled to the absorption tower for absorption of sulfur compounds. According to a typical industrial process, the absorption temperature is speci fi ed at 40 ℃ for these side-stream experiments.
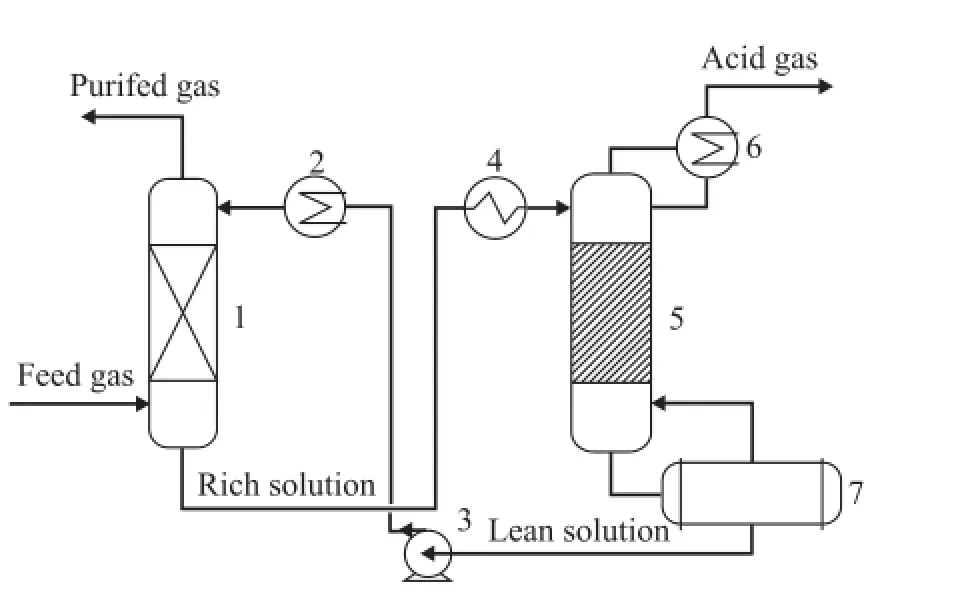
Figure 1 Flowchart of industrial side-stream unit forhighly sour natural gas puri fi cation
2.3 Analytical method
A Clarus 500 gas chromatograph equipped with a fl ame photometric detector (FPD) (Perkin Elmer Chromatograph Instrument Co., Ltd., USA) was used to analyze the sulfur content in the feed gas and the puri fi ed gas. An SE-30 capillary column (Lanzhou Institute of Chemical Physics, China) was used to separate the sulfur compounds.
2.4 Ef fi ciency for COS removal
The ef fi ciencyE(%) for COS removal is calculated using the following expression:
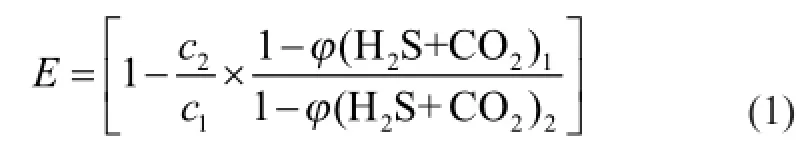
wherec1andc2are the concentration of COS in the feed gas and the purified gas, respectively, mg/m3;φ(H2S+CO2)1andφ(H2S+CO2)2are the total volume fraction of H2S and CO2in the feed gas and in the purified gas, respectively, %.
2.5 Total volume mass transfer coef fi cient
The total volume mass transfer coefficientKga(kmol/ (m3·s·kPa)) for COS is calculated using the following expression:

wherejis the total gas phase velocity, kmol/(m2·s);Pis the gas phase pressure, kPa;His the packing height, m.
3 Results and Discussion
3.1 Mechanism for COS removal
According to the composition analysis results presented in Table 1, COS accounts for the main part of organosulfurs in natural gas feed, suggesting that the overall ef fi ciency of organosulfurs removal can be largely determined by the COS removal rate. Optimization in the formula of solvent should be focused on promoting its ef fi ciency for COS removal. COS can be absorbed into tertiary amine like MDEA through hydrolysis reaction based on the alkali catalytic reaction (see Equation (3)).

In addition to the catalytic hydrolysis reaction of COS, primary amine and secondary amine can also react with COS through forming the zwitterions (RNH2+COS-and R2NH+COS-)[14-16]. On the other hand, the primary amine and secondary amine components can catalyze the hydrolysis reaction of COS more effectively, and therefore, improve the chemical absorptive efficiency for COS removal[17-20]. The reaction can be written as Equations (4) and (5).

Furthermore, heterocyclic amine (R′NH2) is the major component of UDS solvent affecting the removal of COS. Compared with primary amines and tertiary amines, MOR has a faster reaction rate with COS[14,20]. The reaction is shown in Equation (6).

Meanwhile, the UDS solvents have good physical solubility of COS. Components of molecules with the S=O groups in UDS-F solvent can enhance the combination of organosulfurs and solvent molecules, increase the solubility of COS in the solvent, and largely improve the selective absorption of organosulfurs.
Therefore, the UDS solvents have better organosulfurs removal performance than MDEA solvent because of their high physical absorption and chemical absorption nature. Furthermore, according to the compositions of raw natural gas as well as the puri fi cation requirements, the removal performance of UDS solvents can be updated through adjusting the content of UDS-F solvent.
3.2 Puri fi cation performance of UDS solvent
3.2.1 H2S removal under different gas to solvent ratios
Figure 2 shows the H2S removal performance of UDS-I, UDS-II and UDS-III at different gas to solvent volume ratios (V/L). The H2S removal performance of MDEA solvent under the similar operating conditions is also given for comparison. It can be seen from Figure 2 that three kinds of UDS solvents and MDEA solvent all exhibit excellent H2S removal performance. The H2S content in the puri fi ed gas can be reduced up to less than the detection limit at aV/Lratio of less than 200. As theV/Lratio rises to 230, different solvents show a clear distinction in H2S removal performance. As for MDEA solvent, the H2S content in puri fi ed gas is a highest value, 34.7 mg/m3. As regards the UDS solvents, the H2S content in puri fi ed gas is 6.5 mg/m3, 4.2 mg/m3and 0 for UDS-I, UDS-II and UDS-III solvents, respectively.
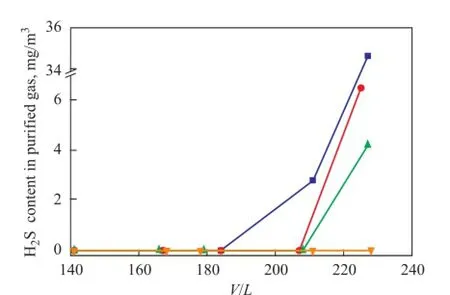
Figure 2 Effect of gas to solvent ratio on the performance of different solvents for H2S removal at 40℃
3.2.2 Total sulfur removal performance under different gas to solvent ratios
At differentV/Lratios, the performance of solvents for removal of total sulfur is shown in Figure 3. With theV/Lratio increasing from 140 to 230, the total sulfur content in the purified gas treated by MDEA is in the range of 150 to 290 mg/m3. The total sulfur content of the puri fi ed gas is below 80 mg/m3at the sameV/Lratio range upon using the UDS solvents. Three kinds of UDS solvents show significantly higher total sulfur removal performance as compared with MDEA solvent. It is mainly because the UDS solvents are designed according to the mechanism for removal of different types of sulfur compounds. Therefore, the UDS solvents not only can effectively remove H2S, but also reduce the total sulfur content in the puri fi ed gas to a lower level and improve the quality of puri fi ed gas.
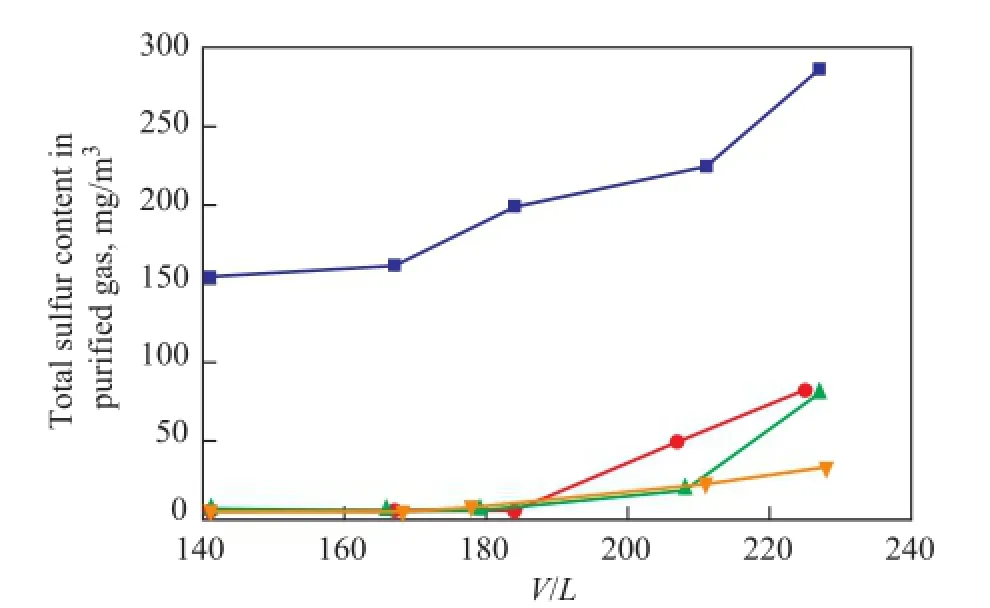
Figure 3 Effect of gas to solvent ratio on the performance for removal of total sulfur at 40
3.2.3 CO2removal performance at different gas to solvent ratios
Figure 4 shows the relationship between the CO2content in the purified gas andV/Lof different solvents. With the increase ofV/Lratio, the CO2content in puri fi ed gas follows a different rising speed. Because of the similar molecular structure of CO2and COS, the mechanism for removal of CO2is similar to that of COS in the absorption process. The composition of UDS solvents that can improve the physical and chemical dissolution performance of COS could also increase the CO2removal rate to a certain extent. Therefore, in comparison with MDEA solvent, the UDS solvents possess higher CO2absorption rate. As a result, varying performance for removal of CO2can also be achieved by adjusting the composition of UDS solvents based on different process requirements.

Figure 4 Effect of gas to solvent ratio on the performanceof solvent for CO2removal at 40℃
3.2.4 Comparison of COS removal performance between UDS and MDEA solvents
Judging from the analysis of sulfur compounds in feed gas shown in Table 1, the organosulfurs in raw materials contain mainly COS, so the organosulfurs removal performance of the solvent is mainly determined by the COS removal rate. The COS removal ef fi ciency and total volume mass transfer coef fi cient of UDS solvents are compared with those of MDEA at aV/Lratio of 230, with the results presented in Figure 5. It can be seen that the COS removal efficiency and total volume mass transfer coefficient increase with an increasing content of UDS-F solvent. The COS removal ef fi ciency of UDS solvents is by more than 30 percentage points higher than that of MDEA solvent. At aV/Lratio of 230, the total volume mass transfercoef fi cient of UDS-I is 8.55×10-7kmol/(m3·s·kPa), which is twice higher than that of MDEA solvent, viz. 3.02×10-7kmol/(m3·s·kPa).

Figure 5 Comparison on removal of COS between UDS and MDEA solvents at 40(atV/L=230)
3.3 Mass transfer performance model for COS absorption
The UDS solvents have definite chemical and physical solubility of COS. But in the presence of H2S and CO2, the chemical solubility of COS will be affected by different degree of inhibition. For the case of highly sour gas absorption process, there is a high concentration of H2S and CO2. The acidic component load in UDS solution is higher. Therefore, we set up an absorption model for removing organosulfurs from highly sour natural gas by the UDS solvents[21-22](see Equation (7)).

wherebis the mass transfer performance factor of COS, mol0.3·m-0.6·s-0.3·kPa-1; a is the packing speci fi c area, m2/m3; H is the height of absorption tower, m;Gis the gas phase fl ow rate, mol/s;Lis the liquid fl ow rate, mol/s;mis the Henry constant of COS;y1andy2are the organosulfurs concentration in the gas feed and in the puri fi ed gas, respectively.
The Henry constantmand the mass transfer performance factorbof COS in UDS and MDEA solvents are calculated using the above model, with the results listed in Table 3. The Henry constantmis used to characterize the capacity of COS dissolved, and the smaller m indicates the higher solubility of COS in the solvent. The mass transfer performance factorbcharacterizes the mass transfer performance of COS in the absorption process. Under the same condition, the greater mass transfer performance factorbof COS is more bene fi cial to the absorption process. The model parameters in Table 3 show that the Henry constant of COS for the UDS solvents is less than that of MDEA solvent, which indicates that the solubility of COS in the UDS solvent is higher than that in MDEA under the same absorption condition. At the same time, the mass transfer performance factor of COS in UDS solvent is higher than that in MDEA. The UDS-F component, which can improve the chemical absorption rate and the physical solubility of COS, provides UDS solvent with higher COS solubility and mass transfer performance. The industrial side-stream test results confirm our conclusion that the UDS solvents can improve the COS removal efficiency by more than 30 percentage points as compared with MDEA solvent.
Table 3 Henry's constant and mass transfer factors of organosulfur for UDS solvents at 40
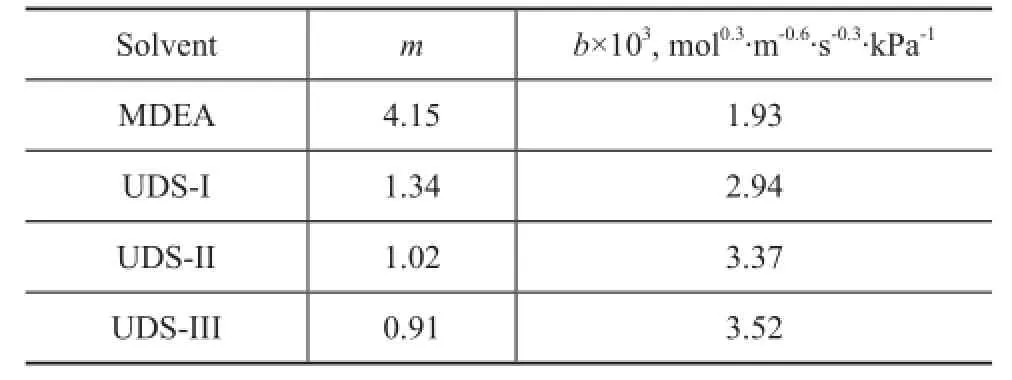
Table 3 Henry's constant and mass transfer factors of organosulfur for UDS solvents at 40
Solventmb×103, mol0.3·m-0.6·s-0.3·kPa-1MDEA 4.15 1.93 UDS-I 1.34 2.94 UDS-II 1.02 3.37 UDS-III 0.91 3.52
The model analysis is carried out to study the effect of operating conditions on the puri fi cation performance. For the same set of devices, the main adjustable parameters are the gas liquid ratio, namely the gas phase fl ow and the liquid phase flow. The effect of gas phase flow and liquid phase fl ow on the puri fi cation performance using the UDS-III solvent is shown in Figure 6. With the decrease of the gas phase fl ow or the increase of liquid phase fl ow, the puri fi cation performance becomes better. At a low gas velocity, a higher solvent circulation volume can improve the puri fi cation performance. But at a high gas velocity, an increasing solvent fl ow, which means reduction of the gas liquid ratio, does not have obvious effect on improvement of the purification performance. Therefore, an appropriate gas liquid ratio not only can improve the effect of puri fi cation, but also reduce the energy consumption.
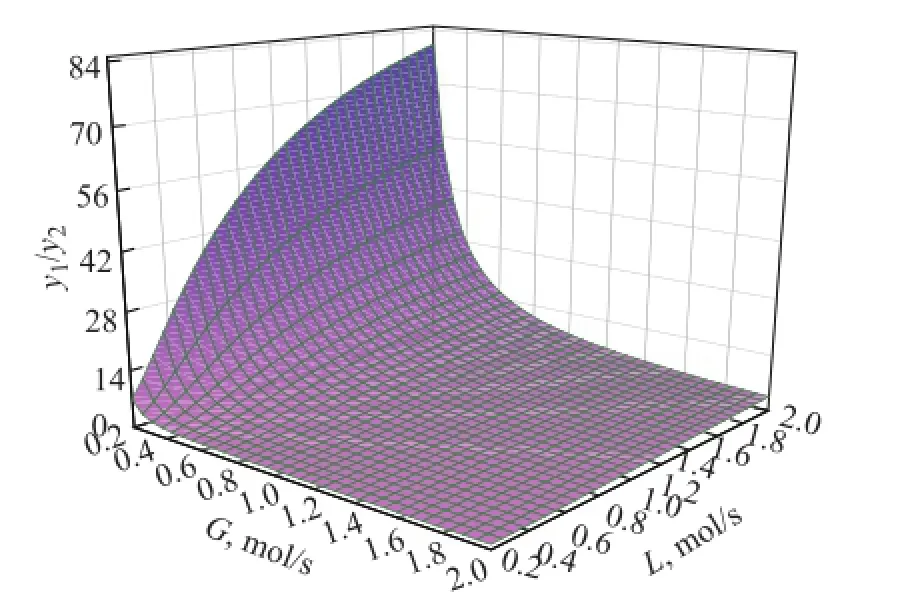
Figure 6 Model analysis on effect of operating conditions on puri fi cation performance
3.4 Regeneration performance of UDS solvents
The regeneration performance of the solvent in the re-cycling process is an important factor that can affect the absorption performance of the solvent, and the content of acid components in the lean solution will directly in fl uence the quality of the puri fi ed gas.
Industrial side-stream experiment examines the regeneration performance of the UDS and MDEA solvents. The results are listed in Table 4. Regenerative steam consumption is speci fi ed at 0.35 kg per kg of solution circulated. Under the same regeneration conditions, the contents of H2S and CO2in the UDS and MDEA lean solutions are maintained at under 0.04 mol/L, indicating to the good regeneration performance.
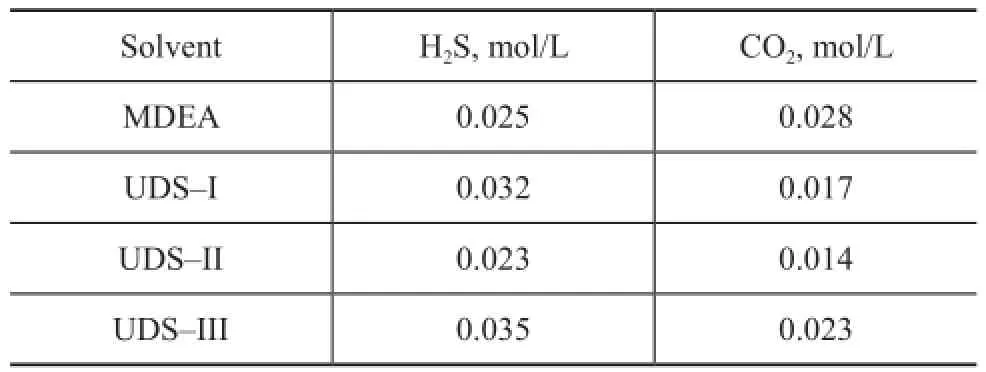
Table 4 Content of H2S and CO2in the UDS and MDEA lean solutions
4 Conclusions
The desulfurization performance of MDEA solvent and three kinds of UDS solvents was studied in an industrial side-stream unit. At an absorption temperature of 40 ℃, an absorption pressure of 8.0 MPa, aV/Lratio of about 230, the H2S content in puri fi ed gas was reduced to 34.7 mg/m3, 6.5 mg/m3, 4.2 mg/m3, and 0 by using MDEA, UDS-I, UDS-II and UDS-III, respectively. In comparison with MDEA solvent, three kinds of the UDS solvents not only showed good effect of H2S removal, but also achieved better COS removal performance. When theV/Lratio changed within the range from 140 to 230, the total sulfur content in the purified gas ranged from 150 mg/m3to 290 mg/m3for MDEA solvent, while the total sulfur content in the purified gas was below 80 mg/m3for the UDS solvents. The UDS solvents achieved by more than 30 percentage points higher in COS removal ef fi ciency as compared to that of MDEA. In addition, the total volume mass transfer coef fi cient of UDS solvent was found to be twice higher than that of MDEA. Furthermore, the UDS solvents exhibited satisfactory regeneration performance.
Acknowledgements: The authors are grateful for the fi nancial support from the National Key Science and Technology Project of China (2011ZX05017-005) and the Fundamental Research Funds for the Central Universities (No.22A201514010).
[1] Long S X, Zhu H, Zhu T, et al. Prospect of Sinopec's exploration for natural gas [J]. Natural Gas Industry, 2008, 28(1): 17-21 (in Chinese)
[2] Li L, Chen J F, Xu L H. Component and carbon isotope characteristics of natural gas in the Puguang gas fi eld, Sichuan [J]. Inner Mongolia Petrochem Indus, 2008(4): 106-108 (in Chinese)
[3] Ma Y S. Geochemical characteristics and origin of natural gases from Puguang gas fi eld in Eastern Sichuan Basin [J]. Natural Gas Geoscience, 2008, 19(1): 1-7 (in Chinese)
[4] Ghanbarabadi H, Khoshandam B. Simulation and comparison of Sulfinol solvent performance with amine solvents in removing sulfur compounds and acid gases from natural sour gas [J]. Journal of Natural Gas Science and Engineering, 2015, 22: 415-420
[5] Luo X W. Development and application of natural gas puri fi cation techniques [J]. Natural Gas and Oil, 2006, 24(2): 30-34 (in Chinese)
[6] Angaji M T, Ghanbarabadi H, Gohari F K Z. Optimizations of sulfolane concentration in propose sulfinol-M solvent instead of MDEA solvent in the refineries of Sarakhs [J]. Journal of Natural Gas Science and Engineering, 2013, 15: 22-26
[7] Zong L, Chen C C. Thermodynamic modeling of CO2and H2S solubility in aqueous DIPA solution, aqueous sulfolane-DIPA solution, and aqueous sulfolane-MDEA solution with electrolyte NRTL model [J]. Fluid Phase Equilibria, 2011, 306(2): 190-203
[8] Rivera-Tinoco R, Bouallou C. Reaction kinetics of carbonyl sulfide (COS) with diethanolamine in methanolic solutions[J]. Industrial & Engineering Chemistry Research, 2008, 47(19): 7375-7380
[9] Yu M, Zhou L. Review and forecast of puri fi cation of H2S in natural gas[J]. Tianjin Chemical Industry, 2002(5): 18-20 (in Chinese)
[10] Shen B X, Zhang J H, Chu Z, et al. High-ef fi ciency puri fication desulfurizer for high-acid oil and gas: China Patent, ZL200910233505.1[P], 2014-04-02
[11] Zhang J H, Shen B X, Liu J C, et al. Study on removing organosulfur from highly sour natural gas by medium pressure absorption using XDS solvent[J]. Petroleum Processing and Petrochemicals, 2009, 40(3): 65-68 (in Chinese)
[12] Zhang J H, Shen B X, Sun H, et al. A study on the desulfurization performance of solvent UDS for purifying highly sour natural gas [J]. Petroleum Science and Technology, 2011, 29(1): 48-58
[13] Zhang J H, Shen B X, Liu J C, et al. Absorption selectivity of solvents for organosulfurs in highly sour natural gas [J]. Energy Sources, Part A: Recovery, Utilization and Environmental Effects, 2014, 36(8): 822-829
[14] Sharma M M. Kinetics of reactions of carbonyl sulphide and carbon dioxide with amines and catalysis by Brönsted bases of the hydrolysis of COS [J]. Transactions of the Faraday Society, 1965, 61: 681-688
[15] Lee S C, Snodgrass M J, Park M K, et al. Kinetics of removal of carbonyl sul fi de by aqueous monoethanolamine [J]. Environmental Science & Technology, 2001, 35(11): 2352-2357
[16] Amararene F, Bouallou C. Kinetics of carbonyl sulfide (COS) absorption with aqueous solutions of diethanolamine and methyldiethanolamine [J]. Industrial & Engineering Chemistry Research, 2004, 43(19): 6136-6141
[17] Asit K S, Symalendu S B, Saju J P. Selective removal of H2S from gases containing H2S and CO2by absorption into aqueous solutions of 2-amino-2-methyl-1-propanol [J]. Industrial & Engineering Chemistry Research, 1993, 32(12): 3051-3055
[18] Hu T Y. Study on solvent CT8-20 on removing organosulfur from highly sour natural gas [J]. Gas Purification, 2005, 5(4): 38-44 (in Chinese)
[19] Littel R J, Versteeg G F, Van Swaaij W P M. Kinetic study of COS with tertiary alkanolamine solutions. 2. Modeling and experiments in a stirred cell reactor [J]. Industrial & Engineering Chemistry Research, 1992, 31(5): 1269-1274
[20] Littel R J, Versteeg G F, Swaaij W P M. Kinetics of COS with primary and secondary amines in aqueous solutions [J]. AIChE Journal, 1992, 38(2): 244-250
[21] Zhang J H, Shen B X, Liu J C, et al. An absorption model for solvent XDS on removing organosulfurs from highly sour natural gas [J]. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 2010, 32(17): 1625-1633
[22] Zhang Feng, Shen Benxian, Sun Hui, et al. Simultaneous removal of H2S and organosulfur compounds from liquefied petroleum gas using formulated solvents: Solubility parameter investigation and industrial test[J]. China Petroleum Processing and Petrochemical Technology, 2015, 17(1): 75-81
Received date: 2015-10-21; Accepted date: 2015-12-25.
Prof. Shen Benxian, Telephone: +86-21-64252851; E-mail: sbx@ecust.edu.cn.
杂志排行
中国炼油与石油化工的其它文章
- Enhanced Performance of Denitrifying Sul fi de Removal Process by 1,2-Naphthoquinone-4-Sulphonate
- Effects of Silylation on Zn-IM5 and Its Catalytic Activity for Butane Aromatization
- Preparation of Core-Shell Composite of Y@Mesoporous Alumina and Its Application in Heavy Oil Cracking
- Investigation of Different Coke Samples Adhering to Cyclone Walls of a Commercial RFCC Reactor
- Numerical Study of Air Nozzles on Mild Combustion for Application to Forward Flow Furnace
- Highly Active and Stable Ni2P/SiO2Catalyst for Hydrogenation of C9Petroleum Resin
