Molecular characterization and functional analysis of a piscidin gene in large yellow croaker (Larimichthys crocea)
2016-02-28JingYANGXinJiangLUFangChaoCHAIJiongCHEN
Jing YANG, Xin-Jiang LU, Fang-Chao CHAI, Jiong CHEN,*
1Laboratory of Biochemistry and Molecular Biology, School of Marine Sciences, Ningbo University, Ningbo 315211, China
2Collaborative Innovation Center for Zhejiang Marine High-Efficiency and Healthy Aquaculture, Ningbo University, Ningbo 315211, China
Molecular characterization and functional analysis of a piscidin gene in large yellow croaker (Larimichthys crocea)
Jing YANG1,#, Xin-Jiang LU1,#, Fang-Chao CHAI1,2, Jiong CHEN1,2,*
1Laboratory of Biochemistry and Molecular Biology, School of Marine Sciences, Ningbo University, Ningbo 315211, China
2Collaborative Innovation Center for Zhejiang Marine High-Efficiency and Healthy Aquaculture, Ningbo University, Ningbo 315211, China
The piscidin family, which includes potent antimicrobial peptides with broad-spectrum activity, plays an important role in the innate immune system of fish. In this study, we cloned piscidin-5-like type 3 (Lcpis5lt3) in large yellow croaker (Larimichthys crocea). Multiple alignments with other known piscidins revealed amino acid conservation throughout the fish, especially at the signal peptide (22 amino acids). The phylogenetic tree confirmed that Lcpis5lt3 and large yellow croaker piscidin-5-like proteins were grouped together to form a branch. Quantitative real-time PCR revealed that Lcpis5lt3 was expressed in a wide range of tissues, including the brain, muscle, gill, head kidney, intestine, kidney, liver, and spleen. The highest mRNA expression level of Lcpis5lt3 was found in the spleen. After Vibrio alginolyticus infection, mRNA expression was rapidly upregulated in the liver, head kidney, gill, kidney, and intestine at 4, 8, 12, and 24 h post infection (hpi), whereas there were no significant changes in the spleen. The antimicrobial spectrum showed that the synthetic mature peptide of Lcpis5lt3 exhibited different activity in vitro against various bacteria, such as Aeromonas hydrophila, V. anguillarum, V. alginolyticus, V. parahaemolyticus, Staphylococcus aureus, and Listeria monocytogenes. In addition, survival rates from the in vivo assay indicated that the synthetic peptide of Lcpis5lt3 increased the survival rate of large yellow croaker after V. alginolyticus challenge, resulting in a decline in bacterial burden and mRNA expression levels of interleukin-1β, interleukin-10, and tumor necrosis factor-α. These data suggest that Lcpis5lt3 plays an important role in innate immunity in large yellow croaker and might represent a potential therapeutic agent against pathogen invasion.
Antimicrobial activity; Large yellow croaker; Piscidin; Survival rate; Vibrio alginolyticus
INTRODUCTION1
Large yellow croaker (Larimichthys crocea) is an economically important marine species of cultured fish (Niu et al., 2013). However, the aquaculture of large yellow croaker has suffered significant economic losses due to various infectious diseases caused by marine microorganisms such as Vibrio alginolyticus (Chen et al., 2003; Liu et al., 2016). Vibrio alginolyticus is an important pathogen that can cause disease in marine cultured fish (Samad et al., 2014). Infection leads to the upregulation of inflammatory cytokines, such as interleukin-1β (IL-1β), interleukin-10 (IL-10), and tumor necrosis factor-α (TNF-α) (Kayansamruaj et al., 2014; Ringø, 2011; Seppola et al., 2008). Infection also increases bacterial burden, which triggers multiple inflammatory mechanisms. Therefore, bacterial burden is an important indicator for innate host immunity in response to infection (Gomes et al., 2013). Increased lethality is also observed in various teleosts infected with pathogens (Chen et al., 2014; Li et al., 2014a). Nowadays, a variety of effective vaccines and medicines have been developed to control marine pathogens; however, these drugs often negatively affect the marine environment and fish themselves (Cabello et al., 2013). Therefore, there is an increasing demand for effective and environmentally friendly commercial therapeutics against marine microorganisms. In this respect, considerable attentionhas been paid to antimicrobial polypeptides (AMPs), known as endogenous antibiotics (Mukherjee & Hooper, 2015).
AMPs, a family of peptides and proteins with low molecular weight, are present in virtually all life forms (from prokaryotes to eukaryotic plants and animals) (Zasloff, 2002). These peptides are critical components of the innate immune system in low vertebrate hosts (Corrales et al., 2010; Lauth et al., 2002). Many AMPs have been identified from fish, including cathelicidin, histone-derived peptides, defensin, and hepcidin (Katzenback, 2015). Piscidin family proteins possess antimicrobial activity and include pleurocidin, moronecidin, chrysophsin, and dicentracin (Masso-Silva & Diamond, 2014; Umasuthan et al., 2016). In teleosts, piscidin genes have been cloned and reported in some species, including Atlantic cod (Gadus morhua) (Fernandes et al., 2010; Ruangsri et al., 2012), rock bream (Oplegnathus fasciatus) (Umasuthan et al., 2016), tilapia (Oreochromis niloticus) (Lin et al., 2016; Peng et al., 2012), hybrid striped bass (Noga et al., 2009; Salger et al., 2011; Silphaduang & Noga, 2001), and mandarin fish (Siniperca chuatsi) (Sun et al., 2007). The piscidin gene shares a common prepropeptide structure consisting of a signal peptide, a mature peptide, and a C-terminal prodomain of varied sequence and length (Lauth et al., 2002; Sun et al., 2007). As the major class of AMPs, piscidin displays potent broad-spectrum activity against bacteria (Silphaduang & Noga, 2001), fungi (Sung et al., 2008), parasites (Colorni et al., 2008), and even viruses (Chinchar et al., 2004).
Recently, a piscidin-like antimicrobial peptide was isolated from large yellow croaker, and was determined to be a typical gill-expressed peptide distributed in various tissues (Niu et al., 2013). Furthermore, two types of piscidin-5-like sequences have been found in large yellow croaker, with their gene structure and sequence characteristics described (Zhou et al., 2014). The large yellow croaker piscidin-5-like gene and hybrid striped bass piscidin-5 gene are reported to be most abundant in the head kidney and intestine, respectively (Salger et al., 2011; Zhou et al., 2014). The synthetic piscidin-4 peptide of hybrid striped bass shows antimicrobial activity against Staphylococcus aureus, Streptococcus iniae, Escherichia coli, and V. anguillarum (Noga et al., 2009), and the synthetic piscidin-like peptide of large yellow croaker exhibits broad antimicrobial activity against S. aureus, E. coli, Aspergillus niger, and Cryptocaryon irritans in parasitic stages (Niu et al., 2013). These results show the high diversity of piscidin in mRNA expression and function in different fish. However, the effects of teleost piscidin on host defenses against pathogens in vivo are still unclear.
In this study, we characterized the cDNA sequence encoding a piscidin-like peptide, Lcpis5lt3, from large yellow croaker. Its mRNA expression in different tissues post V. alginolyticus infection was studied using quantitative PCR. In addition, the antimicrobial activity of synthetic peptides was also investigated in vitro and in vivo.
MATERIALS AND METHODS
Fish rearing
Healthy large yellow croaker, without pathological signs and weighing 35–40 g (fish age 7–9 months), were obtained from a commercial farm in Ningbo, China. Each 10 fish were kept in 100 L tanks at 25–27 °C in a recirculating system with filtered sea water. Detection was performed to ensure no bacteria were present in the seawater during the experiment. After acclimating for one week, the fish were used in the experiments described below. All experiments were approved by the Experimental Animal Management Law of China and the Animal Ethics Committee of Ningbo University.
Bacterial challenge
Overnight cultures of V. alginolyticus ATCC 17749 were diluted to 1: 100 in Tryptic Soy Broth Medium (TSB) (Sigma, Shanghai, China), grown at 28 °C with shaking, and harvested in the logarithmic phase of growth. The cells were washed, resuspended, and diluted to the appropriate concentration in sterile PBS. Sixteen fish were challenged by intraperitoneal injection with 5×106colony forming units (CFUs) of V. alginolyticus (in 100 μL PBS) per fish, and sixteen other fish were injected with 100 μL of PBS per fish as a negative control. The liver, spleen, head kidney, kidney, intestine, muscle, brain, and gill were collected from four fish at each time point at 4, 8, 12, and 24 h post-injection (hpi), as previously reported (Wu et al., 2015), then preserved at -70 °C until examination.
Sequence analysis
Gene sequences used for multiple alignment and phylogenetic analysis are listed in Table 1. The similarity between the obtained sequences with other known sequences was analyzed using BLAST search (http: //blast.ncbi.nlm.nih.gov/Blast.cgi). The cleavage site of signal peptides was predicted by the SignalP4.1 program (http: //www.cbs.dtu.dk/services/SignalP/). Protein analysis was performed using online software on the ExPASy Server (http: //www.expasy.org/tools/). Multiple sequence alignment was analyzed using the ClustalW program (http: //clustalw.ddbj.nig.ac.jp/), and phylogenetic and molecular evolutionary analyses were conducted using MEGA version 5.0 (Tamura et al., 2011).
Quantitative PCR (qPCR)
Changes in mRNA expression of Lcpis5lt3 following V. alginolyticus infection were analyzed by qPCR, as previously described (Lu et al., 2016; Wu et al., 2015). Total RNA was extracted from large yellow croaker tissues using RNAiso reagents (TaKaRa). Gene-specific primers were designed based on the cloned cDNA fragments of Lcpis5lt3, LcIL-1β, LcTNF-α, and LcIL-10 (Table 2). Lcpis5lt3 and two types of piscidin-5-like gene sequences were subjected to nucleotide sequence alignment, and the primers of Lcpis5lt3 were designed in the ORF region of a 153–262 bp portion with low sequence identity. BLAST searching indicated that these primer sequences did not share sequence homology with any known large yellow croaker gene sequence, per the large yellow croaker genome (Wu et al., 2014). As an internal PCR control, primers 18S rRNA F and 18S rRNA R were used to amplify a 200-bp fragment of the housekeeping large yellow croaker 18S rRNA (Lc18S rRNA) gene (Accession No. JN211788.1) (Table2). QPCR was conducted on an ABI StepOne Real-Time PCR System (Applied Biosystems, USA) using SYBR premix Ex Taq (Perfect Real Time) (TaKaRa) in accordance with the manufacturer’s instructions. The reaction mixture was incubated for 300 s at 95 °C, followed by 40 amplification cycles of 30 s at 95 °C, 30 s at 60 °C, and 30 s at 72 °C. After amplification, melt curves were obtained by slow heating from 60 °C to 95 °C at 0.1 °C/s, with continuous fluorescence collection, confirming that only our specific product peaks were detected. Fish were kept in eight aquaria. Two fish in each aquarium were biologically repeated twice, and the fish experiment was repeated once, for a total of four biological replicates. The mRNA expression of Lcpis5lt3 was normalized against that of 18S rRNA using the 2-ΔΔCTmethod.
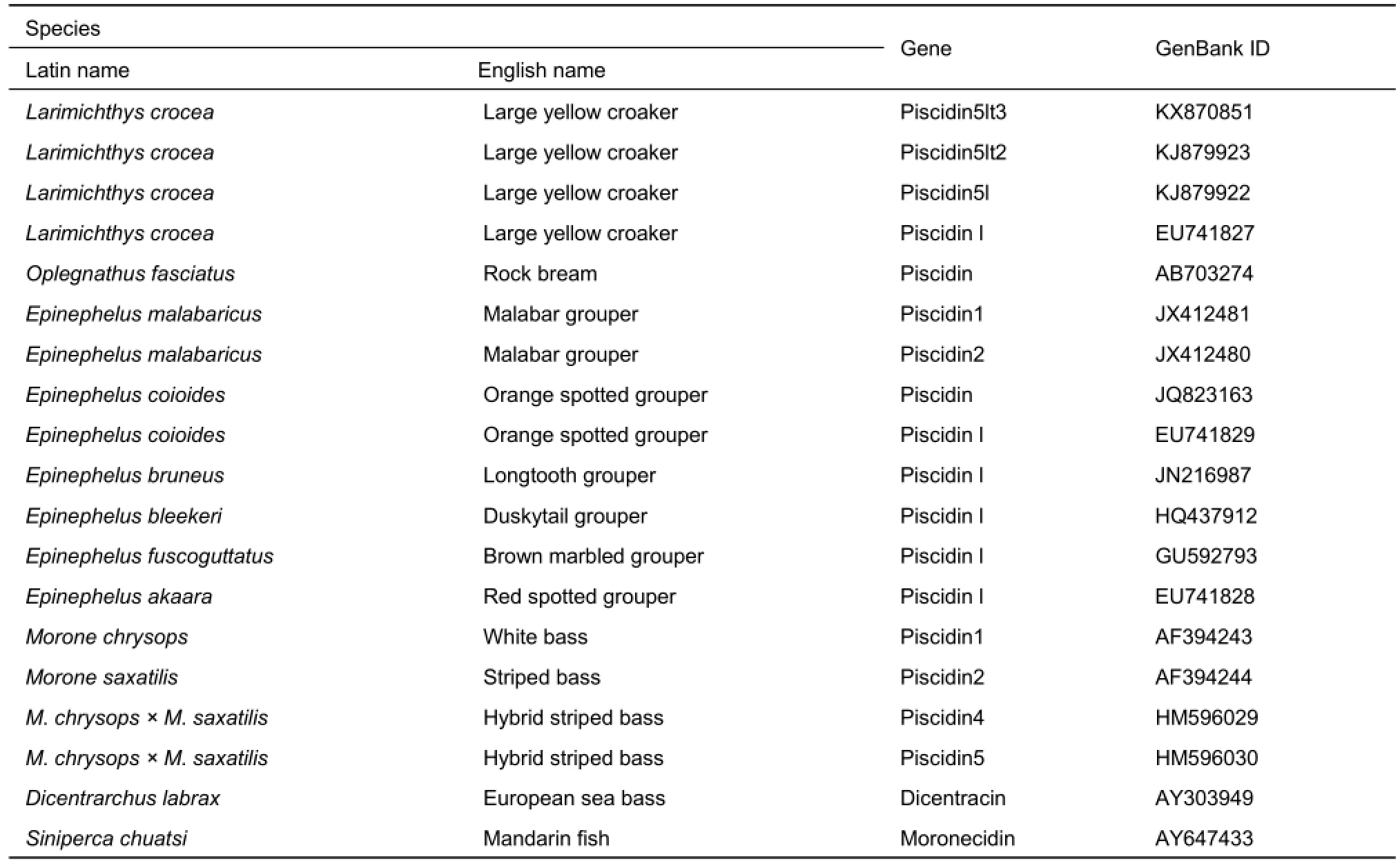
Table 1 Piscidin sequences used for multiple sequence alignment and phylogenetic tree analysis

Table 2 Oligonucleotide primers used in this work
Antimicrobial activity assays
The mature peptide of Lcpis5lt3 was chemically synthesized with over 95% purity (GL Biochem, Shanghai, China). The antimicrobial activity was determined against a panel of microorganisms. A micro-dilution assay was used to determine the minimal inhibitory concentration (MIC) of the various agents, as previously described with some modification (Li et al., 2014b). Inhibition was defined as growth lesser or equal to onehalf of the growth observed in control wells where no peptide
was added (Douglas et al., 2003). Briefly, serial dilutions of the peptides were made at 100, 50, 25, 12.5, 6.25, 3.125, and 1.563 μg/mL in 96-well microtiter plates. Each well contained 100 μL of a bacterial cell suspension at 1×105CFU/mL and 11 μL of test peptide. After incubating for 24 h at the appropriate temperature, microbial growth was examined. All tests were performed in triplicate and each individual experiment was replicated in quadruplicate. For each series of experiments, PBS was employed as a negative control.
Fish survival assay
Fish were divided into three groups (each containing 16 fish) for survival assay. Fish were injected intraperitoneally (ip) with 5×106CFU/g V. alginolyticus. After 30 min, fish received ip injections of 1.0 μg/g Lcpis5lt3 or 0.1 μg/g Lcpis5lt3 of fish weight, while the control group received PBS 30 min post injection. Fish were observed every 24 h for death or moribund state for 8 d.
Bacterial burden in tissues
Three groups, each containing six fish, were ip-injected with V. alginolyticus (5×106CFU/g). At 30 min post-infection, the fish received ip injections of different doses of Lcpis5lt3 or PBS, respectively. Fish were sacrificed 12 h after ip injection, and the liver, kidney, spleen, and blood were collected. The tissues from each large yellow croaker were weighed and homogenized in 1 mL of sterile PBS (pH 7.2). Homogenates and blood were serially diluted in sterile PBS (pH 7.2) and then plated onto separate Thiosulfate Citrate Bile Salts (TCBS) agar plates for 18 h at 28 °C. CFUs were then calculated in all plates and multiplied by the dilution factor. Tissue samples were normalized to tissue weight (0.1 g), and blood samples were normalized to blood volume (0.1 mL).
Statistical analysis
All data were described as mean±SEM. Statistical analysis of results was conducted by one-way analysis of variance (ANOVA) with SPSS version 13.0 (SPSS Inc, Chicago, USA). P-values of less than 0.05 were considered statistically significant.
RESULTS
Lcpis5lt3 gene analysis
Using the liver transcriptome analysis of large yellow croaker, the cDNA sequence of the Lcpis5lt3 gene was identified by BLAST search and submitted to the DDBJ/EMBL/GenBank databases under accession number KX870851. Computer analysis showed that the large yellow croaker pis5lt3, piscidin-5-like, and piscidin-5-like type 2 cDNA sequences contained open reading frames (ORFs) of 264 bp, 213 bp, and 210 bp that encoded an 88, 71, and 70 amino acid peptide, respectively. The peptide of Lcpis5lt3 had an estimated molecular weight (MW) of 9.78 kDa and theoretical isoelectric point (pI) of 8.93. All piscidin-5s were comprised of an N-terminal signal peptide (22 amino acids), a mature peptide (22 amino acids), and a C-terminal prodomain (Figure 1). The deduced cleavage site for the signal peptide wasbetween positions 22 and 23 (GEC-LG), like that of most piscidin sequences referenced, terminating at the motif GEC, GES, or GEG (Figure 1) (Douglas et al., 2003). However, the peptide length of Lcpis5lt3 was longer than that of piscidin-5-like type 2 and piscidin-5-like.

Figure 1 Multiple alignment of the predicted Lcpis5lt3 amino acid sequence with other known piscidins
Amino acid sequence alignment of Lcpis5lt3 with closely related sequences revealed conservation in the signal peptide region (Figure 1), but low similarity in the mature peptide and prodomain. In general, Lcpis5lt3 showed low identity (less than 62.5%) to other known piscidin sequences. Based on the known fish piscidin amino acid sequences, a phylogenetic tree was constructed using the neighbor-joining method (Figure 2). Results showed that Lcpis5lt3 and other large yellow croaker piscidin-5s grouped together to form a large yellow croaker piscidin-5 cluster.
Antimicrobial spectrum
The antibacterial activity of the synthesized mature peptide was determined against a panel of microorganisms using the MIC method. The MIC values obtained are reported in Table 3. The synthesized mature peptide of Lcpis5lt3 exhibited activity against Aeromonas hydrophila, V. anguillarum, and V. alginolyticus at 100 μg/mL. It also displayed antibacterial activity with MICs at 50 μg/mL and 6.25 μg/mL against V. parahaemolyticus and S. aureus, respectively. Lcpis5lt3 had antibacterial activity against Listeria monocytogenes with MIC at 3.125 μg/mL. However, this peptide had no effect on Edwardsiella tarda, V. vulnificus, V. harveyi, or S. iniae at the concentration tested.

Figure 2 Phylogenetic (neighbor-joining) analysis of the complete amino acid sequences of a piscidin protein using the MEGA5.0 program

Table 3 Antimicrobial activity of synthetic Lcpis5lt3
Constitutive and induced expression in different tissues
QPCR was performed to analyze the temporal expression profile of Lcpis5lt3 in different tissues of healthy large yellow croaker. The results showed that Lcpis5lt3 exhibited constitutive expression in all examined tissue, including brain, muscle, liver, intestine, gill, kidney, head kidney, and spleen. The highest expression level of Lcpis5lt3 was detected in the spleen, followed by the head kidney and kidney (Figure 3A). After V. alginolyticus infection, the mRNA expression of Lcpis5lt3 was rapidly upregulated in liver, head kidney, gill, kidney, and intestine at 4, 8, 12, and 24 hpi, whereas no significant changes were found in the spleen at 4, 8, 12, and 24 hpi (Figure 3B).
Effect of Lcpis5lt3 on the survival rate of V. alginolyticusinfected fish
We investigated the bactericidal effects of synthesized mature peptide in vivo by monitoring the survival of large yellow croaker infected with V. alginolyticus prior to treatment with different concentrations of Lcpis5lt3. All PBS-treated large yellow croaker infected with V. alginolyticus died within 7 d after infection. Large yellow croaker treatment with Lcpis5lt3 decreased the mortality rate (Figure 4). At 8 d after V. alginolyticus infection, the survival rates were 6% and 50% for large yellow croaker treated with 0.1 μg/g and 1.0 μg/g Lcpis5lt3, respectively (Figure 4).

Figure 3 QPCR analysis of Lcpis5lt3 mRNA expression in different tissues

Figure 4 Effect of Lcpis5lt3 on the survival rate of large yellow croaker
Bacterial burden in tissues and blood
To examine the impact of the synthesized mature peptide of Lcpis5lt3 on bacterial proliferation and dissemination in vivo, the bacterial loads were quantitated in the liver, spleen, kidney, and blood following ip-injection with 0.1 μg/g Lcpis5lt3 or 1.0 μg/g Lcpis5lt3 in V. alginolyticus-challenged fish. Fish treated with 0.1 μg/g Lcpis5lt3 and 1.0 μg/g Lcpis5lt3 all showed a reduction in V. alginolyticus load in the liver, spleen, kidney, and blood 12 hpi after V. alginolyticus challenge in comparison with the PBS-treated control group (Figure 5). There were significant differences between the control and 1.0 μg/g Lcpis5lt3 groups in all tested tissues, whereas the 0.1 μg/g Lcpis5lt3-treated group showed only small variation (Figure 5).
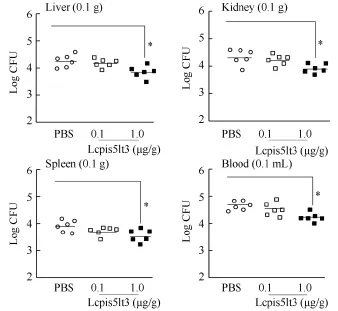
Figure 5 Effect of Lcpis5lt3 on bacterial burden in large yellow croaker liver, spleen, kidney, and blood
Effect of Lcpis5lt3 on cytokine expression following infection
To explore the effect of Lcpis5lt3 on inflammatory gene expression in vivo, the mRNA levels for inflammatory cytokines LcTNF-α, LcIL-1β, and LcIL-10 were evaluated in tissues collected from the fish after Lcpis5lt3 treatment and PBS-treated controls following infection with V. alginolyticus. QPCR analysis revealed a significant decrease in the expressions of LcTNF-α, LcIL-1β, and LcIL-10 transcripts in the tissues of fish treated with 0.1 μg/g or 1.0 μg/g Lcpis5lt3 compared with fish treated with PBS (Figure 6).
DISCUSSION

Figure 6 Effect of Lcpis5lt3 on mRNA levels of LcTNF-α, LcIL-1β, and LcIL-10
In the present study, Lcpis5lt3 was identified and characterized in large yellow croaker. Lcpis5lt3 comprised a signal peptide, mature peptide, and prodomain, as shared by other piscidin paralogues (Buonocore et al., 2012; Niu et al., 2013). Similar to other piscidins (Zhou et al., 2014), Lcpis5lt3 showed more conservation in the signal peptide and less conservation in the mature peptide and prodomain. The phylogenetic tree confirmed that Lcpis5lt3 was grouped together with large yellow croaker piscidin-5-like type 2 and piscidin-5-like to form a cluster. This result revealed that large yellow croaker pis5lt3 was a variation type of the piscidin-5-like peptide. According to previous research, several piscidin paralogues can be found in a single fish species. For example, piscidin-4 and piscidin-5 genes are found in hybrid striped bass (Salger et al., 2011); piscidin-1, piscidin-2, piscidin-3, piscidin-4, and piscidin-5 genes are found in tilapia (Peng et al., 2012); and piscidin-1, piscidin-2, and piscidin-2-β genes are found in Atlantic cod (Ruangsri et al., 2012). There are also several piscidin genes found in large yellow croaker, such as piscidin-like, piscidin-5-like, and piscidin-5-like type 2 (Zhou et al., 2014). These results show a high diversity of piscidin in different fish.
The transcripts of piscidin genes are widely distributed in various tissues (Buonocore et al., 2012; Salger et al., 2011). In rock bream, the piscidin gene is highly expressed in the gills of healthy fish (Bae et al., 2016). In tilapia, piscidin-2 is abundant in the skin, head kidney, and spleen; piscidin-3 is abundant in the skin, head kidney, and gill; and piscidin-4 is abundant in the intestine (Peng et al., 2012). In the large yellow croaker, the piscidin-like gene is most abundantly expressed in the gill of unchallenged fish (Niu et al., 2013), and the piscidin-5-like is most abundant in the head kidney (Zhou et al., 2014). In this study, the Lcpis5lt3 transcript was highly expressed in the spleen, head kidney, and kidney of the large yellow croaker, suggesting that piscidin genes have a variety of tissue expression patterns. In mandarin fish, piscidin gene mRNA expression is upregulated in the intestine, spleen, kidney, liver, skin, and gill after stimulation with LPS (Sun et al., 2007). In large yellow croaker, piscidin-like gene mRNA expression is significantly upregulated in the gill, skin, spleen, head kidney, liver, and intestine after C. irritans infection (Niu et al., 2013). In the current study, the mRNA expression levels of Lcpis5lt3 were upregulated in the liver, head kidney, gill, kidney, and intestine after V. alginolyticus infection. These results suggest that most fish piscidin in a variety of tissues were upregulated after infection. Furthermore, we found there were no significant changes in Lcpis5lt3 in the spleen after infection. However, this mechanism needs further investigation.
Unique for the field of fish research, AMPs have potential applications to prevent pathogenic microbes in aquaculture (Masso-Silva & Diamond, 2014). The constant risk of largescale microbial infection that can lead to significant economic losses requires new strategies to prevent or treat these pathogens (Masso-Silva & Diamond, 2014). In rock bream, the synthetic piscidin peptide exhibits antimicrobial activity against E. tarda, V. vulnificus, V. harveyi, and S. iniae (Bae et al., 2016). In tilapia, five synthetic piscidin peptides have been shown to exhibit antimicrobial activity against V. vulnificus, V. alginolyticus,A. hydrophila, and Pseudomonas aeruginosa (Peng et al., 2012). In contrast, our results showed that Lcpis5lt3 had no effect on E. tarda, V. vulnificus, V. harveyi, or S. iniae at the antimicrobial activity tested. However, Lcpis5lt3 exhibited antimicrobial activity against L. monocytogenes, S. aureus, V. anguillarum, V. alginolyticus, V. parahaemolyticus, and A. hydrophila in vitro. These results demonstrate that Lcpis5lt3 possesses broad-spectrum antimicrobial activity, and different piscidins in teleosts possess spectrum variety in antimicrobial activity.
To evaluate the antimicrobial activity of Lcpis5lt3 against V. alginolyticus in vivo, we performed the survival rate assay. Our results showed that the synthesized mature peptide of Lcpis5lt3 can increase the survival rate of fish infected with V. alginolyticus at different concentrations. The overall survival rate of the 1.0 μg/g Lcpis5lt3 group was higher than that of the 0.1 μg/g Lcpis5lt3 group after infection; this effect was accompanied by a lower bacterial burden and a decline in LcTNF-α, LcIL-1β, and LcIL-10 mRNA expression. The cytokines TNF-α and IL-1β in teleosts are powerful proinflammatory cytokines released by several immune cells during infection or tissue damage and are involved in a diverse range of inflammatory and infectious conditions (Roca et al., 2008; Seppola et al., 2008; Wu et al., 2015). However, IL-10 is a critical anti-inflammatory cytokine, whose expression is induced after proinflammatory mediators. It helps control immune responses and thereby minimize tissue damage (Secombes et al., 2011; Wang and Secombes, 2013; Zhu et al., 2013). As an autoregulatory mediator, IL-10 has important regulatory effects on immunological and inflammatory responses due to its capacity to inhibit the production of proinflammatory cytokines by monocytes (Ringø, 2011). In this study, we found that LcTNF-α, LcIL-1β, and LcIL-10 mRNA expression were all downregulated in the 0.1μg/g and 1.0 μg/g Lcpis5lt3 groups after infection, suggesting that inflammation in fish after V. alginolyticus infection was relieved. Similar results were found with tilapia piscidin-4 (TP4). For example, mice treated with TP4 resulted in the downregulation of TNF-α, IL-1β, and IL-10 (Narayana et al., 2015). However, it is still unclear whether piscidins can directly downregulate inflammatory cytokines, such as the AMPS of other fish (Chen et al., 2016; Li et al., 2015), or only kill pathogens to reduce the expression of inflammatory cytokines. Further investigation is needed to elucidate the role of Lcpis5lt3 in the immune responses of large yellow croaker after infection.
In conclusion, we characterized Lcpis5lt3 as a member of the piscidin family in large yellow croaker. Results showed that V. alginolyticus infection led to the alteration of Lcpis5lt3 mRNA expression in different tissues. Antimicrobial assays in vitro and in vivo also showed that the synthetic mature peptide had broad spectrum antimicrobial activity in vitro and increased fish survival upon bacterial infection in vivo. These data provide new insights into the innate immunity of large yellow croaker against pathogens and reveal the value of piscidin as a therapeutic agent to control microbial infections.
REFERENCES
Bae JS, Jung JM, An CM, Kim JW, Hwang SD, Kwon MG, Park MA, Kim MC, Park CI. 2016. Piscidin: antimicrobial peptide of rock bream, Oplegnathus fasciatus. Fish & Shellfish Immunology,51: 136-142.
Buonocore F, Randelli E, Casani D, Picchietti S, Belardinelli MC, de Pascale D, De Santi C, Scapigliati G. 2012. A piscidin-like antimicrobial peptide from the icefish Chionodraco hamatus (Perciformes: Channichthyidae): molecular characterization, localization and bactericidal activity. Fish & Shellfish Immunology,33(5): 1183-1191.
Cabello FC, Godfrey HP, Tomova A, Ivanova L, Dölz H, Millanao A, Buschmann AH. 2013. Antimicrobial use in aquaculture re-examined: its relevance to antimicrobial resistance and to animal and human health. Environmental Microbiology,15(7): 1917-1942.
Chen J, Chen Q, Lu XJ, Li CH. 2014. LECT2 improves the outcomes in ayu with Vibrio anguillarum infection via monocytes/macrophages. Fish & Shellfish Immunology,41(2): 586-592.
Chen J, Chen Q, Lu XJ, Chen J. 2016. The protection effect of LEAP-2 on the mudskipper (Boleophthalmus pectinirostris) against Edwardsiella tarda infection is associated with its immunomodulatory activity on monocytes/macrophages. Fish & Shellfish Immunology,59: 66-76.
Chen XH, Lin KB, Wang XW. 2003. Outbreaks of an iridovirus disease in maricultured large yellow croaker, Larimichthys crocea (Richardson), in China. Journal of Fish Diseases,26(10): 615-619.
Chinchar VG, Bryan L, Silphadaung U, Noga E, Wade D, Rollins-Smith L. 2004. Inactivation of viruses infecting ectothermic animals by amphibian and piscine antimicrobial peptides. Virology,323(2): 268-275.
Colorni A, Ullal A, Heinisch G, Noga EJ. 2008. Activity of the antimicrobial polypeptide piscidin 2 against fish ectoparasites. Journal of Fish Diseases,31(6): 423-432.
Corrales J, Mulero I, Mulero V, Noga EJ. 2010. Detection of antimicrobial peptides related to piscidin 4 in important aquacultured fish. Developmental & Comparative Immunology,34(3): 331-343.
Douglas SE, Patrzykat A, Pytyck J, Gallant JW. 2003. Identification, structure and differential expression of novel pleurocidins clustered on the genome of the winter flounder, Pseudopleuronectes americanus (Walbaum). European Journal of Biochemistry,270(18): 3720-3730.
Fernandes JMO, Ruangsri J, Kiron V. 2010. Atlantic cod piscidin and its diversification through positive selection. PLoS One,5(3): e9501.
Gomes RN, Teixeira-Cunha MGA, Figueiredo RT, Almeida PE, Alves SC, Bozza PT, Bozza FA, Bozza MT, Zimmerman GA, Castro-Faria-Neto HC. 2013. Bacterial clearance in septic mice is modulated by MCP-1/CCL2 and nitric oxide. Shock,39(1): 63-69.
KATZENBACK BA. 2015. Antimicrobial peptides as mediators of innate immunity in teleosts. Biology,4(4): 607-639.
Kayansamruaj P, Pirarat N, Hirono I, Rodkhum C. 2014. Increasing of temperature induces pathogenicity of Streptococcus agalactiae and the upregulation of inflammatory related genes in infected Nile tilapia (Oreochromis niloticus). Veterinary Microbiology,172(1-2): 265-271.
Lauth X, Shike H, Burns JC, Westerman ME, Ostland VE, Carlberg JM, Van Olst JC, Nizet V, Taylor SW, Shimizu C, Bulet P. 2002. Discovery and characterization of two isoforms of moronecidin, a novel antimicrobial peptide from hybrid striped bass. Journal of Biological Chemistry,277(7): 5030-5039.
Li CH, Lu XJ, Li DF, Chen J. 2014a. Passive protective effect of chicken egg yolk immunoglobulins against experimental Vibrio anguillarum infection in ayu (Plecoglossus altivelis). Fish & Shellfish Immunology,37(1): 108-114. Li CH, Lu XJ, Li MY, Chen J. 2015. Cathelicidin modulates the function of monocytes/macrophages via the P2X7 receptor in a teleost, Plecoglossus altivelis. Fish & Shellfish Immunology, 47(2): 878-885.
Li HX, Lu XJ, Li CH, Chen J. 2014b. Molecular characterization and functional analysis of two distinct liver-expressed antimicrobial peptide 2 (LEAP-2) genes in large yellow croaker (Larimichthys crocea). Fish & Shellfish Immunology,38(2): 330-339.
Lin WC, Chang HY, Chen JY. 2016. Electrotransfer of the tilapia piscidin 3 and tilapia piscidin 4 genes into skeletal muscle enhances the antibacterial and immunomodulatory functions of Oreochromis niloticus. Fish & Shellfish Immunology,50: 200-209.
Liu L, Ge MF, Zheng XY, Tao Z, Zhou SM, Wang GL. 2016. Investigation of Vibrio alginolyticus, V. harveyi, and V. parahaemolyticus in large yellow croaker, Pseudosciaena crocea (Richardson) reared in Xiangshan Bay, China. Aquaculture Reports,3: 220-224.
Lu XJ, Zhang H, Yang GJ, Li MY, Chen J. 2016. Comparative transcriptome analysis on the alteration of gene expression in ayu (Plecoglossus altivelis) larvae associated with salinity change. Zoological Research,37(3): 126-135. Masso-Silva JA, Diamond G. 2014. Antimicrobial peptides from fish. Pharmaceuticals,7(3): 265-310.
Mukherjee S, Hooper L V. 2015. Antimicrobial defense of the intestine. Immunity,42(1): 28-39.
Narayana JL, Huang HN, Wu CJ, Chen JY. 2015. Efficacy of the antimicrobial peptide TP4 against Helicobacter pylori infection: in vitro membrane perturbation via micellization and in vivo suppression of host immune responses in a mouse model. Oncotarget,6(15): 12936-12954.
Niu SF, Jin Y, Xu X, Qiao Y, Wu Y, Mao Y, Su YQ, Wang J. 2013. Characterization of a novel piscidin-like antimicrobial peptide from Pseudosciaena crocea and its immune response to Cryptocaryon irritans. Fish & Shellfish Immunology,35(2): 513-524.
Noga EJ, Silphaduang U, Park NG, Seo JK, Stephenson J, Kozlowicz S. 2009. Piscidin 4, a novel member of the piscidin family of antimicrobial peptides. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology,152(4): 299-305.
Peng KC, Lee SH, Hour AL, Pan CY, Lee LH, Chen JY. 2012. Five different piscidins from Nile tilapia, Oreochromis niloticus: analysis of their expressions and biological functions. PLoS One,7(11): e50263.
Ringø E. 2011. Evaluation of probiotic strain Bacillus subtilis C-3102 as a feed supplement for koi carp (Cyprinus carpio). Journal of Aquaculture Research & Development, S1: 005.
Roca FJ, Mulero I, López-Muñoz A, Sepulcre MP, Renshaw SA, Meseguer J, Mulero V. 2008. Evolution of the inflammatory response in vertebrates: fish TNF-α is a powerful activator of endothelial cells but hardly activates phagocytes. The Journal of Immunology,181(7): 5071-5081.
Ruangsri J, Salger SA, Caipang CMA, Kiron V, Fernandes JMO. 2012. Differential expression and biological activity of two piscidin paralogues and a novel splice variant in Atlantic cod (Gadus morhua L.). Fish & Shellfish Immunology,32(3): 396-406.
Salger SA, Reading BJ, Baltzegar DA, Sullivan CV, Noga EJ. 2011. Molecular characterization of two isoforms of piscidin 4 from the hybrid striped bass (Morone chrysops x Morone saxatilis). Fish & Shellfish Immunology,30(1): 420-424.
Samad APA, Santoso U, Lee MC, Nan FH. 2014. Effects of dietary katuk (Sauropus androgynus L. Merr.) on growth, non-specific immune and diseases resistance against Vibrio alginolyticus infection in grouper Epinephelus coioides. Fish & Shellfish Immunology,36(2): 582-589.
Secombes CJ, Wang T, Bird S. 2011. The interleukins of fish. Developmental & Comparative Immunology,35(12): 1336-1345.
Seppola M, Larsen AN, Steiro K, Robertsen B, Jensen I. 2008. Characterisation and expression analysis of the interleukin genes, IL-1β, IL-8 and IL-10, in Atlantic cod (Gadus morhua L.). Molecular Immunology,45(4): 887-897.
Silphaduang U, Noga EJ. 2001. Peptide antibiotics in mast cells of fish. Nature,414(6861): 268-269.
Sun BJ, Xie HX, Song Y, Nie P. 2007. Gene structure of an antimicrobial peptide from mandarin fish, Siniperca chuatsi (Basilewsky), suggests that moronecidins and pleurocidins belong in one family: the piscidins. Journal of Fish Diseases,30(6): 335-343.
Sung WS, Lee J, Lee DG. 2008. Fungicidal effect and the mode of action of piscidin 2 derived from hybrid striped bass. Biochemical and Biophysical Research Communications,371(3): 551-555.
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution,28(10): 2731-2739.
Umasuthan N, Mothishri MS, Thulasitha WS, Nam BH, Lee J. 2016. Molecular, genomic, and expressional delineation of a piscidin from rock bream (Oplegnathus fasciatus) with evidence for the potent antimicrobial activities of Of-Pis1 peptide. Fish & Shellfish Immunology,48: 154-168.
Wang TH, Secombes CJ. 2013. The cytokine networks of adaptive immunity in fish. Fish & Shellfish Immunology,35(6): 1703-1718.
Wu CW, Zhang D, Kan MY, Lv ZM, Zhu AY, Su YQ, Zhou DZ, Zhang JS, Zhang Z, Xu MY, Jiang LH, Guo BY, Wang T, Chi CF, Mao Y, Zhou JJ, Yu XX, Wang HL, Weng XL, Jin JG, Ye JY, He L, Liu Y. 2014. The draft genome of the large yellow croaker reveals well-developed innate immunity. Nature Communications,5: 5227.
Wu J, Shi YH, Zhang XH, Li CH, Li MY, Chen J. 2015. Molecular characterization of an IL-1β gene from the large yellow croaker (Larimichthys crocea) and its effect on fish defense against Vibrio alginolyticus infection. Zoological Research,36(3): 133-141.
Zasloff M. 2002. Antimicrobial peptides of multicellular organisms. Nature,415(6870): 389-395.
Zhou QJ, Su YQ, Niu SF, Liu M, Qiao Y, Wang J. 2014. Discovery and molecular cloning of piscidin-5-like gene from the large yellow croaker (Larimichthys crocea). Fish & Shellfish Immunology,41(2): 417-420.
Zhu LY, Nie L, Zhu G, Xiang LX, Shao JZ. 2013. Advances in research of fish immune-relevant genes: a comparative overview of innate and adaptive immunity in teleosts. Developmental & Comparative Immunology,39(1-2): 39-62.
Received: 08 October 2016; Accepted: 09 November 2016
Foundation items: This project was supported by the National 863 Project (2012AA10A403), the Scientific Research Foundation of Graduate School of Ningbo University (G16089), and KC Wong Magna Fund in Ningbo University
*Corresponding author, E-mail: jchen1975@163.com
#Authors contributed equally to this work
10.13918/j.issn.2095-8137.2016.6.347
杂志排行
Zoological Research的其它文章
- The geographical distribution of grey wolves (Canis lupus) in China: a systematic review
- A new species of genus Fejervarya (Anura: Dicroglossidae) from northern Thailand
- Patterns of change in the population and spatial distribution of oriental white storks (Ciconia boyciana) wintering in Poyang Lake
- Re-evaluating data quality of dog mitochondrial, Y chromosomal, and autosomal SNPs genotyped by SNP array
- Meeting report: the 4thsymposium on animal models of non-human primates in Kunming, Yunnan, China
- A clarification of authorship in an old publication
