ReLEx®飞秒激光微小切口基质透镜取出术治疗近视或近视散光术后屈光情况研究
2016-02-27SriGaneshRishikaGupta
Sri Ganesh, Rishika Gupta
(作者单位:1印度,班加罗尔 560082,Nethradhama超级专业眼科医院人事管理部;2印度,班加罗尔 560082,Nethradhama超级专业眼科医院眼科)
ReLEx®飞秒激光微小切口基质透镜取出术治疗近视或近视散光术后屈光情况研究
Sri Ganesh1, Rishika Gupta2
(作者单位:1印度,班加罗尔 560082,Nethradhama超级专业眼科医院人事管理部;2印度,班加罗尔 560082,Nethradhama超级专业眼科医院眼科)
摘要
目的:研究分析采用ReLEx®全飞秒激光微小切口基质透镜取出术(SMILE)治疗近视或近视散光术后视力对比敏感度、像差及干眼发生情况。
Citation:Ganesh S, Gupta R. Visual and refractive outcomes with ReLEx®SMILE in 600 eyes.GuojiYankeZazhi(IntEyeSci) 2016;16(2):218-223
INTRODUCTION
In the present scenario,photo-refractive keratectomy (PRK) and laser-assistedinsitukeratomileusis (LASIK) are the most widely practiced procedures for myopic correction.
PRK offers the advantage of retaining corneal biomechanical strength but entails a surface insult to the eye, causing patient discomfort postoperatively and haze and regression in the long run[1]. LASIK allows rapid visual rehabilitation and wound healing, but biomechanically it results in an unstable system.
Until recently, femtosecond lasers have primarily been used as an alternative to microkeratomes to cut thinner and planar corneal flaps in LASIK patients, thus enhancing the safety of correction in thinner corneas[2-3]. Even with its high success rates, side effects such as increased postoperative dryness, reduced biomechanical corneal strength, induction of higher-order aberrations (HOA), postoperative glare, haloes, and flap-related complications remain[4-6].
More recently developed is therefractive lenticule extraction, which uses femtosecond laser to correct myopia by creating an intrastromal corneal lenticule, the removal of which, alters the shape of the cornea, thereby correcting myopia. It encompasses two different techniques: femtosecond lenticule extraction (FLEX) and small incision lenticule extraction (SMILE).
FLEX, first reported by Sekundoetal[7]in 2008, essentially mimics a LASIK-type procedure in that it involves the creation of a flap. The procedure begins with creation of a deeper plane of lenticule, followed by the superficial cut, which is extended beyond the limit of the deeper cut to form the anterior corneal flap. The hinged flap is then lifted above the lenticule in a similar fashion to the LASIK flap, the refractive lenticule is peeled away, and the flap is repositioned.
SMILE is a more recent, flapless subtype, in which the lenticule is extracted through a small arcuate incision (2-4 mm). This allows for greater stability as most of the Bowman’s layer remains intact. In this paper, we present our clinical experience with ReLEx®SMILE performed in 600 eyes.
SUBJECTS AND METHODS
This prospective, non-randomized study included patients presenting to our hospital for correction of myopia between Dec. 2013 and May 2014. The study was approved by the local ethics committee and performed in accordance with the tenets of the Declaration of Helsinki. Informed consent was obtained from all patients.
Inclusion criteria were:spherical equivalent up to -10 D, age of 21y or older, stable refraction for at least 1y, soft contact lens discontinued for 1wk and rigid gas permeable lens discontinued for 3wk prior to the procedure, minimum corneal thickness of 480 μm, residual stromal bed of at least 250 μm, ability to understand and willingness to participate in all follow-up visits.
Exclusion criteria included: evidence of residual or active ocular diseases such as herpetic keratitis, uveitis, glaucoma,visually significant cataract, retinal diseases such as retinal dystrophies or diabetic retinopathy, corneal dystrophies, keratoconus, history of corneal trauma or surgery, severe dry eyes (Schirmer 2 test value less than 10 mm), use of systemic medications likely to affect wound healing (e.g. corticosteroids or antimetabolites), an immuno-compromised state, or females who were pregnant or nursing.
Table 1Patient Demographics
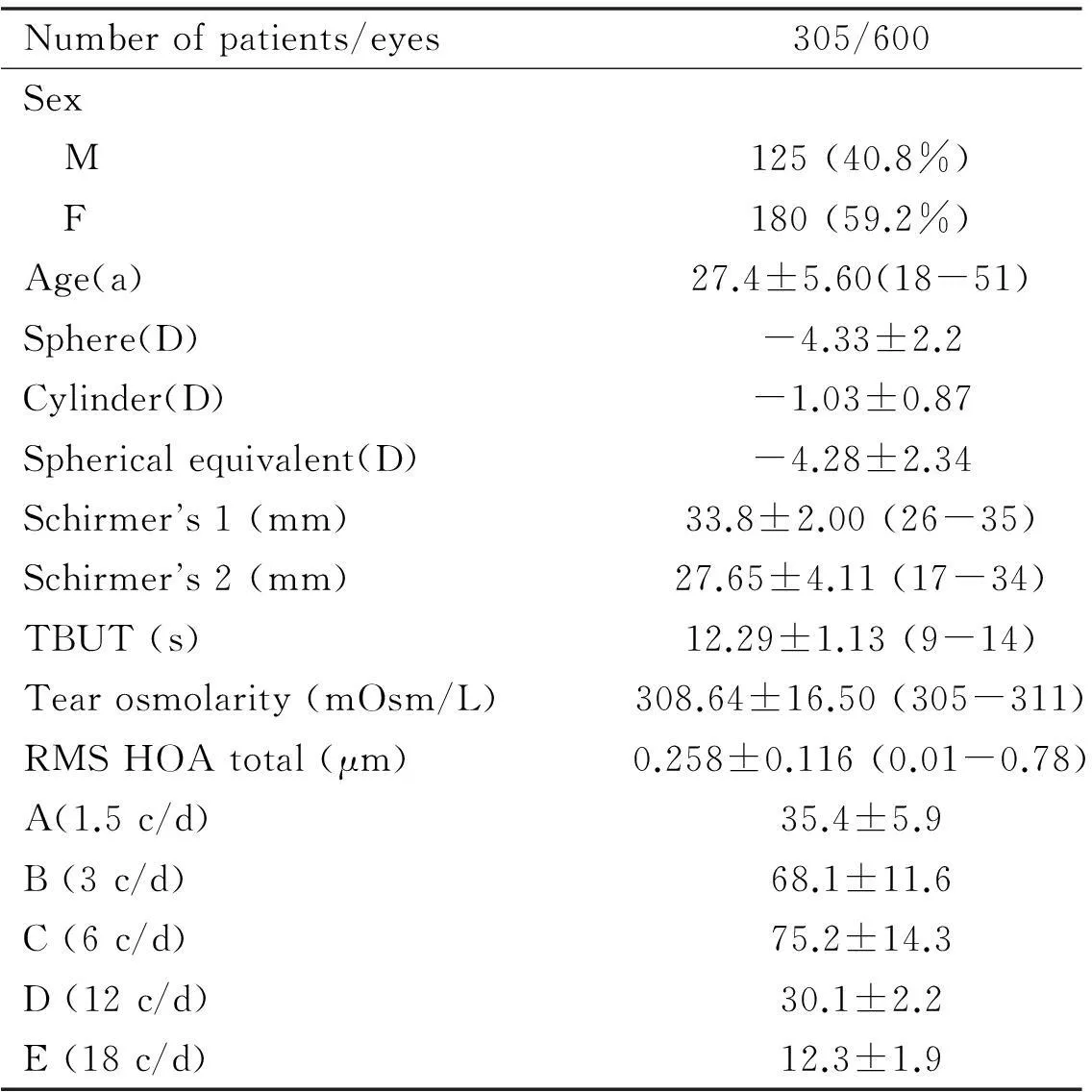
Numberofpatients/eyes305/600Sex M125(40.8%) F180(59.2%)Age(a)27.4±5.60(18-51)Sphere(D)-4.33±2.2Cylinder(D)-1.03±0.87Sphericalequivalent(D)-4.28±2.34Schirmers1(mm)33.8±2.00(26-35)Schirmers2(mm)27.65±4.11(17-34)TBUT(s)12.29±1.13(9-14)Tearosmolarity(mOsm/L)308.64±16.50(305-311)RMSHOAtotal(μm)0.258±0.116(0.01-0.78)A(1.5c/d)35.4±5.9B(3c/d)68.1±11.6C(6c/d)75.2±14.3D(12c/d)30.1±2.2E(18c/d)12.3±1.9
A thorough preoperative examination was conducted on each eye including Snellen corrected distance visual acuity (CDVA), manifest refraction (sphere, cylinder and spherical equivalent)(Table 1), corneal topography (Orbscan and Pentacam), functional acuity contrast test (F.A.C.T), Schirmer’s 1 and 2, tear breakup time (TBUT), tear osmolarity (TearLab) and aberrometry (iTrace).
Vision Sciences Research Corporation’s (VSRC) patented F.A.C.T Chart is a sine-wave grating chart that tests 5 spatial frequencies (sizes) and 9 levels of contrast. Tested monocularly, the patient determines the last grating seen for each row (A, B, C, D, and E). The test is performed at normal room illumination (30-70 foot lamberts/68-240 cd/m2) at a distance of 10 feet. Absolute values of contrast sensitivity were obtained for each patient, and mean and standard deviation values were then calculated.
Wavefront measurements were taken using the Hoya iTrace-combination ray- tracing wavefront aberrometer (Tracey Technologies Corporation, Houston, Texas, USA) with a 5.0 mm analysis diameter and included the root mean square (RMS) HOA: Total, coma, and spherical aberrations. The iTrace sequentially projects 256 near-infrared laser beams into the eye to measure forward aberrations. Data is displayed in the form of wavefront maps showing RMS value for each aberration.
Patients were given a subjective questionnaire on 1d to assess for any pain, pricking sensation, watering and redness and at 15d to assess glare and overall patient satisfaction. The level of pain was assessed using the Wong-Baker faces pain rating scale. Patients either indicated “yes” or “no” for the presence or absence of pricking sensation, watering, and redness. Patient satisfaction was measured on a scale of 1 (excellent) to 4 (poor), and glare was graded on a scale of 0 (no difficulty) to 4 (severe difficulty).
ReLEx®SMILE ProcedureReLEx®SMILE was performed using VisuMax femtosecond laser (Carl Zeiss Meditec, Jena, Germany). The following parameters were used: 100 μm cap thickness, 7.5 mm cap diameters, 6.0-6.5 mm optical zone, 500 kHz repetition rate, 35-37 (130 nJ) energy cut index, a 2 mm side cut incision, and 4.5 μm spot and track distance.
Patient was positioned under the curved contact glass of the femtosecond laser and asked to fixate on a blinking target. When appropriate centration was achieved, suction was applied and laser started. Following creation of the lenticule, incision was opened, and the two planes of the lenticule were identified. A thin blunt spatula was used to dissect the superficial and deep planes of the lenticule and to break the remaining tissue bridges, thus separating the lenticule from the surrounding stroma. This lenticule was grasped with a pair of forceps and extracted through the 2 mm incision.
The corneal interface was then flushed with balanced salt solution. Postoperative regimen included prednisolone acetate, ofloxacin and lubricating eyedrops.
Follow-upFollow-up visits were on 1, 15d and 3mo. UCVA, CDVA, and refraction was tested on 1d, corneal topography, wavefront measurement (iTrace, Hoya), Schirmer 1, 2, TBUT, and TearLab and F.A.C.T for contrast sensitivity was tested at 15d and at 3mo.
Data Collection and Statistical AnalysisData analysis was done with the help of a computer using SPSS for Windows Software (version 17.0, SPSS, Inc., New York, USA). Using this software, range, frequencies, percentages, means, standard deviations, Chi square andPvalues were calculated. A pairedt-test was used to test the significance of difference between quantitative variables and Yate’s Chi-square test for qualitative variables.Pvalue less than 0.05 denoted a significant relationship.
RESULTS
Six hundred eyes of 305 patients underwent SMILE for correction of myopia and/or myopic astigmatism. One hundred and twenty five (40.8%) patients were male and 180 (59.2%) were female (Table 1). Patients were between 21 and 51y of age (mean age: 27.4 ±5.6y).
RefractionPreoperative mean spherical equivalent was -4.28±2.34 D (range: -0.50 D to -10.0 D; Table 1). Postoperative mean spherical equivalent was -0.14 ± 0.28 D (range: -0.12 D to -0.75 D).
Refractive EfficacyPostoperatively, at 1d, 47 eyes (7.83%) achieved a UCVA of 20/25 or better, 536 eyes (89.33%) achieved 20/20 and, 17 eyes (2.83%) achieved 20/15.At 3mo postoperatively, 7 eyes (1.17%) achieved a UCVA of 20/25, 556 eyes (92.67%) had achieved a UCVA of 20/20, and 37 eyes (6.17%) had achieved 20/15 (Figure 1).
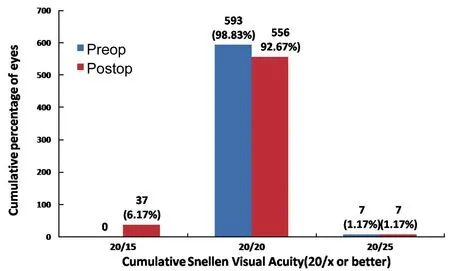
Figure 1Cumulative percentages of eyes in which target refraction was zero attaining specified levels of uncorrected distance visual acuity (UDVA) 3mo after small-incision lenticule extraction (SMILE).
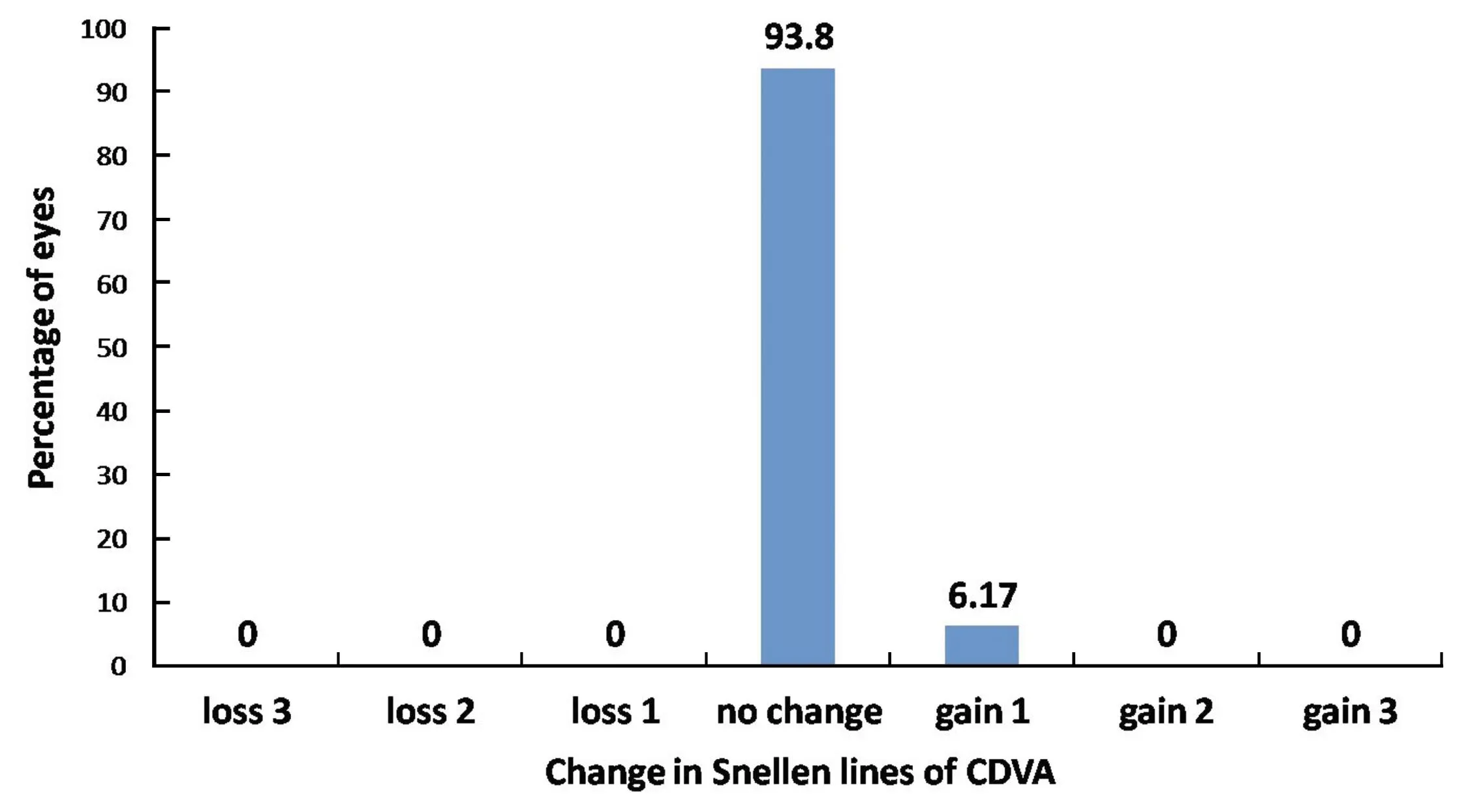
Figure 2The percentage of eyes (y-axis) in which there was a gain/loss of specified number of Snellen visual acuity lines 3mo after SMILE.
SafetyOn 1d postoperatively, 40 eyes (6.67%) showed loss of 1 line in CDVA, 543 eyes (90.5%) showed no difference between preoperative CDVA and postoperative CDVA, and 17 eyes (2.83%) showed a 1-line gain in CDVA. By 15d, 10 eyes (1.67%) showed loss of 1 line in CDVA, 561 eyes (93.5%) had no difference, and 29 eyes (4.83%) showed a gain of 1 line. By 3mo, 37 eyes (6.17%) showed a gain of 1 line, while 563 eyes (93.8%) showed no change. No eye had a loss of CDVA at 3mo postoperatively (Figure 2).
PredictabilityA scatter plot of the attempted correction versus the actual correction achieved(manifest spherical equivalent) at 3mo after SMILE is shown in Figure 3. All patients were within ±0.75 D of the attempted correction.
At 3mo after surgery, 93.17% (559) of eyes were within -0.14 D to -0.50 D, 6.17% (37 eyes) were within -0.13 to +0.13D, and 0.67% (4 eyes) were within -0.50 to-1.00D of the attempted correction (Figure 4).
Contrast SensitivityContrast sensitivity reduced postoperatively at all spatial frequencies (P<0.001). An improvement was observed in the absolute values of contrast sensitivity from 1d to 15d and from 15d to 3mo postoperatively, particularly at lower spatial frequencies (P=0.43, 0.47, 0.46) (Figure 5).
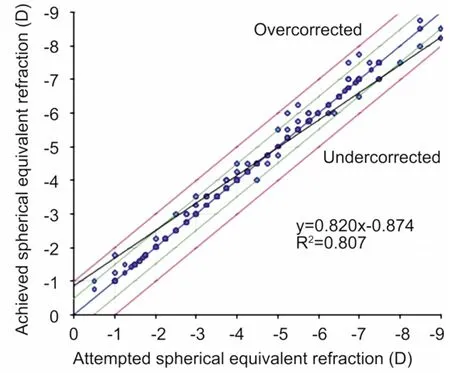
Figure 3Predictability: the scatter plot of the attempted versus the actual manifest spherical equivalent correction 3mo after SMILE (600 eyes).
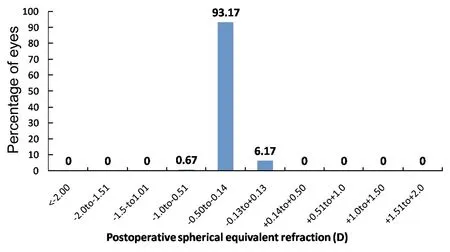
Figure 4Percentage of eyes within different diopter ranges of the attempted correction (spherical equivalent) 3mo after SMILE.
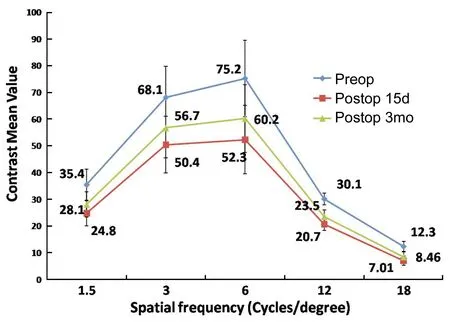
Figure 5The preoperative and 3mo postoperative FACT contrast sensitivity following SMILE.
Corneal WavefrontHOA increased from a preoperative mean RMS (Table 1) of 0.258± 0.116μm (range: 0.011 to 0.78 μm) to 0.36±0.109 μm at 15d and 0.31±0.115 μm 3mo postoperatively (P<0.001; Figure 6). A 0.03 μm and 0.08 μm increase in mean coma and spherical aberrations was observed.
Dry EyeA significant reduction in Schirmer’s 1 and 2 and TBUT were seen from a preoperative mean (Table 1) of 33.8±2 mm, 27.65±4.1 mm, and 12.29±1.13s to a postoperative mean of 32.67±2.28 mm, 24.59±2.65 mm, and 10.4±1.24s respectively at 3mo (P<0.001). Similarly, TearLab significantly increased from 308.64±16.5 mOsm/L to 309.37± 17.2 mOsm/L (P<0.001; Figure 7).
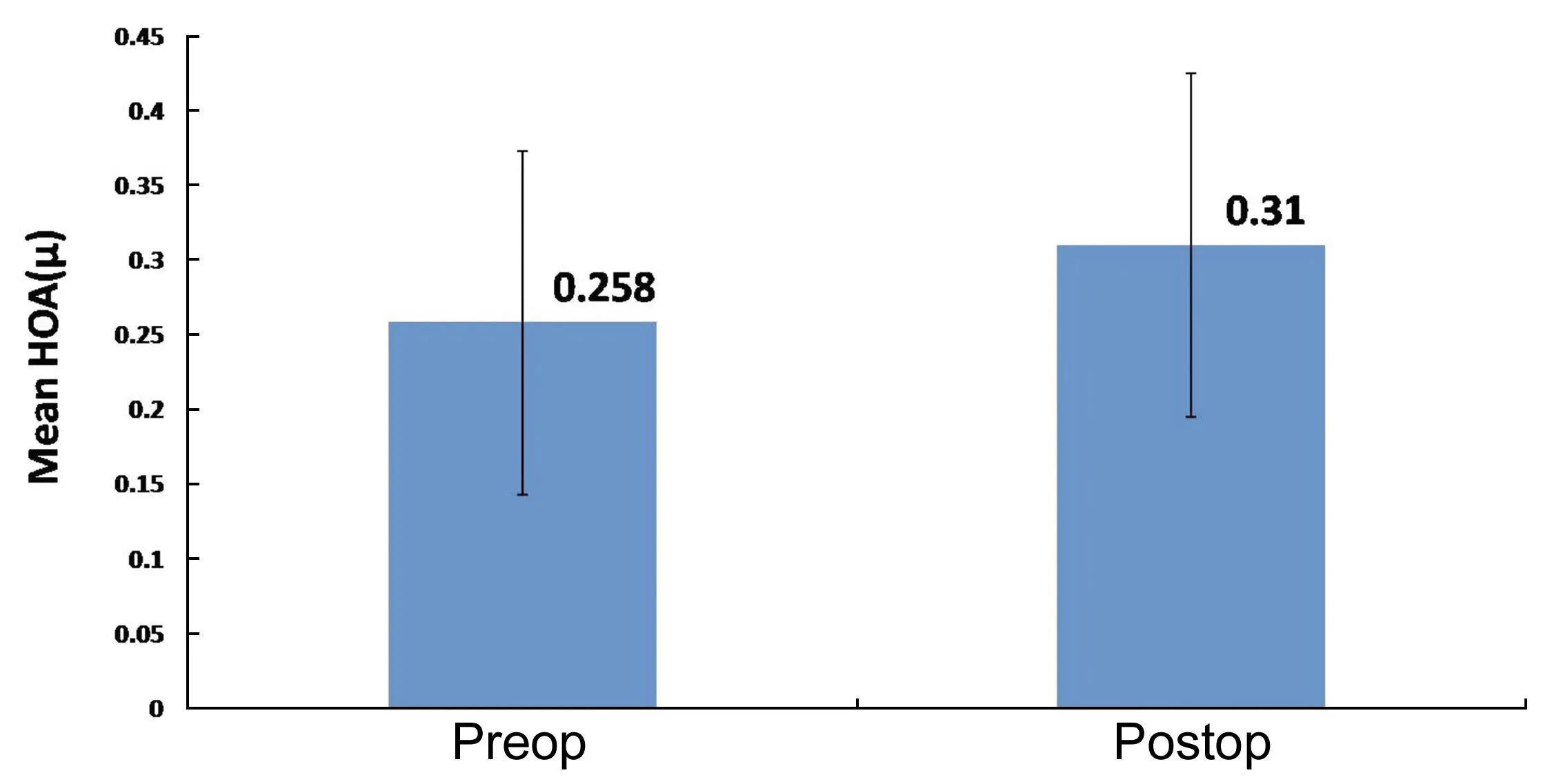
Figure 6Preoperative and postoperative higher order aberrations following SMILE.
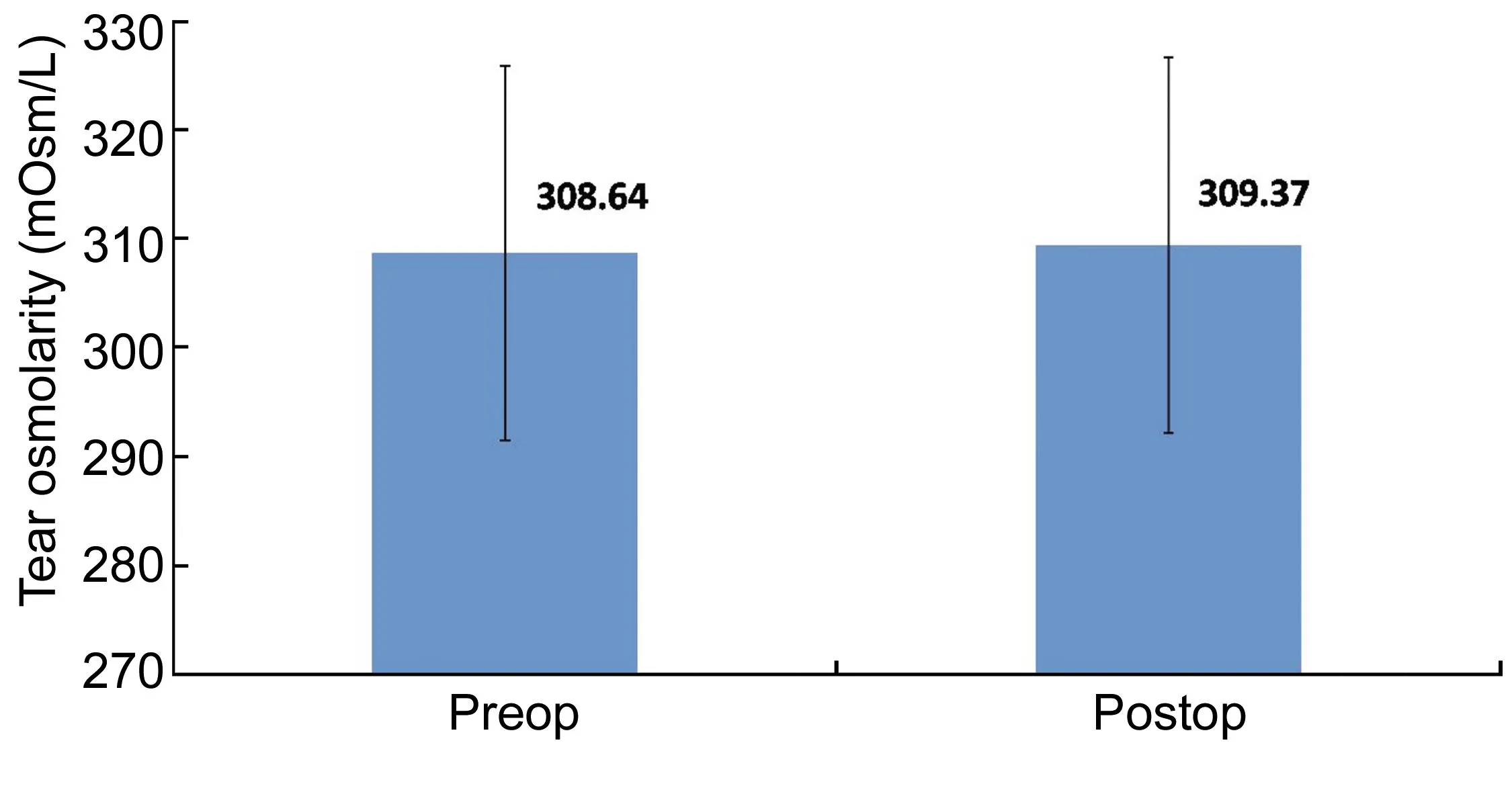
Figure 7 The preoperative and postoperative tear osmolarity (mOsm/L) following SMILE.
Patient QuestionnaireThe mean score on the subjective questionnaire on pain, redness, watering, pricking sensation was 0.21±0.36. Patient satisfaction score was 3.57±0.41. Score for glare complaints was 1.89±0.73.
ComplicationsDuring the procedure, 5 eyes (0.833%) experienced suction loss. Four suction breaks were corrected by redocking and reapplying the laser. The fifth eye experienced suction loss before the deeper plane of the lenticule was cut and hence could not be re-docked. A flap had to be created in this eye using the VisuMax femtosecond laser, and the eye was treated with excimer laser ablation for correction of refractive error. This eye was excluded from the study as the eye underwent a flap procedure similar to the one seen in LASIK rather than SMILE.
DISCUSSION
To date, LASIK has been considered the “gold standard” for laser refractive correction of myopia, with flaps fashioned in more than 50% of cases with the femtosecond laser, according to a 2009 industry survey[8]. Various studies have been published about limitations of LASIK. It is estimated that the incidence of corneal ectasia following LASIK surgery ranges between 0.2-0.6%[9-10]. Other drawbacks such as increased postoperative dryness, reduced biomechanical corneal strength, induction of HOA causing postoperative glare, haloes, and flap-related complications remain[4-6].
So far, relatively little is known about the refractive outcomes, contrast sensitivity, aberrations, and dry eyes in a large sample size of SMILE patients.
Vestergaardetal[11]found that 95% patients attained a UCVA of 20/40 or better 3mo following ReLEx®SMILE, while 2 eyes showed a gain of 2 lines of CDVA at 3mo. Hjortdaletal[12]found that 97.2% patients achieved a UCVA of 20/40 or better at 3mo following SMILE.In our study, 98.83% of the eyes (593 eyes) achieved a UCVA of 20/20 or better. Results of SMILE are better than those found in literature for PRK. Two long-term studies of PRK have shown that between 50.7% and 67% of eyes achieve a UCVA of 20/20 or better[13-14]. In a comparative study, Huetal[15]found 48 eyes showing a gain of 1 line in SMILE group, and 37 eyes in LASIK group. No patient in SMILE group showed loss of CDVA while 1 eye in LASIK group had loss of CDVA by 1 line. In our study, no eye had loss of CDVA. By 3mo, 37 eyes (6.17%) showed a gain of 1 line, while 563 eyes (93.8%) showed no change in CDVA.
We believe this is because refractive accuracy depends on the accurate removal of stromal tissue intraoperatively. A variation in hydration of the corneal stroma is the most likely cause for under- or overablation of stromal tissue[16-18]. In LASIK, the flap needs to be lifted before excimer laser ablation can be performed. This exposes the stroma to hydration changes before refractive correction. On the other hand, in SMILE, refractive lenticule is cut by femtosecond laser prior to any disturbance of the stroma, thus enhancing the procedure’s predictability.
Increase in corneal HOA postoperatively was statistically significant (P<0.01) which can be attributed to a larger sample size (n=600) and lower standard deviation. We found a 0.03 μm and 0.08 μm increase in mean coma and spherical aberrations. Similar results were reported by Shahetal[19]where in increase in mean coma and spherical aberrations was 0.07 μm and 0.11 μm, respectively.
A comparison of our results with those available in the literature for LASIK shows fewer aberrations induced postoperatively following SMILE compared with LASIK.
Studies done on LASIK byde Orteutaetal[20]showed an increase of 0.10 μm in coma and 0.17 μm in spherical aberrations. Kohnenetal[21]found an increase of 0.13 μm in spherical aberrations.
Decentration of ablation intraoperatively can lead to induction of coma postoperatively. However, low suction pressure during SMILE enables the patient to see the blinking target, thereby allowing a good and steady centration. This could be the reason for the low RMS coma induced postoperatively.
With regard to contrast sensitivity, Sekundoetal[22]in a study on SMILE, found no significant decrease in mesopic contrast postoperatively, while in another comparative study between ReLEx®and FS-LASIK, Gertnereetal[23]found better mesopic contrast sensitivity in ReLEx®group than LASIK group. Montés-Micóetal[24]found a statistically significant reduction (P<0.01) in contrast sensitivity at high spatial frequencies (12 and 18 c/d) under mesopic conditions following LASIK. In the present study, a significant reduction in contrast sensitivity was observed at postoperative 1d (P<0.001), but that improved by 15d and by 3mo postoperatively and was clinically insignificant for lower spatial frequencies (1.5, 3, and 6 c/d) (P=0.43, 0.47, 0.46).
Postoperative dry eye following ReLEx®SMILE was seen with less frequency than that seen following PRK or LASIK[25-26]. This can be attributed to the small side-cut incision, approximately one-eighth the length of a LASIK flap, which means a smaller likelihood of cutting corneal nerves, hence leading to less dry eye postoperatively.
Adverse event was noted only in 1 out of 5 eyes that experienced suction loss intraoperatively. SMILE procedure had to be abandoned and conversion to flap, followed by excimer laser for correction of refractive error was done.In the study done by Shahetal[19]1 eye incurred suction loss intraoperatively. Postoperatively, 5 eyes showed corneal cap edema, which resolved subsequently. Perioperative complications noted by Ivarsenetal[27]in a study of 1500 eyes which underwent SMILE, included epithelial abrasions (6%), small tears at the incision (1.8%), and difficult lenticule extraction (1.9%). Cap perforation in 4 eyes, and a major tear in 1 eye was noted.
ReLEx®SMILE is the most significant development in corneal refractive surgery since the introduction of LASIK. In the hands of experienced surgeons, the ReLEx®SMILE technique provides the combined advantages of LASIK and PRK with a minimal risk of complications. Our results demonstrate high refractive accuracy, predictability, safety and patient satisfaction associated with ReLEx®SMILE.
REFERENCES
1 Lipshitz I, Loewenstein A, Varssano D, Lazar M. Late onset corneal haze after photorefractive keratectomy for moderate and high myopia.Ophthalmology1997;104(3): 369-373
2 Slade SG. The use of the femtosecond laser in the customization of corneal flaps in laserinsitukeratomileusis.CurrOpinOphthalmol2007;18(4): 314-317
3 Kim P, Sutton GL, Rootman DS. Applications of the femtosecond laser in corneal refractive surgery.CurrOpinOphthalmol2011;22(4): 238-244
4 Bailey MD, Mitchell GL, Dhaliwal DK, Boxer Wachler BS, Zadnik K. Patient satisfaction and visual symptoms after laserinsitukeratomileusis.Ophthalmology2003;110(7): 1371-1378
5 Knorz MC. Flap and interface complications in LASIK.CurrOpinOphthalmol2002;13(4): 242-245
6 Battat L, Macri A, Dursun D, Pflugfelder SC. Effects of laserinsitukeratomileusis on tear production, clearance, and the ocular surface.Ophthalmology2001;108(7): 1230-1235
7 Sekundo W, Kunert K, Russmann C, Gille A, Bissmann W, Stobrawa G, Sticker M, Bischoff M, Blum M. First efficacy and safety study of femtosecond lenticule extraction for the correction of myopia: six-month results.JCataractRefractSurg2008;34(9): 1513-1520
8 Duffey RJ, Leaming D. U.S. trends in refractive surgery: The 2009 Survey. ASCRS Symposium on Cataract IOL and Refractive Surgery, Boston, Massachusetts, USA, 2009. Summary in Cataract and Refractive Surgery Today May 2010, page 10. (http://bmctoday.net/crstoday/2010/05/article.asp?f=2009-ascrs-trends-in-refractive-surgery-survey-reported)
9 Pallikaris IG, Kymionis GD, Astyrakakis NI. Corneal ectasia induced by laserinsitukeratomileusis.JCataractRefractSurg2001;27(11):1796-1802
10 Rad AS, Jabbarvand M, Saifi N. Progressive keratectasia after laserinsitukeratomileusis.JCataractRefractSurg2004;20(5 Suppl):S718-722
11 Vestergaard A, Ivarsen AR, Asp S, Hjortdal JØ. Small-incision lenticule extraction for moderate to high myopia: Predictability, safety, and patient satisfaction.JCataractRefractSurg2012;38(11): 2003-2010
12 Hjortdal JØ, Vestergaard AH, Ivarsen A, Ragunathan S, Asp S. Predictors for the outcome of small-incision lenticule extraction for Myopia.JRefractSurg2012;28(12): 865-871
13 Rajan MS, Jaycock P, O’Brart D, Nystrom HH, Marshall J. A long-term study of photorefractive keratectomy; 12-year follow-up.Ophthalmology2004;111(10): 1813-1824
14 O’Connor J, O’Keeffe M, Condon PI. Twelve-year follow-up of photorefractive keratectomy for low to moderate myopia.JRefractSurg2006;22(9): 871-877
15 Hu YK, Li WJ, Gao XW, Guo YL, Dong J. Comparison of small incision lenticule extraction andfemtosecond laser assisted LASIK for myopia.GuojiYankeZazhi(IntEyeSci) 2013;13(10):2074-2077
16 Kim WS, Jo JM. Corneal hydration affects ablation during laserinsitukeratomileusis surgery.Cornea2001;20(4): 394-397
17 Patel S, Alió JL, Artola A. Changes in the refractive index of the human corneal stroma during laserinsitukeratomileusis. Effects of exposure time and method used to create the flap.JCataractRefractSurg2008;34(7):1077-1082
18 Patel S, Alió JL, Pérez-Santonja JJ. Refractive index change in bovine and human corneal stroma before and after lasik: a study of untreated and re-treated corneas implicating stromal hydration.InvestOphthalmolVisSci2004;45(10): 3523-3530
19 Shah R, Shah S, Sengupta S. Results of small incision lenticule extraction: All-in-one femtosecond laser refractive surgery.JCataractRefractSurg2011;37(1):127-137
20 de Ortueta D, Arba Mosquera S, Baatz H. Comparison of standard and aberration-neutral profiles for myopic LASIK with the SCHWIND ESIRIS platform.JRefractSurg2009;25(4): 339-349
21 Kohnen T, Mahmoud K, Bühren J. Comparison of corneal higher-order aberrations induced by myopic and hyperopic LASIK.Ophthalmology2005;112(10): 1692
22 Sekundo W, Gertnere J, Bertelmann T, Solomatin I. One-year refractive results, contrast sensitivity, high-order aberrations and complications after myopic small-incision lenticule extraction (ReLEx®SMILE).GraefesArchClinExpOphthalmol2014;252(5): 837-843
23 Gertnere J, Solomatin I, Sekundo W. Refractive lenticule extraction (ReLEx®flex) and wavefront-optimized Femto-LASIK: comparison of contrast sensitivity and high-order aberrations at 1 year.GraefesArchClinExpOphthalmol2013;251(5): 1437-1442
25 Shoja MR, Besharati MR. Dry eye after LASIK for myopia: Incidence and risk factors.EurJOphthalmol2007;17(1): 1-6
26 Yu EY, Leung A, Rao S, Lam DS. Effect of laserinsitukeratomileusis on tear stability.Ophthalmology2000;107(12): 2131-2135
27 Ivarsen A, Asp S, Hjortdal J. Safety and complications of more than 1500 small-incision lenticule extraction procedures.Ophthalmology2014;121(4): 822-828
方法:前瞻性非随机临床研究。收集符合研究标准的600眼接受全面眼科检查,包括矫正远视力、对比敏感度、像差和干眼的评估。SMILE采用VisuMax全飞秒激光系统。术后1,15d及3mo对患者进行随访,并记录术前和术后3mo内的裸眼视力、矫正远视力、像差、干眼和对比敏感度。数据分析采用SPSS(美国,版本17.0)软件。计量资料采用配对t检验统计,计数资料采用Yate卡方检验统计。P<0.05则差异有统计学意义。
结果:本研究包含305例(600眼)患者。其中,10位患者由于屈光参差仅单眼接受SMILE。术后3mo,98.83%眼裸眼视力达到或超过20/20。所有患者矫正远视力无下降并且有37眼(6.17%)提高了一行。术后产生的彗差和球面像差最小。术后早期对比敏感度减少(P<0.01),但3mo后增加,特别是低频尤为明显(P=0.43、0.47、0.46)。
结论:结果表明,ReLEx®SMILE治疗近视和近视散光精确安全,与其他屈光手术一样,术后干眼和像差有所增加,但低频对比敏感度的下降不明显。
关键词:ReLEx®微小切口基质透镜取出术;飞秒激光;近视矫正;屈光结果
引用:Ganesh S, Gupta R. ReLEx®飞秒激光微小切口基质透镜取出术治疗近视或近视散光术后屈光情况研究.国际眼科杂志2016;16(2):218-223
Abstract
•AIM:To study the outcomes of ReLEx®small incision lenticule extraction (SMILE) for correction of myopia or myopic astigmatism in terms of visual acuity, contrast sensitivity, aberrations, and dry eye.
•METHODS: In this prospective, non-randomized clinical study, done at Nethradhama Super Speciality Eye Hospital, a total of 600 eyes that met the inclusion criteria underwent a thorough preoperative examination, including corrected distance visual acuity (CDVA), contrast sensitivity, aberrometry, and dry eye assessment. VisuMax femtosecond laser system was used to perform SMILE. Patients were followed up on 1, 15d and 3mo. Pre and postoperative uncorrected visual acuity (UCVA), CDVA, aberrations, dry eye, and contrast sensitivity during 3mo of follow-up were recorded. Data analysis was done with the help of a computer using SPSS for Windows Software (version 17.0, SPSS, Inc., New York, USA). A pairedt-test was used to test the significance of difference between quantitative variables and Yate’s Chi-square test for qualitative variables.Pvalue less than 0.05 denoted a significant relationship.
•RESULTS: The study enrolled 600 eyes of 305 patients, of which 10 patients underwent SMILE in 1 eye only due to anisometropia. At 3mo, 98.83% of eyes had attained a UCVA of 20/20 or better. No patient had a loss of CDVA, and 37 eyes (6.17%) showed a gain in 1 line in postoperative CDVA. Postoperative induction of coma and spherical aberrations was minimal. Contrast sensitivity reduced immediate postoperatively (P<0.001) but showed improvement by 3mo, especially at lower spatial frequencies (P=0.43, 0.47, 0.46)
•CONCLUSION:Our results demonstrate the high refractive accuracy and safety of ReLEx®SMILE for the treatment of myopia and myopic astigmatism. Increase in postoperative dryness and aberrations, both accepted drawbacks of any corneal refractive surgery were observed, while decrease in contrast sensitivity was insignificant at lower spatial frequencies.
KEYWORDS:•ReLEx®small incision lenticule extraction; femtosecond laser; myopic correction; refractive outcomes
DOI:10.3980/j.issn.1672-5123.2016.2.05
通讯作者:Rishika Gupta. rishikagupta55@yahoo.com
杂志排行
国际眼科杂志的其它文章
- VEGF, HIF-1α and PEDF expression in the retina of streptozotocin-induced diabetics rats treated with ozone
- 比较糖尿病和非糖尿病患者超声乳化术超乳参数和黄斑厚度的变化
- Retinal asymmetry in Chinese adults measured by cirrus high definition optical coherence tomography
- Lens-sparing vitrectomy for shaken baby syndrome
- Eaf2基因敲除对紫外线诱导的鼠白内障形成的影响
- 两种不同术式白内障摘除术后泪膜稳定性的检测
