抗菌医药左氧氟沙星在有机电致发光二极管中的应用
2015-12-29苗艳勤高志翔武钰铃杜晓刚李源浩刘慧慧贾虎生刘旭光
苗艳勤 高志翔 武钰铃 杜晓刚 李源浩刘慧慧 贾虎生 王 华,* 刘旭光
(1太原理工大学新材料界面科学与工程教育部重点实验室,太原030024;2山西大同大学物理与电子科学学院,山西大同037009;3太原理工大学新材料工程技术研究中心,太原030024;4太原理工大学材料科学与工程学院,太原030024;5太原理工大学化学化工学院,太原030024)
抗菌医药左氧氟沙星在有机电致发光二极管中的应用
苗艳勤1,3高志翔2武钰铃1,3杜晓刚1,3李源浩1,3刘慧慧1,3贾虎生1,4,*王 华1,3,*刘旭光5
(1太原理工大学新材料界面科学与工程教育部重点实验室,太原030024;2山西大同大学物理与电子科学学院,山西大同037009;3太原理工大学新材料工程技术研究中心,太原030024;4太原理工大学材料科学与工程学院,太原030024;5太原理工大学化学化工学院,太原030024)
左氧氟沙星(LOFX)是一种知名的抗菌药物,它的价格非常便宜,且有成熟的合成和纯化技术.本文中首次将LOFX作为一种蓝光发光材料和电子传输材料应用于有机电致发光器件(OLED)中.通过热重分析、UVVis吸收光谱、发射光谱以及循环伏安曲线详细地表征了LOFX的热学及光物理特性.LOFX有高的分解温度,为327°C;HOMO、LUMO能级分别为-6.2和-3.2 eV,光学带隙为3.0 eV.以LOFX作为客体材料,掺杂在主体材料4,4'-二(9-咔唑)联苯(CBP)中制备了蓝光OLED,该器件的电致发光(EL)发射峰位于452 nm,最大亮度为2315 cd·m-2.进一步,选择8-羟基喹啉铝(Alq3)作为参考材料,分别以LOFX和Alq3作为电子传输材料制备了结构相同的单载流子器件和绿色磷光OLED.在相同的电压下,以LOFX作为电子传输材料的单载流子器件的电流密度比以Alq3作为电子传输材料的单载流子器件更高.同时,以LOFX作为电子传输材料的绿色磷光OLED获得更高的器件效率.从这些EL性能可以看出,LOFX同时也是一很好的电子传输材料.
有机电致发光器件;左氧氟沙星;蓝光发光材料;电子传输材料;电致发光光谱;电致发光性能
1 Introduction
Organic light-emitting diodes(OLEDs)have attracted attention because of applications to full-color flat-panel displays,lighting, and sensor.1-7The fabrication cost is one of the important factors for the mass-production OLED companies.Organic materials, which are less-expensive and established in the synthesis and purification technologies,are preferable for the mass production of OLED devices.In our research to find such a material,beyond the field of chemical compounds for conventional light emitting semiconductors,we have extended our attention to a field of medicament and noticed a blue fluorescence emitting antimicrobial medicament,levofloxacin.
Levofloxacin C18H20FN3O4((S)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methylpiperazin-1-yl)-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid,called LOFX hereafter)is an oral broad spectrum antibiotic of the fluoroquinolone drug,which is widely used in the treatment of certain bacterial infections including pneumonia,urinary tract infections,and abdominal infections.8-12A blue emission has been reported for LOFX.13The molecular structure is shown in Fig.1.

Fig.1 Molecular structures of materials involved in this paper
Gunasekaran et al.14have reported that the intense absorption bands from LOFX were observed at 323,281,and 255 nm.The absorption bands which appeared below 323 nm were confirmed from the photoluminescence excitation(PLE)spectra of LOFX in solution,which contain the 330 and 286 nm PLE bands corresponding to the 323 and 281 nm absorption bands,respectively.13Here,the 286 nm band is attributed to π-π*transition,while the 330 nm band to the n-π*transition.14
Although the infrared vibrational,UV-Vis absorption,photoluminescence(PL),and PLE spectra have been reported for LOFX,13,14the optical properties have not fully established yet.Its application to OLED has never been examined.In the present paper,we report the detailed optical properties of LOFX and the possibility of application to OLED materials.
Here,we investigate the physical properties(spectroscopic properties,electronic energy levels,and thermal stability)of LOFX,and fabricate three types of OLEDs,which are named as blue-light Device series-B,electron-only Device series-E,and green-light Device series-G,to examine whether LOFX is useful as OLED materials such as blue emitter and electron transporter. Molecular structures of all materials involved in this paper are shown in Fig.1.Schematic device structures and the energy levels of functional materials used in this work are shown in Fig.2.
2 Experimental details
LOFX was purchased from J&K Chemical and other materials involved in devices were purchased from Luminescence Technology Corp.The purity of all materials is>98%and directly used without further purification.
The thermogravimetry analysis(TGA)of LOFX was performed in a NETZSCH STA409CTGAsystem at a ramping rate of 10°C· min-1under an argon flow of 10 mL·min-1from room temperature to 600°C.The differential scanning calorimetry(DSC)of LOFX was performed in a NETZSCH STA409C TGAsystem at a ramping rate of 10°C·min-1under an argon flow rate of 10 mL·min-1from room temperature to 300°C.The UV-Vis absorption spectrum of LOFX aqueous solution(10-5mol·L-1)was recorded by Hitachi U3900 UV-Vis spectrophotometer.The photoluminescence spectrum of LOFX solid powder was measured by Cary Eclipse fluorescence spectrophotometer.The photoluminescence quantum yield(PLQY)of LOFX was measured by a FluoroMax-4 fluorescence spectrophotometer equipped with an integrating sphere. To examine the highest occupied molecular orbital(HOMO)and the lowest unoccupied molecular orbital(LUMO)energies,the cyclic voltammetry(CV)curve was measured with Autolab/PG STAT302 electrochemical workstation in a three-electrode cell containing tetrabutyl perchloric acid amine(TBAP)(0.1 mol·L-1in the mixed solution of acetonitrile and methylene chloride(2:1, molar ratio))as an electrolyte at the scan speed of 50 mV·s-1.A platinum wire,a platinum electrode,and a calomel electrode were used as a working electrode,a counter electrode,and a reference electrode,respectively.
OLEDs with the emission area of 3 mm×3 mm were fabricated on the pre-patterned indium tin oxide(ITO)glass substrate with sheet resistance of 15 Ω·□-1.ITO substrates were cleaned by ultrasonication in baths of detergent water,deionized water,and acetone for 15 min successively,and then blown dry nitrogen and treated with UV ozone for 8 min,respectively.Then,the substrates were transferred into a vacuum chamber for sequential deposition of all organic functional layers by thermal evaporation below a vacuum of 5×10-4Pa.The deposition rate for organic materials,LiF,and Al were about 0.1,0.01,and 0.6 nm·s-1,respectively.The device performances of OLEDs were characterized by Keithley 2400 source meter combined with Photo Research PR655 spectrometer simultaneously.All measurements were performed at room temperature in ambient atmosphere without device encapsulation.
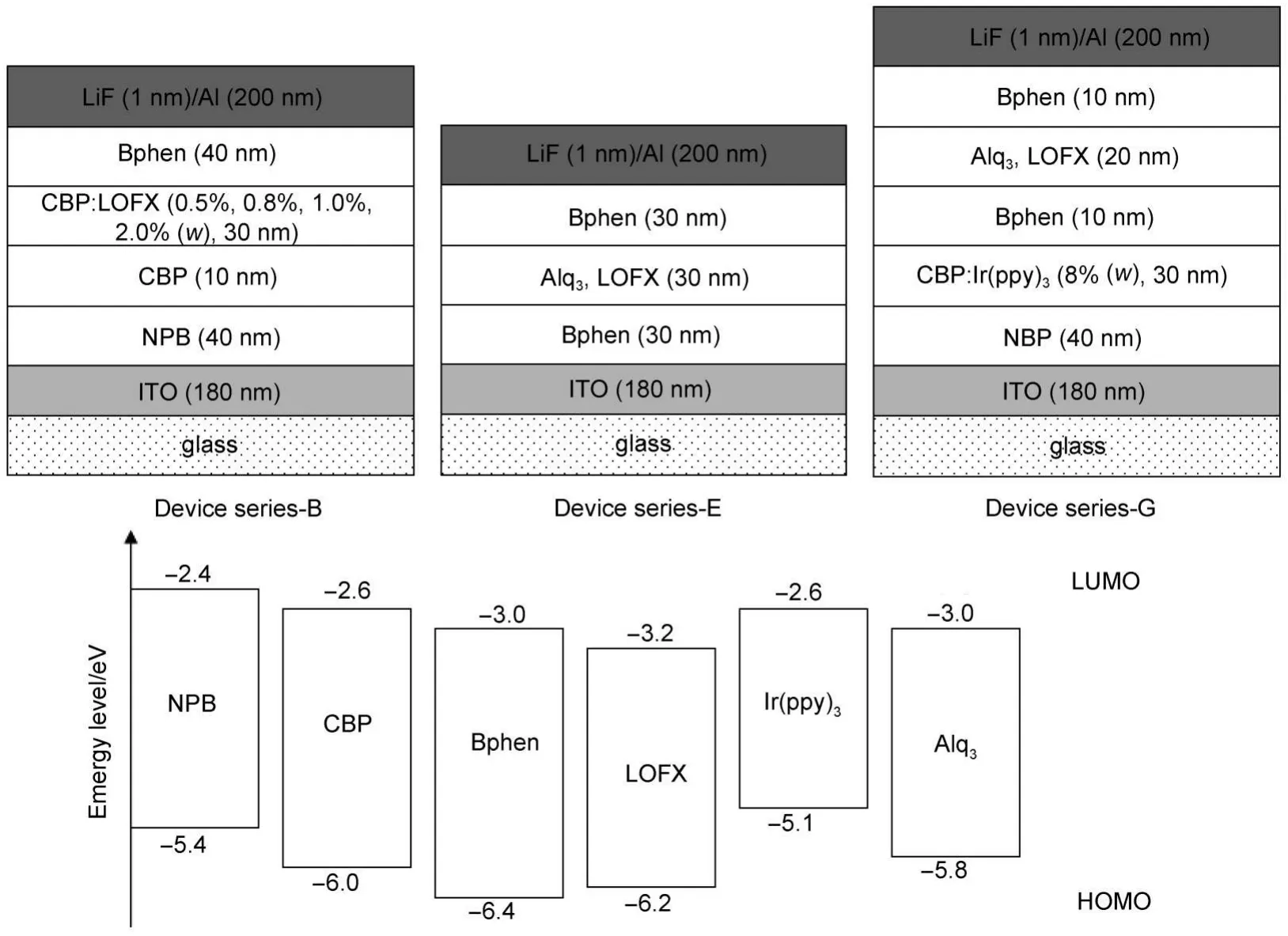
Fig.2 Schematic device structures of Device series-B,Device series-E,and Device series-G,and the energy levels of functional materials used in these devices
3 Results and discussion
3.1 Physical properties of LOFX
Fig.3 shows the UV-Vis absorption and PL spectra of LOFX in deionized water.Absorption begins from about 400 nm,giving an absorption band with peak at 302 nm and a sideband at about 330 nm.Much intense band continues from about 230 nm.An intense PL band with peak at 445 nm is observed.Our absorption spectrum of LOFX in solution is consistent with the PLE spectrum of LOFX in aqueous solution by Polishchuk et al.13,although our PL spectrum is shifted from 485.3 nm13to 445 nm.However,our absorption spectrum is not consistent with the absorption spectrum by Gunasekaran et al.14who observed an absorption band at 400 nm.It seems that the 400 nm band is due to an aggregate.
The thermal stability is an important factor for organic electroluminescent materials.Fig.4(a)shows the TG and differential thermogravimetry(DTG)curves of LOFX,and Fig.4(b)shows the DSC curves of LOFX.In TG curve,a 5%weight loss was observed at 327°C,indicating a high decomposition temperature (Td)and good thermal stability of LOFX.Meanwhile,in DSC curve,LOFX exhibited a high glass transition termperature(Tg) of 161°C.The high Tgand Tdvalues render LOFX an OLED material capable of forming stable amorphous films through vacuum thermal evaporation and upon heating.15

Fig.3 UV-Vis absorption and PLspectra of LOFX in deionized water solution excited at 360 nm,compared with the electroluminescence(EL)spectrum

Fig.4 (a)TG and DTG curves of LOFX;(b)DSC curve of LOFX
Fig.5 shows the CV curve of LOFX.From the CV curve,two oxidation peaks at about 1.0 and 1.5 V can be observed.The oxidation peaks at about 1.0 V and the onset oxidation potential at about 0.8 V should be ascribed to the oxidation of acetonitrile. And the oxidation peak at about 1.5 V and onset oxidation potential at about 1.4 V should be assigned to the oxidation of LOFX.There is an empirical equation EHOMO=-(Eoxonset+4.8)(eV), where Eoxonsetstands for the onset potential for oxidation,the potential of saturated calomel electrode(SCE)relative to the vacuum level is 4.8 eV.16,17Therefore,the HOMO level is-6.2 eV for LOFX.From the absorption spectrum of LOFX(see Fig.3),the wavelength of absorption edge(λedge)is above 414 nm.The optical gap(Eg)was obtained according to the following equation:Eg= 1240/λedge.18So,the Egof LOFX is 3.0 eV,and ELUMO=Eg+EHOMO, is-3.2 eV.Here,the LUMO level of LOFX is lower than that of Alq3(-3.0 eV)but higher than the work function of Al(-4.1 eV),indicating that LOFX has an electron transport characteristic.
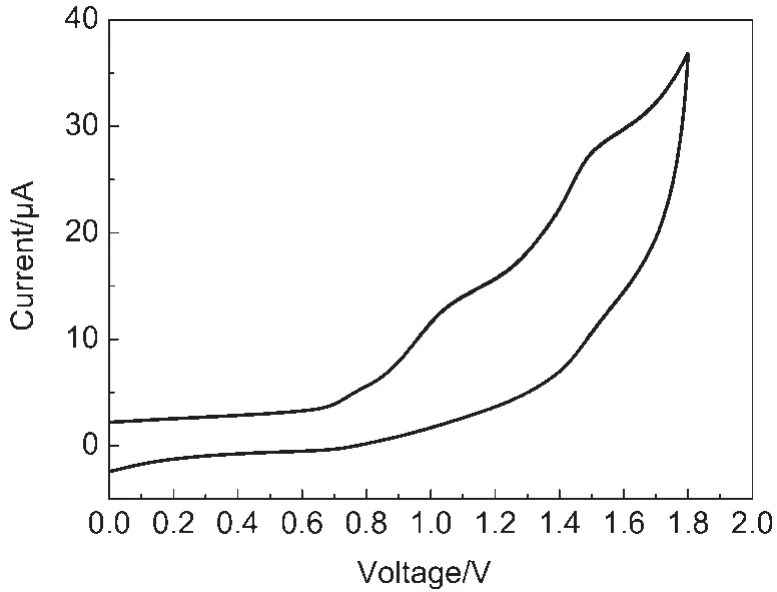
Fig.5 CV curve of LOFX
3.2 LOFX as blue-light emitting material in OLED
From above experimental results,it can be seen that LOFX expresses remarkable blue fluorescence effect,indicates that LOFX has potential application in OLED.Further,we measured the fluorescence quantum yield of LOFX solid powders,pure LOFX film,and CBP doped with 1%LOFX film(in mass fraction).The results indicate that CBP doped with 1%LOFX film shows a high PLQY of 12.53%,is higher than those of LOFX solid powders(5.54%)and pure LOFX film(6.25%).It is expected that LOFX is doped into host material to structure high performance device.For identifying it,a series of blue light OLED(Device series-B)(see Fig.2)are fabricated using LOFX as blue emitter with the device configuration of indium tin oxide (ITO)/NPB(40 nm)/CBP(10 nm)/CBP:wLOFX(30 nm)/Bphen (40 nm)/LiF(1 nm)/Al(200 nm),where the concentrations(in w, mass fraction)of LOFX are changed as 0.5%,0.8%,1.0%,and 2.0%.Here,NPB is N,N′-bis-(naphthyl)-N,N′-diphenyl-1,1′-biphenyl-4,4′-diamine,which is used as hole transport layer(HTL); CBP is 4,4'-bis(carbazol-9-yl)biphenyl,which is used as the exciton-block layer;Bphen is 4,7-diphenyl-1,10-phenanthroline, which is used as electron transport layer(ETL).The layer of CBP doped with 1.0%LOFX is a light emitting layer(EML),while LiF and Al are used as electron injection layer(EIL)and cathode, respectively.
Fig.6(a)shows the luminance-voltage(L-V)curves of Device series-B with different doping concentrations of LOFX in CBP.As doping concentrations increase from 0.5%to 1%,the maximum luminance of Device series-B increases from 1593 cd·m-2at 0.5% to 2315 cd·m-2at 1%.When doping concentration is 2%,the maximum luminance of Device series-B lowers to 736.8 cd·m-2. Lower doping concentrations limit radioactive recombination on dopant sites and induce in inadequate utilization of excited energy origined form CBP.On the contrary,higher doping concentrations result in serious concentration quenching of LOFX.19So,the optimum doping concentration of LOFX in CBP is determined to be 1.0%.The Device series-B(w=1.0%)exhibited a turn-on voltage,defined as the voltage measured at 1 cd·m-2,of around 4.5 V and a maximum luminance of 2315 cd·m-2driven by voltage of 7.5 V.
Fig.6(b)shows the EL spectra of Device series-B(w=1.0%) driven by various bias voltages,inset is the photograph of Device series-B(w=1.0%)under voltage of 8 V.An EL band with emission peak at 452 nm due to LOFX is observed clearly when driven voltage is above 6 V.The EL band red-shifted by 7 nm from the PL band(Fig.3)observed in solution owing to a solid state effect.20From Fig.6(b),it can be seen that no obvious EL band shift was observed by changing the operating voltage.The Device series-B(w=1.0%)emitted a pure blue light with com-mission international del′eclairage(CIE)1931 coordinates coordinates of(0.17,0.14)at 7-10 V,which is close to the National Television System Committee(NTSC)blue standard.21These EL performances indicate that LOFX as blue emitter is useful for OLEDs.

Fig.6 (a)Luminance-voltage(L-V)curves of Device series-B with different doping concentrations of LOFX in CBP;(b)EL spectra of Device series-B(w=1.0%)at various voltages with CIE coordinates
3.3 LOFX as electron transport material in OLED
Further,we investigate if LOFX is useful as electron transporting material in OLEDs.To check this point,we investigated the current density-voltage characteristic by fabricating an electron-carrier-only OLED(called Device E-2)with structure of ITO/Bphen(30 nm)/LOFX(30 nm)/Bphen(30 nm)/LiF(1 nm)/Al (200 nm)(see Fig.2).We compare its characteristics with another electron-carrier-only OLED(called Device E-1)based on Alq3, which is well-known as good electron transporting material. Device E-1 has the quite similar layer structure as Device E-2,i.e., ITO/Bphen(30 nm)/Alq3(30 nm)/Bphen(30 nm)/LiF(1 nm)/Al (200 nm)(see Fig.2).The current densities of these two devices are plotted against applied voltage in Fig.7.Device E-2 with LOFX has higher current density than Device E-1 withAlq3at the same driving voltage.Device E-2 has much lower turn-on voltage than Device E-1.From these results,LOFX is confirmed its superiority toAlq3in electron-transporting capability.
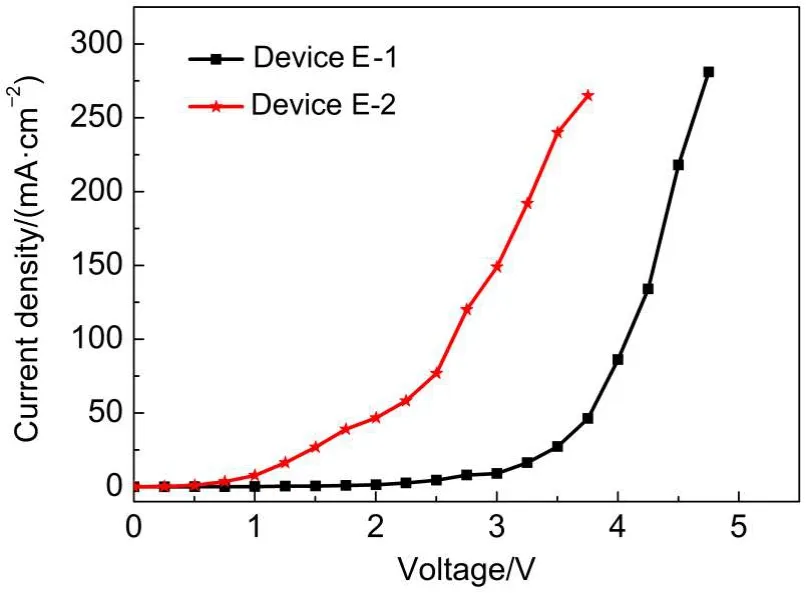
Fig.7 Current density-voltage curves of Device E-1 and Device E-2
To further check the electron-transporting superiority of LOFX to Alq3,we fabricate two green-emitting OLEDs with Ir(ppy)3((fac-tris(2-phenylpyridine)iridium)emitter(Devices G-1 and G-2).Devices G-1 and G-2 have the same structure except ETL layer,i.e.,ITO/NPB(40 nm)/BP:Ir(ppy)3(8%,30 nm)/Bphen(10 nm)/Alq3or LOFX(20 nm)/Bphen(10 nm)/LiF(1 nm)/Al(200 nm)(see Fig.2),respectively,where NPB is used as HTL,CBP: Ir(ppy)3complex)as EML,and Bphen adjacent to LiF as EIL. Fig.8(a,b,c,d)show the luminance-voltage,current densityvoltage,current efficiency-current density,and power efficiencyvoltage characteristics of Device G-1 with Alq3and G-2 with LOFX,respectively.For Devices G-1 and G-2,the turn-on voltages are 3.5 and 4.0 V,the high luminance of 36267 cd·m-2at 7.5 V and 36600 cd·m-2at 8.5 V,the maximum current efficiency of 15 cd·A-1at 47.5 mA·cm-2and 17.7 cd·A-1at 16.7 mA·cm-2,the maximum power efficiency of 8.86 lm·W-1at 4.5 V and 9.96 lm· W-1at 5.5 V,respectively.The current efficiency is higher at low current densities of 8-200 mA·cm-2in Device G-2 than that in Device G-1(Fig.8(a,c)),and the maximum power efficiency is also higher in Device G-2 than that in Device G-1(Fig.8(d)).One of the reasons of higher efficiency of Device G-2 than Device G-1 is higher electron transport of LOFX than Alq3.Another reason is that Device G-2 blocks holes more efficiently thanAlq3because LOFX has lower HOMO level(-6.2 eV)thanAlq3(-5.8 eV).
Regarding the low operational voltage,Alq3is superior to LOFX.This is understood by higher electron injection barrier(0.2 eV)at LOFX/Bphen interface relative to the barrier(0 eV)atAlq3/ Bphen interface,leading to more difficulty to enter electrons from cathode to EML in Device G-2 than that in Device G-1.
The maximum current efficiency of Device G-2 with LOFX is superior to Device G-1 withAlq3.However the efficiency of 17.7 cd·A-1is smaller than the conventional efficiencies of OLEDs with phosphorescence Ir(ppy)3.For example,Baldo et al.22obtained 28 cd·A-1using an OLED with EML of CBP doped with 6%Ir(ppy)3.
To find this reason,we examine the EL spectra of Device G-2 carefully by semi-log plotting.As seen in Fig.9,a small EL band appears at about 445 nm besides the 510 nm EL band due to Ir(ppy)3.We compare the PL spectrum of NPB neat film with the EL spectra(Fig.9).NPB gives PL band with peak at about 445 nm,which coincides with the weak band.Therefore the 445 nm EL band is attributable to NPB.This indicates that electrons fromcathode are leaked to the NPB layer.This leakage is understood from the energy level diagram of Device G-2 which shows that the energy gap(0.2 eV)of LUMO energy between CBP of EML and NPB of HTL is small(Fig.2).
Device G-2 is more enhanced the roll-off effect than Device G-1.This is understood as follows.Better electron transportation leads to higher triplet-triplet annihilation and roll-off.23-25It is suggested that the electron injection to EML is better to LOFX layer than to Alq3layer because of better electron transportation in LOFX layer than in Alq3layer as mentioned above.This leads to higher electron accumulation in EML with phosphorescent Ir(ppy)3emitter of Device G-2 at high current densities than in EML of Device G-1,resulting in stronger roll-off for Device G-2 than for Device G-1.In this way,the superior of LOFX electron transportation toAlq3is confirmed from the roll-off.
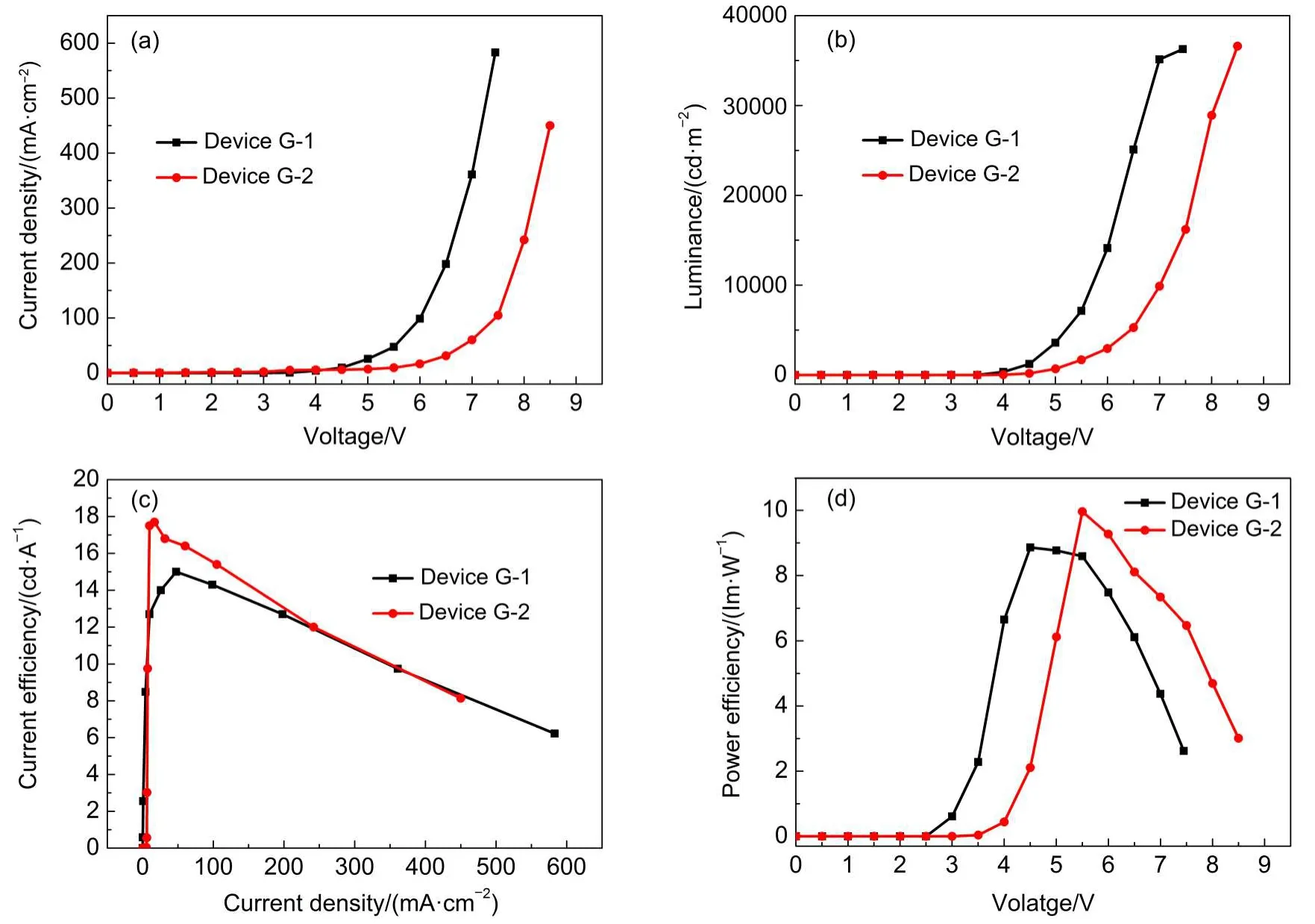
Fig.8 (a)Current density-voltage curves,(b)luminance-voltage characteristic,(c)current efficiency-current density curves,and (d)power efficiency-voltage curves of Devices G-1 and G-2
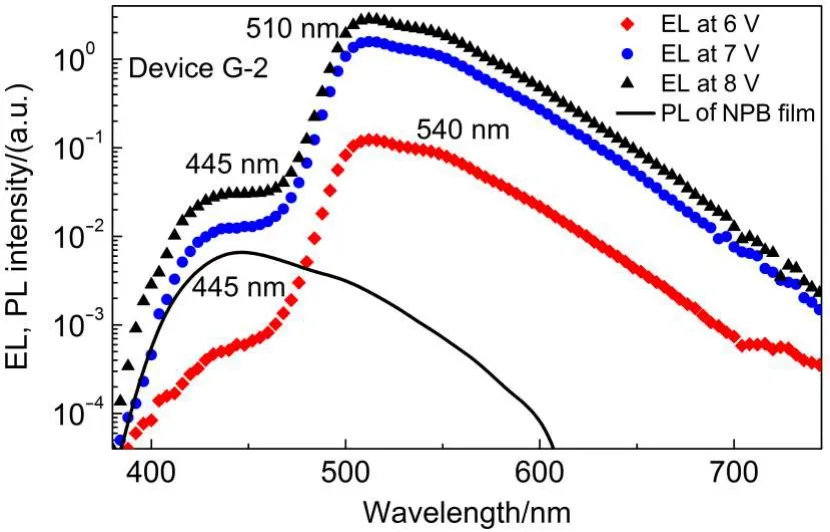
Fig.9 Semi-log plotted ELspectra of Device G-2 at various voltages,compared with the PLspectrum of NPB neat film excited at 350 nm
4 Conclusions
To investigate whether LOFX,well-known as an antimicrobial medicament,is useful as OLED materials such as blue emitter and electron transporter,we have studied the spectroscopic properties, electronic energy levels,thermal stability,electroluminescence, and OLED characteristics.LOFX shows a blue PL band at 446 nm,HOMO and LUMO energies of-6.2 and-3.2 eV,respectively,and high molecule decomposition temperature(Td)at 327°C. The blue OLED with LOFX emitter shows a pure blue emission with a peak at 452 nm and a maximum luminance of 2315 cd·m-2. Further,LOFX is found to be higher in electron-transporting ability thanAlq3,which was obtained using the electron-transportonly device.In the case that LOFX is used as ETL in green emitting OLED with Ir(ppy)3,the maximum current density of 17.7 cd·A-1and the maximum power efficiency of 9.96 lm·W-1are obtained,which are higher than 15.0 cd·A-1and 8.86 lm·W-1in the OLED with ETL of Alq3.The current efficiency is lower than the conventional efficiency of OLEDs with Ir(ppy)3.This is attributed to leakage of electrons from EMLto HTLof NPB in the present OLED device.From the EL performances,it is suggested that LOFX can act as a desired bifunctional material:not only a pure blue emitter,but also a excellent electron transport material in OLED devices,which is useful for OLEDs.
References
(1)Kido,J.;Kimura,M.;Nagai,K.Science1995,267,1332.doi: 10.1126/science.267.5202.1332
(2)D′Andrade,B.W.;Forrest,S.R.Adv.Mater.2004,16,1585.
(3)Yu,S.J.;Suo,F.;Li,W.Z.;Lin,H.;Li,L.;Jiang,Y.D.Acta Phys.-Chim.Sin.2007,23,1821.[于胜军,锁 钒,黎威志,林 慧,李 璐,蒋亚东.物理化学学报,2007,23,1821.] doi:10.3866/PKU.WHXB20071132
(4)Shinar,J.;Shinar,R.J.Phys.D:Appl.Phys.2008,41,133001/ 1.doi:10.1088/0022-3727/41/13/133001
(5)Reineke,S.;Lindner,F.;Schwartz,G.;Seidler,N.;Walzer,K.; Lüssem,B.;Leo,K.Nature2009,459,234.doi:10.1038/ nature08003
(6)Xiao,L.X.;Hu,S.Y.;Kong,S.;Chen,Z.J.;Qu,B.;Gong,Q. H.Acta Phys.-Chim.Sin.2011,27,977.[肖立新,胡双元,孔 胜,陈志坚,曲 波,龚旗煌.物理化学学报,2011,27, 977.]doi:10.3866/PKU.WHXB20110325
(7)Chang,Y.L.;Song,Y.;Wang,Z.B.;Helander,M.G.;Qiu,J.; Chai,L.;Liu,Z.W.;Scholes,G.D.;Lu,Z.H.Adv.Funct. Mater.2013,23,705.doi:10.1002/adfm.v23.6
(8)Nelson,J.M.;Chiller,T.M.;Powers,J.H.;Angulo,F.J.Clin. Infect.Dis.2007,44,977.doi:10.1086/512369
(9)Mandell,L.A.;Wunderink,R.G.;Anzueto,A.Clin.Infect.Dis.2007,44,S27.
(10)Solomkin,J.S.;Mazuski,J.E.;Bradley,J.S.Clin.Infect.Dis.2010,50,133.doi:10.1086/648977
(11)Chien,S.C.;Wong,F.A.;Fowler,C.L.;Callery-D′Amico,S. V.;Williams,R.R.;Nayak,R.;Chow,A.T.Antimicrob.Agents Chemother.1998,42,4885.
(12)Zhang,J.L.;Yang,X.Z.;Han,Y.;Li,W.;Wang,J.K.Fluid Phase Equilib.2012,335,1.doi:10.1016/j.fluid.2012.05.027
(13)Polishchuk,A.V.;Karaseva,E.T.;proskurina,N.A.;Karasev, V.E.High Energy Chem.2008,42,459.doi:10.1134/ S0018143908060076
(14)Gunasekaran,S.;Rajalakshmi,K.;Kumaresan,S.Spectroc. Acta Pt.A-Molec.Biomolec.Spectr.2013,112,351. doi:10.1016/j.saa.2013.04.074
(15)Lin,M.S.;Chi,L.C.;Chang,H.W.;Huang,Y.H.;Tien,K.C.; Chen,C.C.;Chang,C.H.;Wu,C.C.;Chaskar,A.;Chou,S.H.; Ting,H.C.;Wong,K.T.;Liu,Y.H.;Chi,Y.J.Chem.Mater.2012,22,870.doi:10.1039/c1jm13323c
(16)Pomrnerehne,J.;Vestweber,H.;Gun,W.;Muhrt,R.F.;Basler, H.;Porsch,M.;Daub,J.Adv.Mater.1995,7,551.
(17)Zhuang,J.Y.;Su,W.M.;Li,W.F.;Zhou,Y.Y.;Shen,Q.;Zhou, M.Org.Electron.2012,13,2210.doi:10.1016/j. orgel.2012.06.025
(18)Bredas,J.L.;Silbey,R.;Boudreaux,D.S.;Chance,R.R.J.Am. Chem.Soc.1983,105,6555.doi:10.1021/ja00360a004
(19)Lüssem,B.;Riede,M.;Leo,K.Phys.Status Solidi A2013,210, 9.doi:10.1002/pssa.v210.1
(20)Bulovic,V.;Deshpande,R.;Thompson,M.E.;Forrest,S.R. Chem.Phys.Lett.1999,308,317.doi:10.1016/S0009-2614(99) 00580-1
(21)Kang,G.W.;Ahn,Y.J.;Lim,J.T.;Lee,C.H.Synth.Met.2003,137,987.
(22)Baldo,M.;Lamansky,S.;Burrows,P.E.;Thompson,M.E.; Forrest,S.R.Appl.Phys.Lett.1999,75,4.doi:10.1063/ 1.124258
(23)Baldo,M.A.;Adachi,C.;Forrest,S.R.Phys.Rev.B2000,62, 10967.doi:10.1103/PhysRevB.62.10967
(24)Ern,V.;Merrifield,R.E.Phys.Rev.Lett.1968,21,609.doi: 10.1103/PhysRevLett.21.609
(25)Kondakova,M.E.;Deaton,J.C.;Pawlik,T.D.;Giesen,D.J.; Kondakov,D.Y.;Young,R.H.;Royster,T.L.;Comfort,D.L.; Shore,J.D.J.Appl.Phys.2010,107,014515/1.doi:10.1063/ 1.3275053
Antimicrobial Drug Levofloxacin Applied to an Organic Light-Emitting Diode
MIAO Yan-Qin1,3GAO Zhi-Xiang2WU Yu-Ling1,3DU Xiao-Gang1,3LI Yuan-Hao1,3LIU Hui-Hui1,3JIAHu-Sheng1,4,*WANG Hua1,3,*LIU Xu-Guang5
(1Key Laboratory of Interface Science and Engineering in Advanced Materials,Ministry of Education,Taiyuan University of Technology,Taiyuan 030024,P.R.China;2School of Physical Science and Electronics,Shanxi Datong University,Datong 037009, Shanxi Province,P.R.China;3Research Center of Advanced Materials Science and Technology,Taiyuan University of Technology, Taiyuan 030024,P.R.China;4College of Materials Science and Engineering,Taiyuan University of Technology,Taiyuan 030024, P.R.China;5College of Chemistry and Chemical Engineering,Taiyuan University of Technology,Taiyuan 030024,P.R.China)
Levofloxacin(LOFX)is a well-known and inexpensive antimicrobial drug that can be easily synthesized and purified.We report the first application of LOFX to an organic light emitting diode(OLED).Its thermal and photophysical properties were thoroughly investigated using thermogravimetric analysis(TGA), UV-Vis absorption spectra,emission spectra,and cyclic voltammetry.LOFX has HOMO and LUMO energiesof-6.2 and-3.2 eV,respectively,and high molecule decomposition temperature(Td)of 327°C.An OLED with a LOFX emitter shows electroluminescence(EL)at 452 nm and maximum luminance of 2315 cd·A-1,which can be used in a white OLED.To investigate the electron transporting ability of LOFX,an electron-carrier only OLED was made.In addition,a green OLED based on Ir(ppy)3(fac-tris(2-phenylpyridine)iridium)with electron transporting layer of LOFX was made,comparing with that with electron transporting layer of tris(8-hydroxyquinoline)aluminum(Alq3).The former exhibited higher device efficiencies than that of the latter.The results show that LOFX has a higher electron transport ability thanAlq3.©Editorial office ofActa Physico-Chimica Sinica
Organic light-emitting diode;Levofloxacin;Blue-light emitting material;Electron transport material;Electroluminescence spectrum;Electroluminescence performance
O649
10.3866/PKU.WHXB201501051www.whxb.pku.edu.cn
Received:October 27,2014;Revised:January 4,2015;Published on Web:January 5,2015.
∗Corresponding authors.WANG Hua,Email:wanghua001@tyut.edu.cn;Tel:+86-13613477492.JIAHu-Sheng,Email:jia_husheng@126.con;
Tel:+86-351-6014852.
The project was supported by the Program for New Century Excellent Talents in University of Ministry of Education of China(NCET-13-0927),
International Science&Technology Cooperation Program of China(2012DFR50460),National Natural Science Foundation of China(61307029, 21101111),and Shanxi Provincial Key Innovative Research Team in Science and Technology,China(2012041011).
教育部新世纪人才计划(NCET-13-0927),科技部国际科技合作专项项目(2012DFR50460),国家自然科学基金(61307029,21101111)和山西省科技创新重点团队项目(2012041011)资助
