以氨基化的聚苯乙烯微球为模板制备电致变色性能优良的单层有序多孔PBTB薄膜
2015-12-29欧阳密黄森彪韩延刚吕晓静媛戴玉玉吕耀康浙江工业大学化工学院杭州3004浙江质量检测科学研究院杭州3008
欧阳密 黄森彪 韩延刚 吕晓静 杨 媛戴玉玉 吕耀康,* 张 诚,*(浙江工业大学化工学院,杭州3004; 浙江质量检测科学研究院,杭州3008)
以氨基化的聚苯乙烯微球为模板制备电致变色性能优良的单层有序多孔PBTB薄膜
欧阳密1黄森彪1韩延刚2吕晓静1杨 媛1戴玉玉1吕耀康1,*张 诚1,*
(1浙江工业大学化工学院,杭州310014;2浙江质量检测科学研究院,杭州310018)
通过利用氨基化的聚苯乙烯(PAS)为模板,结合电化学原位聚合法,成功制备了具有高度有序多孔纳米结构的聚1,4-二噻吩苯(PBTB)薄膜.所制得的有序多孔薄膜在掺杂/脱掺杂状态下能够实现颜色从紫色到黄色的可逆转变.相比于平整的PBTB薄膜,有序多孔纳米薄膜在1100 nm处的褪色时间从1.0 s缩短至0.5 s,着色时间从3.6 s缩短至3.1 s;同样地,在620 nm处的褪色时间和着色时间也有所减少,分别从1.6 s减少到0.7 s和从4.5 s减少到了3.8 s.同时,电化学阻抗图谱显示有序多孔纳米薄膜具有更小的电荷转移阻抗,这主要归功于多孔纳米结构的引入增加了聚合物薄膜的比表面积,有利于离子的注入与抽出,并有效缩短了离子的扩散距离,从而提高了聚合物的响应速度.因此,多孔有序纳米结构的引入可以显著提升导电聚合物薄膜的电致变色性能.
电致变色;有序多孔结构;响应时间;离子扩散;电化学聚合
©Editorial office ofActa Physico-Chimica Sinica
Key Words:Electrochromism;Ordered porous structure;Switching time;Ion diffusion; Electropolymerization
1 Introduction
Currently,the development of organic electronic devices has been one of the central issues in material science.Electrochromic (EC)devices1-3have attracted great interests in the fields of antiglare rear mirrors,4energy-saving smart windows,5thin flat panel displays,6and active camouflage7due to low power consumption and stable memory effect under open circuit condition.Conducting polymers(CPs)based EC materials,8,9owing to their multicolor-display,10high optical contrast,11and coloration efficiency,12have been widely studied by researchers.Among these properties,the switching speed13is one of the most important parameters for the practical applications of EC materials,which is dependent on the ion-transport rate in the polymer layer. Therefore,a relative plausible solution to improve the switching speed is to reduce the ion-transport distance in the polymer via introducing the nanostructures.Zang et al.14has prepared polypyrrole(PPy)network by template free approach,15-17which reported that the network could reduce the switching time.Lu et al.18also has fabricated the PPy nano-network structure by using 1-pyrenesulfonic acid(PSA)micelles as soft template,19-21which also improves the switching rate of the material.All the work has further indicated that the nanostructures could shorten the iontransport distance and fasten ion diffusion rate,which effectively improved the switching speed of electrochromic materials.
Compared with disordered nanostructure,the highly ordered nanostructure has been recognized to be more promising for the enhancement of electrochromic performance,which offers uniform ion transport channel and facilitate ion doping/dedoping. However,most of current reports about the ordered nanostructure have been focused on the inorganic electrochromic materials owing to its higher stability and easier to control the morphology. Zhang et al.22have prepared ordered WO3porous arrays with enhanced switching speed using polystyrene(PS)colloidal crystals as template via electrodeposition method.Yuan et al.23synthesized the ordered porous nickel oxide(NiO)thin film by self-assembled PS colloidal crystal template-assisted with electrodeposition to improve the electrochromic properties.Both of them have synthesized the highly ordered nanostructure using hard templates,24,25such as PS colloidal crystal template,26,27but it is mainly limited for the inorganic electrochromic materials.The highly ordered organic electrochromic nanostructure28-30has been rarely reported due to the complicated and uncontrollable process. Cho and Lee31demonstrated that the electrochromic performances of poly(3,4-ethylenedioxythiophene)(PEDOT)nanotubes and nanowires prepared by using anodic aluminum oxide(AAO)32,33as template have been significantly enhanced compared with the compact film.However,the electrochromic polymer could only show monotonous colors from dark blue to light blue,which hinders the practical applications on display devices.In addition, there are only few reports on the ordered nanostructure EC polymer,which requires further study on the relationship between ordered nanostructure and EC performance.
In this work,electrochromic poly(1,4-bis(2-thienyl)-benzene) (PBTB)with highly ordered porous nanostructure was successfully fabricated using monolayer amine-modified polystyrene (PAS)as template via electropolymerization method.The ordered porous nanostructure can effectively shorten ion diffusion distance and improve switching speed significantly.In addition,the possible mechanism of the ordered porous structure to the enhanced properties is also discussed herein.
2 Expermental
2.1 Materials
Amine-modified polystyrene microsphere latex(Aldrich,2.5% g·mL-1,diameter:0.6-1 μm),tetrabutylammonium perchlorate (TBAP,Energy Chemical,95%),acetonitrile(ACN,Energy Chemical,98%),tetrahydrofuran(THF,Energy Chemical,98%), dichloromethane(CH2Cl2,Energy Chemical,98%)were used as received.(1,4-bis(2-thienyl)-benzene)(BTB)was synthesized and characterized according to the reference.34Indium tin oxide(ITO) glass substrates(Zhuhai Kaivo Electronic Components Co.,Ltd., sheet resistance(Rs)≤10 Ω·□-1,area:1 cm×4 cm)were used by ultrasonic washing in deionized water,acetone,toluene,and ethanol solutions,respectively.
2.2 Preparation of OP-PBTB film
The preparation of the ordered porousnanostructure′sPBTB film involved three steps.The first one was the preparation of the PAS monolayer template using the vertical deposition method: drop some PAS latex with good dispersion onto the ITO substrate, then spread it slowly and make it homogeneous.After 30 s,put the ITO substrate upright,use the dropper to absorb most of excess latex and keep it vertical for another 30 s.Then put the end of ITO substrate into the deionized water and the rest of excess latex will be rapidly dispersed in the deionized water.At last,dry in the air vertically and put it into an incubator at 50°C for 2 h. The second step was the preparation of PBTB/PAS composite film via the constant potential polymerization of 2 mmol·L-1BTB/ ACN at 1.4 V,which was employed in a conventional three compartment electrolysis cell with PAS-coated ITO substrate as working electrode,a platinum(Pt)sheet(4 cm2)as counter electrode,andAg/AgCl in saturated KCl(aq)solution as reference electrode.The charge capacity of polymerization was controlled at 0.03C to make sure that the polymer PBTB grew along the interspace between PAS microspheres and could not cover the PAS single-layer template.For comparison,the pure PBTB film was prepared under the same condition.All the electrochemical experiments were carried out under the room temperature.The last step was the corrosion of PAS template.The OP-PBTB film was obtained after the immersion of PBTB/PAS composite film into THF bath for 1 h,then washed with THF and dried at 50°C in theoven for 2 h.
2.3 Characterization
A CHI 660E electrochemical analyzer(Chenhua,China)was used to perform the electrochemical measurements.The surface morphologies and microstructures of PAS template,pure PBTB film,and OP-PBTB nanostructure were investigated by a Hitachi S-4800 scanning electron microscope(SEM,Hitachi,Japan).The structural characteristics of samples at neutral state on the ITO electrode were examined by a Nicolet 6700 Fourier transform infrared(FTIR)spectrometer(Thermo Fisher Nicolet,USA). Ultraviolet visible(UV-Vis)spectra,optical contrast,and switching time of pure PBTB film and OP-PBTB nanostructure were carried out on a Shimadzu UV-1800 UV-Vis spectrophotometer(Shimadzu,Japan)integrated with the CHI660E electrochemical analyzer.
3 Results and discussion
The micrographs of PAS monolayer template,pure PBTB film, PBTB/PAS composite film,and corresponding order porous OPPBTB film are shown in Fig.1.Fig.1a shows the PAS microspheres,with the average diameter about 600 nm,are orderly and evenly arranged on the ITO substrate via self-assembly function. As shown in Fig.1b,we can see that the PAS microspheres form a monolayer opal structure without redundant balls.Fig.1d displays the surface morphology of the PBTB/PAS composite film. It is very evident that a layer of polymer film(PBTB)is successfully electrochemical polymerized around the PASball′sarray,while the BTB monomers lead to a compact,uniform,and stable film of PBTB on ITO without the PAS template (Fig.1c).After the immersion in tetrahydrofuran(THF)bath for 1 h,the PAS microspheres in composite films are dissolved,and the PBTB film presents the close-packed voids with uniform size called inverse opals structure(seen in Fig.1e and Fig.1f),and it also reveals some irregular or imperfect skeletons.Therefore,with help of PAS monolayer template,the highly ordered porous PBTB films are achieved for the next electrochemical characterization and electrochromic performance tests.
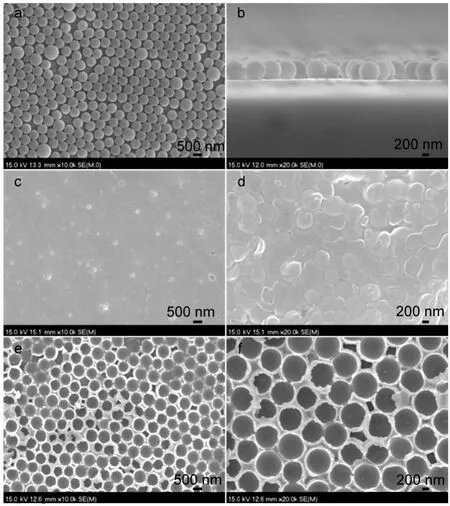
Fig.1 SEM images of top-view(a)and cross-view(b)of PAS monolayer template,pure PBTB film(c),PBTB/PAS composite film(d),and OP-PBTB nanostructure(e,f)
Fig.2 displays the FTIR spectra of PAS template,pure PBTB film,PBTB/PAS composite film,and OP-PBTB film.The peaks around 1600 cm-1should be ascribed to the C—C stretching vibrations in the phenyl ring in all films.35The peak at 794 cm-1is the characteristic of C—S bonds in the thiophene rings,and the peaks of pure PBTB film and PBTB/PAS composite film around 1382 and 1453 cm-1are attributed to the stretching of thiophene rings,36indicating the existence of PBTB.Meanwhile,the PBTB/ PAS composite film and the PAS template possess the same peak at 1726 cm-1,which is attributed to C=O stretching vibrations of benzoyl peroxide(BPO,the initiator of PAS).Therefore,the FTIR spectra demonstrate the effective formation of the PBTB/PAS composite film.Moreover,the OP-PBTB also possesses the similar characteristic bands of pure PBTB such as the bands at 1600,1465,and 794 cm-1.However,from the comparison of the OP-PBTB film and the PBTB/PAS composite film,we can find that the bands at 1726 and 1097 cm-1are disappeared,which are ascribed to the dissolution of PAS and the remove of ClO4-on the film surface in THF,respectively.
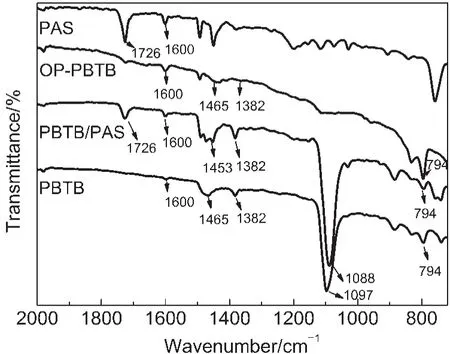
Fig.2 FTIR spectra of the PAS monolayer template,pure PBTB film,PBTB/PAS composite film,and OP-PBTB film
Fig.3 depicts the cycle voltammetry curves of pure PBTB film and OP-PBTB film in ACN containing 0.1 mol·L-1TBAP from 0 to 1.4 V at a scan rate of 125 mV·s-1.The pure PBTB film exhibits an obvious reduction peak at 1.1 V without apparent oxidation peak,while the OP-PBTB film displays a wide reduction peak from 0.9 to 1.2 V without apparent oxidation peak.Both of them present yellow and purple in the reduced state(0 V)and oxidized state(1.4 V).So it indicates that the PBTB film with order porous nanostructure possesses similar electrochemical activity with pure PBTB film,and both films have the same color capability.
Apowerful way to investigate the optical switches and changesof EC conducting polymers upon potential change is the spectroelectrochemical analysis,which provides the insights into the electronic structure of the conducting polymer.Therefore,the UVVis absorption spectra of the pure PBTB film and the OP-PBTB film under various applied potentials from 0 to 1.4 V have been tested and shown in Fig.4.In Fig.4a,the pure PBTB film shows a well-defined maximum absorption band centered at 410 nm(Eg(energy gap)=2.34 eV,calculated by using Eg=1240/λonset;λonset:the onset value of the absorption)at the neutral state(0 V),which is ascribed to the π-π*transition of the polymer backbone.With the increase of potential,it gradually decreases.However,the bands at around 620 and 1100 nm increase with the increase of potential at the same time,which is attributed to the evolution of polaron and bipolaron bands.37As seen from Fig.4b,the maximum absorption peak of the OP-PBTB film locates at 420 nm(Eg=2.14 eV)at the neutral state(0 V),and it also decreases with the increase of the potential.Moreover,the charge carrier bands at 620 and 1100 nm also increase with the increase of the potential. Comparing to the pure PBTB film,the maximum absorption peak of OP-PBTB film at the neutral state(0 V)has a little red-shift about 10 nm due to the introduction of ordered porous nanostructure,which causes the lower energy gap and contributes to the improvement of the electrochromic performance of polymer.

Fig.3 Cyclic voltammogram curves of the pure PBTB film and the OP-PBTB film at a scan rate of 125 mV·s-1

Fig.4 Spectroelectrochemical behavior of pure PBTB film(a) and OP-PBTB film(b)on ITO/glass under various potentials in 0.1 mol·L-1TBAP/ACN solution
Electrochromic optical contrast and switching time are two of the important parameters of electrochromic materials.As shown in Fig.5(a,b),the pure PBTB film exhibits 36%and 20%of optical contrast at 620 and 1100 nm,respectively.The OP-PBTB film presents a similar optical contrast of 32%and 17%at the corresponding wavelengths.It can be speculated that the slight decline in optical contrast may be attributed to two aspects.One is that the actual thickness of OP-PBTB film is thinner than the pure PBTB film due to the porous structure,especially in the middle of holes.The other is the OP-PBTBfilm′srough surface caused by ordered porous structure,and it enhances Bragg scattering.12
Generally,the switching time,defined as the time measured at 95%of full contrast between the colored and bleached states,is monitored at the maximum absorption peaks.From Fig.5(c,d),we can see that the blenched time(tb)and colored time(tc)of the pure PBTB film are 1.0 and 3.6 s at 1100 nm,1.6 and 4.5 s at 620 nm, while the tband tcof the OP-PBTB film are 0.5 and 3.1 s at 1100 nm,0.7 and 3.8 s at 620 nm respectively at the corresponding wavelength(Fig.5(e,f)).The significant improvement of switching speed should be ascribed to the ordered porous nanostructure causing shorter diffusion distance and faster ion transport speed in the polymer film.
To further investigate the underlying causes of the enhanced switching speed,the electrochemical impedance spectra of the compact PBTB film and OP-PBTB film are performed(Fig.6)in ACN solution containing 0.1 mol·L-1TBAP as supporting electrolyte.The impedance spectra can be explained on the basis of an equivalent circuit with electrolyte resistance(Re),charge transfer resistance(Rct),and Warburg impedance(Zw).In the impedance plots,the initial intercept of the spectrum at theZ′axis in high frequency corresponds to Re.The semicircle at low impedance frequencies represents Rct,while the straight line at low frequencies indicates Zw,which depicts the diffusion-controlled process.As shown in Fig.6,the semicircle diameter of the OPPBTB film is 32.3 Ω,which is much lower than that of the pure PBTB film(125.2 Ω),which indicates that the OP-PBTB film has much lower charge transfer resistance.Therefore,it can be concluded that the ordered porous structure can facilitate counterions transport from the surface of polymer film to the anode,which leads to faster switching speed compared with the pure PBTB film.And the detailed mechanism is given as followed.
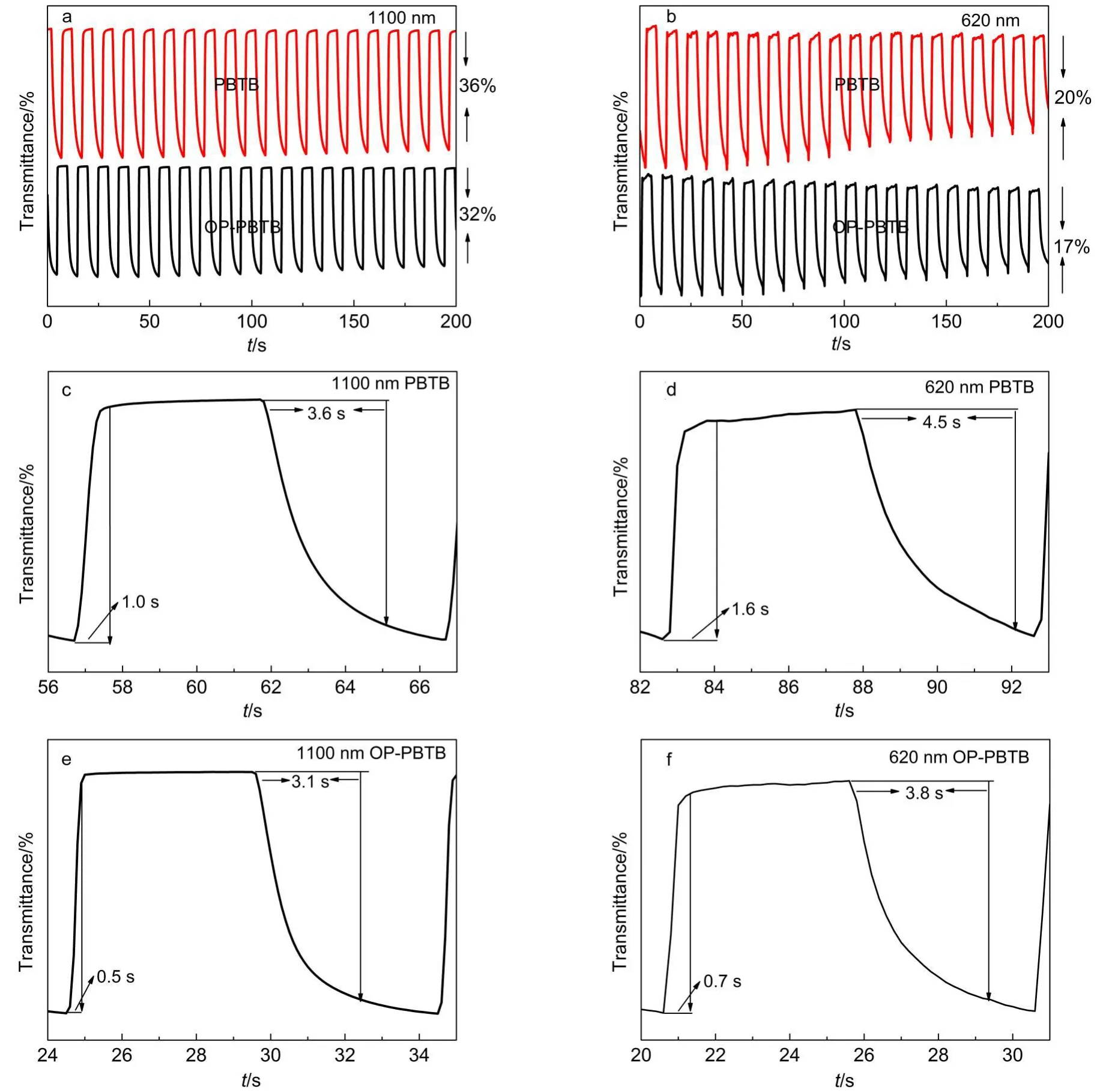
Fig.5 Optical contrast monitored at various absorption wavelengths of(a)1100 nm,(b)620 nm;the bleached and colored time of pure PBTB(c,d)and OP-PBTB film(e,f)at 1100 and 620 nm

Fig.6 Electrochemical impedance spectra of the pure PBTB film and the OP-PBTB film
As well-known,the switching time,which represents the intercalation/extraction rate of the counterions,is decided by two main factors:one is the diffusion coefficient,which is determined by thematerials′chemical structure;another one is the distance of diffusion path limited by the microstructure.Therefore,a relatively plausible solution to decrease the switching time is tailoring the polymer structure,for example,introducing the porous structure to polymer film.As applied potential,the counterions in the polymer film move toward to the anode along any directions in various paths(straight line,twisting routine,and other forms). Fig.7a exhibits the shortest diffusion distance for the counterions (ClO4-)transporting across the polymer layer to the anode,which is namely the thickness of the pure PBTB film.However,with the introducing of ordered porous structure into PBTB film via PAS template,the counterions can transport more easily across the space between polymer film(Fig.7b).Therefore,it can be concluded that,in OP-PBTB film,the ion diffusion distance is significantly shortened and the ion transport between doped andundoped states becomes more rapid,which leads to faster electrochromic switching speed.
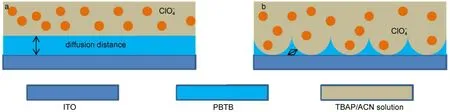
Fig.7 Schematic of counterion transport in the film
4 Conclusions
The PBTB film with highly ordered porous nanostructure using PAS monolayer as template was successfully fabricated via electropolymerization method.The obtained ordered porous polymer(OP-PBTB)film presents reversible color changes(yellow and purple)between the doping/dedoping states.Interestingly,the OP-PBTB film presents faster switching speed(bleached and colored time:0.5 and 3.1 s at 1100 nm,0.7 and 3.8 s at 620 nm, respectively)than that of pure PBTB film.In addition,the electrochemical impedance spectra indicate that the OP-PBTB film has a relatively lower charge transfer resistance,which illustrates that the ion has faster diffusion rate with the introduction of porous structure.It can be speculated that the introduction of ordered porous structure significantly shortens counterion diffusion distance,and the ion transport rate is relatively fastened.In summary, this work provides a convenient method for preparing porous polymer electrochromic materials with enhanced performance.
(1)Song,Y.Y.;Gao,Z.D.;Wang,J.H.;Xi,X.H.;Lynch,R.Adv. Funct.Mater.2011,21,1941.doi:10.1002/adfm.201002258
(2)Scherer,M.R.J.;Steiner,U.Nano Lett.2013,13,3005. doi:org/10.1021/nl303833h
(3)Mortimer,R.J.;Dyer,A.L.;Reynolds,J.R.Displays2006,27, 2.doi:10.1016/j.displa.2005.03.003
(4)Gazotti,W.A.;Casalbore-Miceli,G.;Geri,A.;Berlin,A.;De Paoli,M.A.Adv.Mater.1998,10,1522.doi:10.1002/(SICI) 1521-4095(199812)
(5)Heuer,H.W.;Wehrmann,R.;Kirchmeyer,S.Adv.Funct.Mater.2002,12,89.doi:10.1002/1616-3028(20020201)
(6)Zhang,X.P.;Zhang,H.K.;Li,Q.;Luo,H.L.IEEE Electron Device Lett.2000,21,215.doi:10.1109/55.841300
(7)Beaupre,S.;Breton,A.C.;Dumas,J.;Leclerc,M.Chem.Mater.2009,21,1504.doi:10.1021/cm802941e
(8)Barik,S.;Navarathne,D.;LeBorgne,M.;Skene,W.G.J.Mater. Chem.C2013,1,5508.doi:10.1039/c3tc30494a
(9)Ouyang,M.;Fu,Z.Y.;Lü,X.J.;Chen,H.L.;Hu,B.;Xia,X.F. Zhang,C.Acta Phys.-Chim.Sin.2013,29(5),996. [欧阳密,付志艳,吕晓静,陈欢乐,胡 彬,夏旭峰,张 诚.物理化学学报,2013,29(5),996.]doi:10.3866/PKU.WHXB201302282
(10)Ouyang,M.;Wang,P.J.;Yu,W.Y.;Huang,S.B.;Sun,J.W.; Hu,B.;Lv,X.J.;Fu,Z.Y.;Zhang,C.J.Electrochem.Soc.2014,161,H337.doi:10.1149/2.076405jes
(11)Lv,X.J.;Hu,B.;Sun,J.W.;Ouyang,M.;Yu,C.H.;Fu,Z.Y.; Zhang,C.J.Electrochem.Soc.2013,160,H87.doi:10.1149/ 2.034302jes
(12)Ge,D.T.;Yang,L.L.;Tong,Z.Q.;Ding,Y.B.;Xin,W.H.; Zhao,J.P.;Li,Y.Electrochim.Acta2013,104,191.doi: 10.1016/j.electacta.2013.04.102
(13)Xia,X.H.;Tu,J.P.;Zhang,J.;Xiang,J.Y.;Wang,X.L.;Zhao, X.B.ACS Appl.Mater.Interfaces2010,2,186.doi:10.1021/ am900636g
(14)Zang,J.;Li,C.M.;Bao,S.J.;Cui,X.;Bao,Q.;Sun,C.Q. Macromolecules2008,41,7053.doi:10.1021/ma801345k
(15)Chang,J.;Sun,J.;Xu,C.H.;Xu,H.;Gao,L.Nanoscale2012,4,6786.doi:10.1039/c2nr31725g
(16)Yu,X.;Zhao,Z.L.;Nie,W.;Deng,R.H.;Liu,S.Q.;Liang,R. J.;Zhu,J.T.;Ji,X.L.Langmuir2011,27,10265.doi:10.1021/ la201944s
(17)Okada,K.;Nandi,M.;Maruyama,J.;Oka,T.;Tsujimoto,T.; Kondoh,K.;Uyama,H.Chem.Commun.2011,47,7422.doi: 10.1039/c1cc12402a
(18)Lu,G.W.;Li,C.;Shi,G.Q.Polymer2006,47,1778.doi: 10.1016/j.polymer.2006.01.081
(19)Lehoux,A.;Ramos,L.;Beaunier,P.;Uribe,D.B.;Dieudonné, P.;Audonnet,F.;Etcheberry,A.;José-Yacaman,M.;Remita,H. Adv.Funct.Mater.2012,22,4900.doi:10.1002/ adfm.201200666
(20)Pal,N.;Bhaumik,A.Adv.Colloid Interface Sci.2013,189-190, 21.doi:10.1016/j.cis.2012.12.002
(21)Xu,C.;Zeng,Y.;Rui,X.H.;Xiao,N.;Zhu,J.X.;Zhang,W.Y.; Chen,J.;Liu,W.L.;Tan,H.T.;Hng,H.H.;Yan,Q.Y.ACS Nano2012,6,4713.doi:10.1021/nn2045714
(22)Zhang,J.;Tu,J.P.;Cai,G.F.;Du,G.H.;Wang,X.L.;Liu,P.C. Electrochim.Acta2013,99,1.doi:10.1016/j. electacta.2013.03.099
(23)Yuan,Y.F.;Xia,X.H.;Wu,J.B.;Chen,Y.B.;Yang,J.L.;Guo, S.Y.Electrochim.Acta2011,56,1208.doi:10.1016/j.electacta.2010.10.097
(24)Sumida,T.;Wada,Y.;Kitamura,T.;Yanagida,S.Chem. Commun.2000,1613.doi:10.1039/b003261l
(25)Zhang,T.;Qian,J.;Tuo,X.L.;Yuan,J.;Wang,X.G.Colloid. Sur.A-Physicochem.Eng.Asp.2009,335,202.doi:10.1016/j. colsurfa.2008.11.022
(26)Wagata,H.;Fujisawa,M.;Mizuno,Y.;Zettsu,N.;Oishi,S.; Teshima,K.Cryst.Growth Des.2013,13,3294.doi:10.1021/ cg400714e
(27)Wang,X.D.;Dong,P.;Chen,S.L.Acta Phys.-Chim.Sin.2006,22(7),831.[王晓冬,董 鹏,陈胜利.物理化学学报,2006,22(7),831.]doi:10.1016/S1872-1508(06)60035-1
(28)Abidian,M.R.;Kim,D.H.;Martin,D.C.Adv.Mater.2006,18, 405.doi:10.1002/adma.200501726
(29)Bartlett,P.N.;Birkin,P.R.;Ghanem,M.A.;Toh,C.S.J.Mater. Chem.2001,11,849.doi:10.1039/b006992m
(30)He,X.M.;Li,C.;Chen,F.G.;Shi,G.Q.Adv.Funct.Mater.2007,17,2911.doi:10.1021/ar7002094
(31)Cho,S.I.;Lee,S.B.Accounts Chem.Res.2008,41,699.doi: 10.1021/ar7002094.
(32)Poinern,G.E.J.;Le,X.;Loomes,C.;Fawcett,D.Mater.Lett.2014,131,182.doi:10.1016/j.matlet.2014.05.108
(33)Surawathanawises,K.;Cheng,X.H.Electrochim.Acta2014,117,498.doi:10.1016/j.electacta.2013.11.144
(34)Yang,M.J.;Zhang,Q.H.;Wu,P.;Ye,H.;Liu,X.Polymer2005,46,6266.doi:10.1016/j.polymer.2005.05.054
(35)Ng,S.C.;Xu,J.M.;Chan,H.S.O.;Fujii,A.;Yoshino,K. J.Mater.Chem.1999,9,381.doi:10.1039/A807329E
(36)Li,C.;Imae,T.Macromolecules2004,37,2411.doi:10.1021/ ma035188w
(37)Zhang,C.;Hua,C.;Wang,G.H.;Ouyang,M.;Ma,C.A. Electrochim.Acta2010,55,4103.doi:10.1016/j. electacta.2010.02.086
Enhanced Electrochromic Performance of Ordered Porous Monolayer PBTB Film Using Amine-Modified Polystyrene Spheres as a Template
OUYANG Mi1HUANG Sen-Biao1HAN Yan-Gang2LÜXiao-Jing1YANG Yuan1DAI Yu-Yu1LÜYao-Kang1,*ZHANG Cheng1,*
(1College of Chemical Engineering,Zhejiang University of Technology,Hangzhou 310014,P.R.China;2Zhejiang Institute of Quality Inspection Science,Hangzhou 310018,P.R.China)
Poly(1,4-bis(2-thienyl)-benzene)(PBTB)with a highly ordered porous nanostructure was successfully made using monolayer amine-modified polystyrene(PAS)as template in an electropolymerization method.The ordered porous polymer(OP-PBTB)film obtained can exhibit reversible color changes(yellow and purple)between the doping/dedoping states.Compared with the switching time of pure PBTB(colored time(tc) and bleached time(tb):1.0 and 3.6 s at 1100 nm;1.6 and 4.5 s at 620 nm),the switching time of OP-PBTB film is significantly shortened to 0.5 s(tc)and 3.1 s(tb)at 1100 nm,and 0.7 s(tc)and 3.8 s(tb)at 620 nm with the introduction of the porous nanostructure.In addition,the electrochemical impedance spectra(EIS)show that the ordered porous nanostructure offers OP-PBTB film a relatively low charge transfer resistance due to the larger surface area and shorter ion diffusion distance.This leads to higher intercalation/extraction capacities and faster ion diffusion speed,thus further shortening the switching time.Therefore,an effective way to improve the electrochromic performance of conducting polymers may be to construct ordered nanostructures using PAS as a template.
10.3866/PKU.WHXB201501121www.whxb.pku.edu.cn
O646
Received:November 14,2014;Revised:January 12,2015;Published on Web:January 12,2015.
∗Corresponding authors.ZHANG Cheng,Email:czhang@zjut.edu.cn,Tel:+86-571-88320508.LÜYao-Kang,Email:Yaokanglv@zjut.edu.cn.
The project was supported by the International Science and Technology Cooperation Program,China(2012DFA51210)and National Natural Science Foundation of China(51203138,51273179).
国际科技合作计划(2012DFA51210)和国家自然科学基金(51203138,51273179)资助项目
