Formation of Uniform Oil-Soluble Fe3O4 Nanoparticles via Oil-Water Interface System
2015-12-20XUEJingcheng薛精诚WANGHuiZHENGXueshuang郑学双WUYingZHOUXingping周兴平
XUE Jingcheng(薛精诚),WANG Hui(王 会),ZHENG Xueshuang(郑学双),WU Ying(吴 瑛),2,ZHOU Xingping(周兴平)*
1College of Chemistry,Chemical Engineering and Biotechnology,Donghua University,Shanghai 201620,China
2College of Life Science,Tarim University,Alar 843300,China
Introduction
Fe3O4nanoparticles(NPs),possessing unique physical and chemical properties,show a lot of sparking traits in comparison with the conventional magnetic materials.It is widely applied in the fields of bio-separation[1],biomedicine[2-3], hyperthermia[4-5], bio-labels[6], etc.Nowadays,due to their promising prospects on practical applications,many studies have focused on the synthesis of Fe3O4[7-8].So far,a wide variety of methods have been developed to produce Fe3O4NPs,such as high temperature pyrolysis[9],chemical co-precipitation method[10],gel-sol method[11]and microemulsion method[12].However,high temperature pyrolysis requires complex reaction conditions and has a certain toxicity.The uniformity of the products obtained by co-precipitation method was unsatisfactory,limiting the usage of Fe3O4NPs in practical application.The products synthesized by gel-sol method are easy to aggregate,causing many defective sites on their surfaces.The products prepared from microemulsion have some excellent properties,but the output is relatively low with a high cost.
Oil-water interface method was employed firstly to prepare QDs by Alexander et al.[13]and Kasuyaet al.[14]In Alexander's work,uniform CdS NPs sized as about 3.0nm in a cubic crystal structure were prepared successfully.By the same method,highly luminescing and monodispersed CdSe QDs were also successfully prepared by Kasuya et al.[14]In the previous work,the oil-water interface method has been employed to prepare TiO2NPs[15]and ZnS NPs[16]and the possible formation mechanism by this method has been proposed that the adsorption of surfactant on the nuclei plays a crucial role in determining their formation.
In the novel oil-water interface method,which is somewhat similar to that of upper-phase microemulsion,oil and water phases coexist,and the reactants exist in different phases.By controlling the reaction conditions,nucleation and growth can only occur at the oil-water interface.In the presence of surfactant,when the nucleation happens,the pre-formed nuclei are capped by the surfactant to form a hydrophobic surface and then spontaneously enter into the oil phase to stop growing[16].Hereafter,the growth period could be shortened and the size distribution of products could be narrowed.Therefore, nearly monodispersed nanoparticles sized usually as several nanometers can be formed by the oil-water interface method,with a high output in comparison with that from microemulsion due to the existence of a large amount of both oil and water including reactants.Meanwhile,the surface defect sites of the product decrease markedly,owing to the vital decrease of free cations and anions in oil phase by comparison with that in water phase.Distinguishing from the defects of the other methods above,the novel oil-water method can avoid the shortcomings and combine the advantages successfully such as mild reaction conditions,low cost,narrow size distribution and good oil-solubility.
Additionally,effects of reaction conditions,nucleation and growth on size and shape control of Fe3O4NPs formed by coprecipitation,gel-sol method, hydrothermal method and microemulsion method have been fully investigated[17-23],which is very useful and significant to the preparation of Fe3O4NPs from the oil-water interface method.In this work,reaction conditions such as NH3·H2O concentration,amount of oleic acid,reaction temperature and time were altered to investigate their effects on the properties of Fe3O4NPs.The as-prepared Fe3O4NPs owned the advantages of good oil-solubility and narrow size-distribution under mild reaction conditions and simple operations.More importantly,the formation mechanism of Fe3O4NPs synthesized by oil-water interface method was firstly proposed on the bases of those of oil-soluble TiO2and ZnS NPs.
1 Experimental
1.1 Materials
Absolute alcohol(AR,99.7%),aqueous ammonia(CP,97%),ferric sulfate(AR,99%),cyclohexane(AR,99.5%),oleic acid(CP,97%),and sodium stearate(CP,98%)were purchased from Sinopharm Chemical Reagent Co.,Ltd.(Shanghai,China)Green copperas(AR,99%)was purchased from Shanghai Gongxuetuan No.2Experiment Factory(China).
1.2 Synthesis of oil-soluble Fe3O4 NPs
Some amount of sodium stearate was firstly added into a three-necked flask containing about 60 mL of de-ionized water.Then ferric sulfate solution was injected into the flask and reacted for 2h.The upper oil phase was filtered by step funnel and washed by hot water for three times,and then dried at 80 ℃for 24h.
The standard conditions for preparing Fe3O4NPs by the oilwater interface method were established as follows[8-11].The above prepared ferric stearate was dissolved in 40 mL of cyclohexane to achieve 0.067mol/L solution.Then 5mL oleic acid was injected into the three-necked flask with vigorous stirring to form the oil phase.Ferrous sulfate was dissolved into de-ionized water and injected into the flask with stirring for 20min.The molar ratio of ferric stearate to ferrous sulfate was 2∶1.After that,30mL NH3·H2O of 4.5mol/L was gradually transferred into the oil-water mixture and the system was maintained at 80℃for 2h.The upper oil phase was washed centrifugally by the solvent of ethanol:water(v/v)4∶1mixture for three times and oil-soluble Fe3O4nanoparticles were obtained by drying the centrifugal products at 60 ℃for 24h.
2 Results and Discussion
2.1 Characterization of Fe3O4 NPs
2.1.1 Transmission electron microscopy(TEM)and X-ray diffraction(XRD)
Figure 1(a)displays the TEM image of as-synthesized oil-soluble Fe3O4NPs under the standard conditions.As can be seen,the prepared Fe3O4NPs were spherical-like with an average size of 3.6nm.The relative average derivation was 18.9%based on the calculation for about 100particles.The capping of oleic acid on the surface makes the Fe3O4NPs well-dispersed with a uniform morphology.Figure 1(b)shows the XRD profile,suggesting the production of Fe3O4NPs successfully.
2.1.2 Vibrating sample magnetometer(VSM)
The presence of magnetization is proven by the measurement of superconducting quantum interference device magnetometer at room temperature as shown in Fig.2.The magnetite remanence was an S-like curve,while the saturation magnetization was 0.865 emu·g-1with the coercivity of 0 Oe which demonstrated the ferromagnetic behavior of the Fe3O4NPs.The obtained Fe3O4NPs exhibited some certain of deficiency for the relatively weak ferromagnetic behavior compared with other methods.
2.1.3 Fourier transform infrared spectroscopy(FT-IR)
Figure 3 exhibits the FT-IR spectrum of Fe3O4NPs obtained under the standard conditions.The characteristic peak corresponding to the stretching vibration of Fe—O bond was 591.74cm-1and the peak at 1 534.44cm-1attributing to the characteristic stretching vibration of the carbonyl unit demonstrated that oleic acid was successfully combined to the surface of Fe3O4NPs.In addition,the 3 445.98cm-1peak may relate to the OH-of unmoved water,resulting from air-slake during the storing.
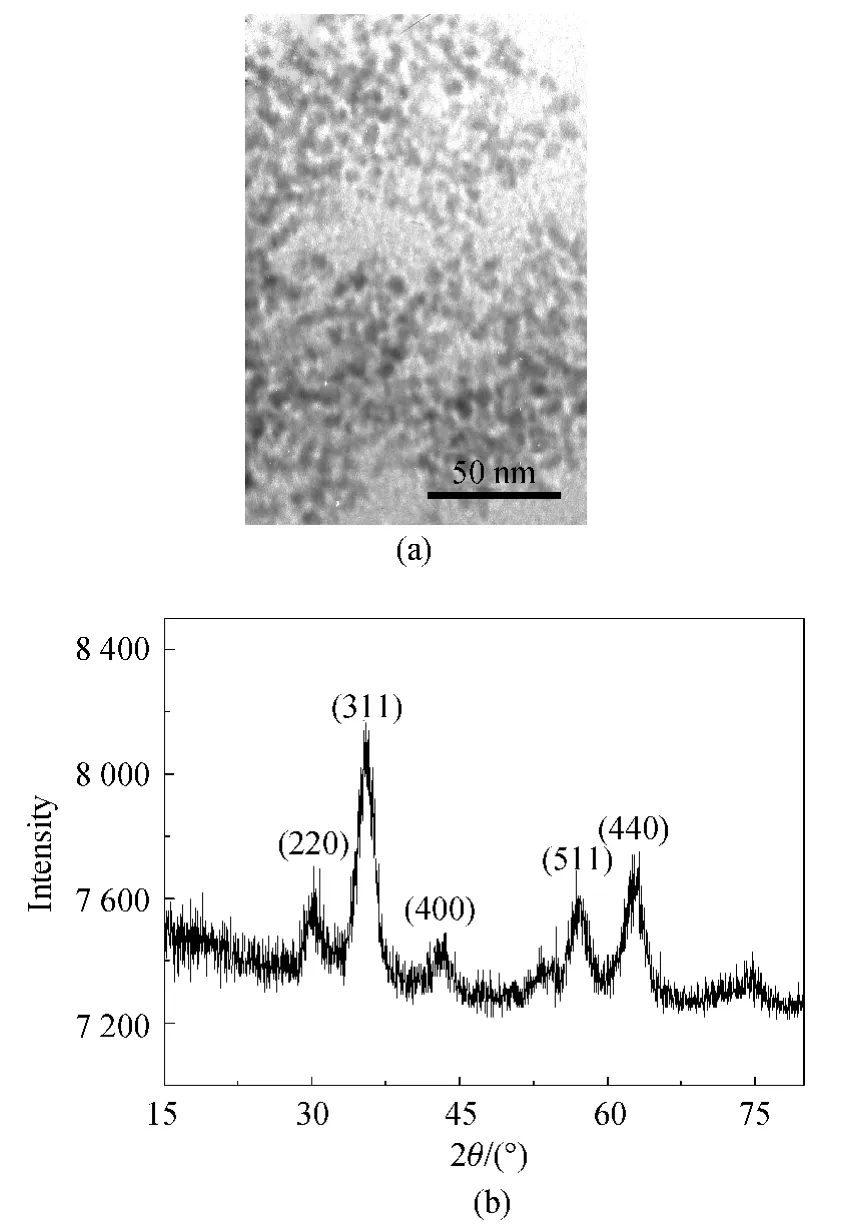
Fig.1 TEM image(a)and XRD profile(b)of the oil-soluble Fe3 O4 NPs
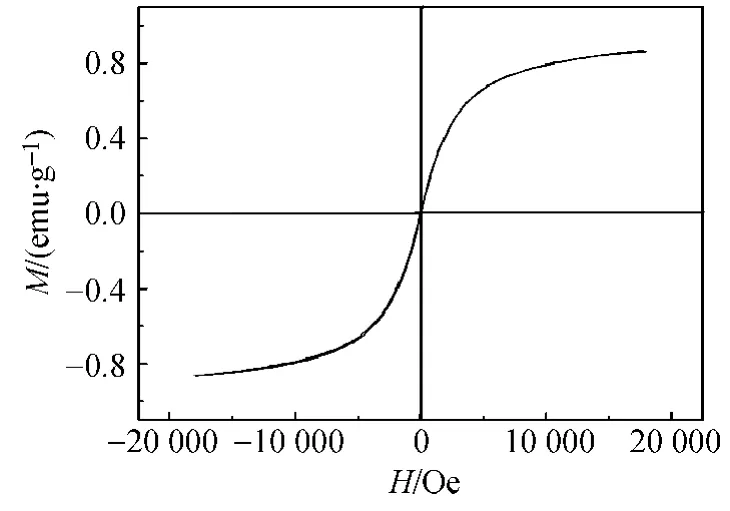
Fig.2 Magnetic hysteresis curve of Fe3O4 NPs
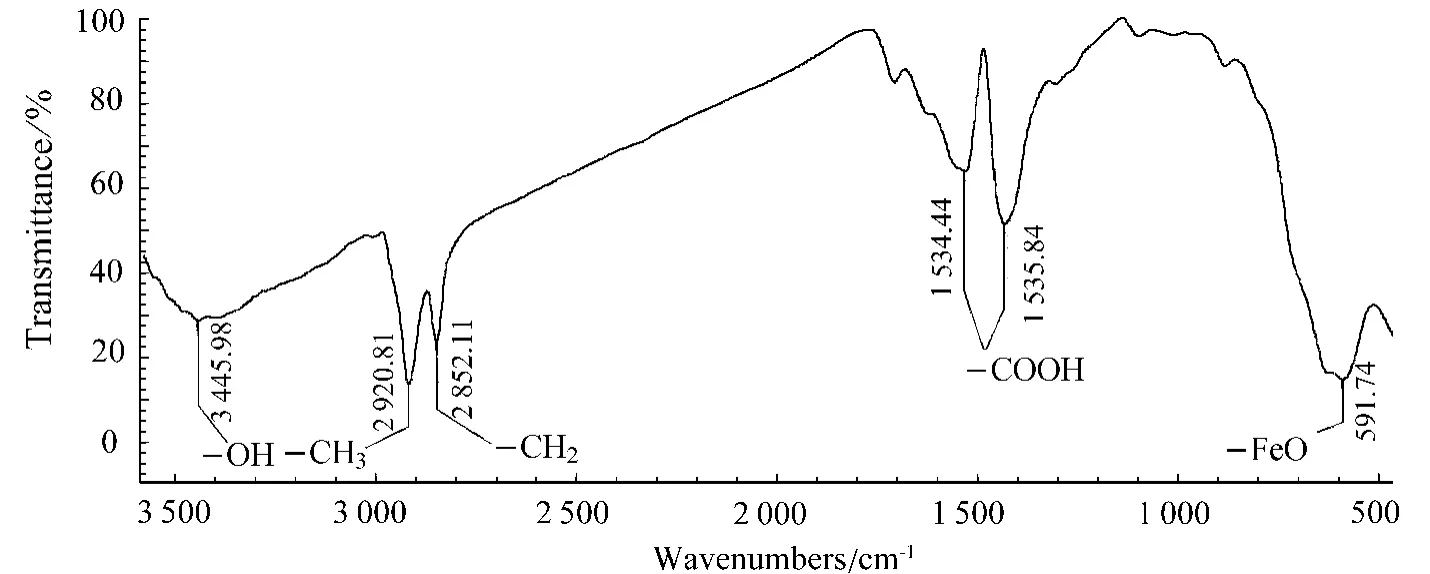
Fig.3 FT-IR spectrum of Fe3O4 NPs
2.1.4 Thermogravimetric analyzer(TGA)
TGA measurement is performed for a typical product as showed in Fig.4together with that of the commercial Fe3O4.The weight loss of Fe3O4NPs was a four-stage process.During the first stage(30-240℃),the loss of weight was mainly caused by the evaporation of water,ethanol,and cyclohexane.In the second stage(300-400 ℃),containing the boiling point of oleic acid,the evaporation of the free oleic acid led to a loss of 11%of the total weight.In the third stage(700-790℃),the weight loss of 6%was corresponding to that of the oleic acid closely combined with Fe3O4NPs.During the final stage(820-900 ℃),the curve rebounded slightly due to the partially oxidizing of Fe3O4.
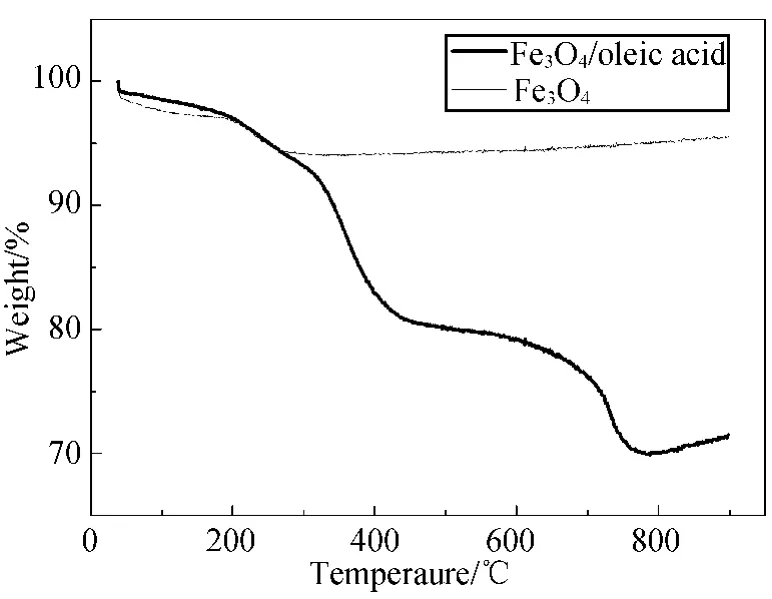
Fig.4 TGA curves of Fe3O4NPs
2.2 Effects of reaction conditions on synthesizing oil-soluble Fe3O4
2.2.1 Effect of NH3·H2O concentration
Figure 5(a)demonstrates XRD profiles of solid products obtained under different concentrations of NH3·H2O.The products were changed,accompanied by the changing of NH3·H2O concentration.An unstable intermediate product was firstly formed and could transform into two kinds of substances according to different conditions.The product was mainlyα-FeOOH particles when the concentration of NH3·H2O was lower than 3.0mol/L.While higher concentration of NH3·H2O promoted the products changed to be Fe3O4NPs.The reason may be due to the existence of different precursory complexes for the formation ofα-FeOOH and Fe3O4NPs and the excessive NH3·H2O accelerated the formation of Fe3O4NPs from α-FeOOH.
The increase in NH3·H2O concentration also resulted in the slightly decreased size of Fe3O4NPs for more crystal nuclei were probably produced as shown in Fig.5(b).As obtained from their TEM images,the particles were spherical-like with the sizes ofα-FeOOH and Fe3O4NPs ranged in 6.9-4.4and 3.9-3.1nm,respectively.NaOH was also used to synthesize Fe3O4NPs but the products were of poor uniformity and dispersion,which was probably due to the too strong alkalinity.
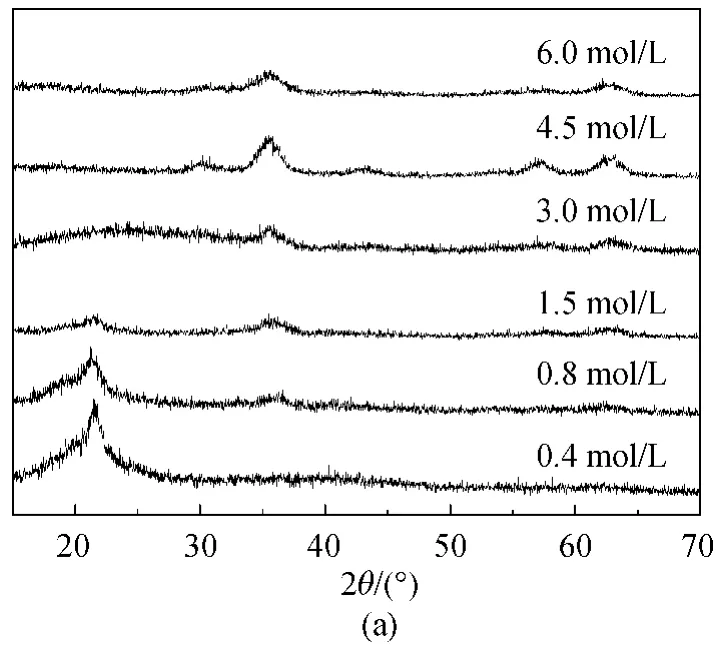
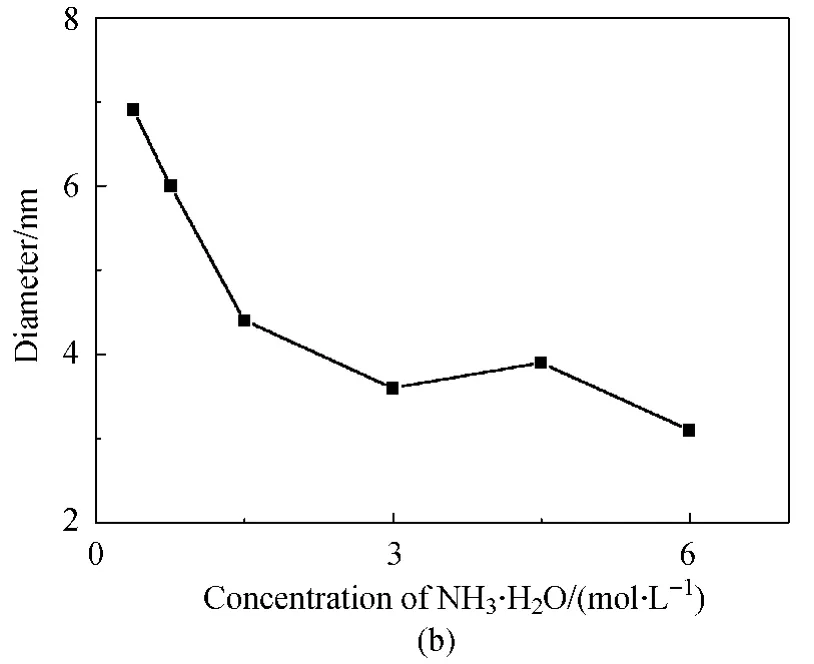
Fig.5 Products of different concentration of NH3·H2O:(a)XRD profiles and(b)size change
2.2.2 Effect of oleic acid amount
The size of Fe3O4NPs decreased at first and then increased with the increase of oleic acid amount as shown in Fig.6.In the beginning,increased amount of oleic acid led to its faster and stronger adsorption on the growing particles,causing their earlier entering into the oil phase and then stopping the growth.Thereafter,size of the formed Fe3O4decreased with the increasing amount of oleic acid.Additionally,with the further increase of oleic acid,more oleate ions were produced,which was combined to the Fe2+in water phase and led to the decrease of free Fe2+ions,resulting in the inhibited nucleation.Therefore,the size of the obtained Fe3O4NPs increased gradually.
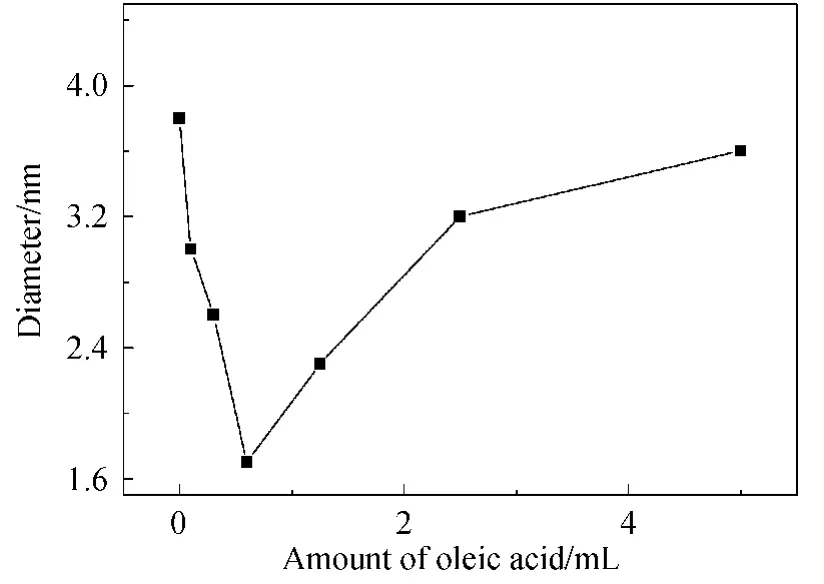
Fig.6 Size change of the products with different amount of oleic acid
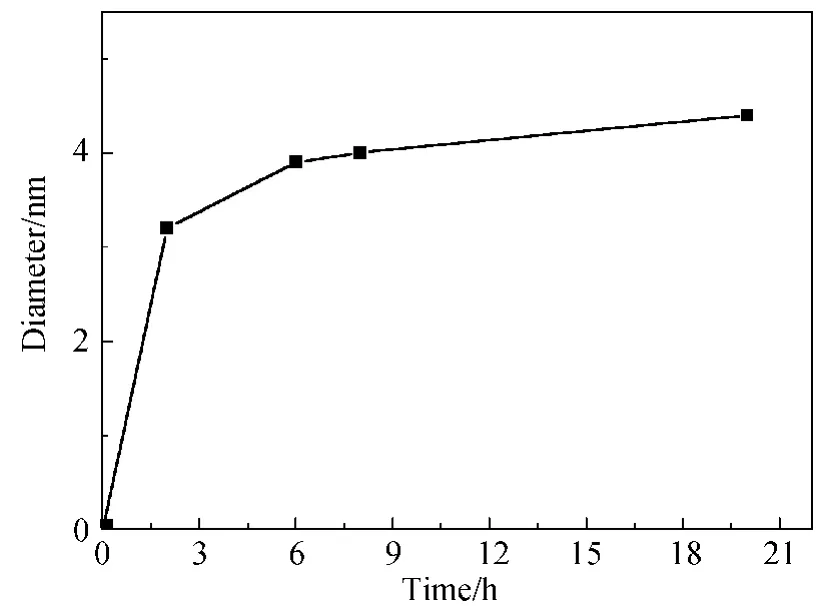
Fig.7 Size change of Fe3O4NPs of time evolution
2.2.3 Time evolution of Fe3O4NPs As shown in Fig.7,the time longer than 6hdid not have much influence on the growing of Fe3O4NPs with the size between 3.8-4.4nm which went incidence with the TEM results.The NPs formed on the oil-water interface were rapidly capped by oleic acid,causing the controlled growth of Fe3O4NPs.
2.2.4 Effect of temperature
Fe3O4NPs were also prepared under different temperatures,but no obvious size distinction was found as shown in Table 1.

Table 1 Effect of temperature on the size of Fe3O4 NPs
The reason may be that the reactions happen in the oil-water interface,and the temperature only exerts little influence on the formation and adsorption of oleic acid,as the surfactant adsorption plays an important role in the production of monodispersed inorganic NPs.In addition,the more uniform Fe3O4NPs were obtained at the temperature of 80 ℃for the competition between the adsorption of surfactant and the particle growth.The Fe3O4NPs capped by the surfactant of oleic acid entered into the oil phase swiftly,and then the more uniform Fe3O4NPs were produced at 80 ℃.
2.3 Formation mechanism of Fe3O4 NPs in the oilwater interface
The previous studies on the formation of TiO2and ZnS NPs in the oil-water interface have laid deep foundation on the formation mechanism of Fe3O4NPs[17-18].As shown in Fig.8,on the surface of the nuclei,there is a competition between the adsorption of the surfactant and the deposition of Fe3+and Fe2+.After the nucleation takes place,the adsorption of surfactant on the nuclei in oil-water interface goes in two ways,the fast adsorption and the slow adsorption.By the fast one,the nuclei are capped by the surfactant as early as the nucleation occurs in oilwater interface and subsequently enter into oil phase to stop growing and mono-dispersed Fe3O4NPs are produced.By the slow one,the nuclei grow by the deposition of reactants,along with the surfactant adsorption on their surfaces.Once the surfactant adsorption is sufficient to minimize their surface Gibbs free energy,the particles spontaneously enter into the oil phase and then the particle growth stops.Therefore,relatively poly-dispersed Fe3O4NPs are generated as the nucleation takes place continually through the reaction period.
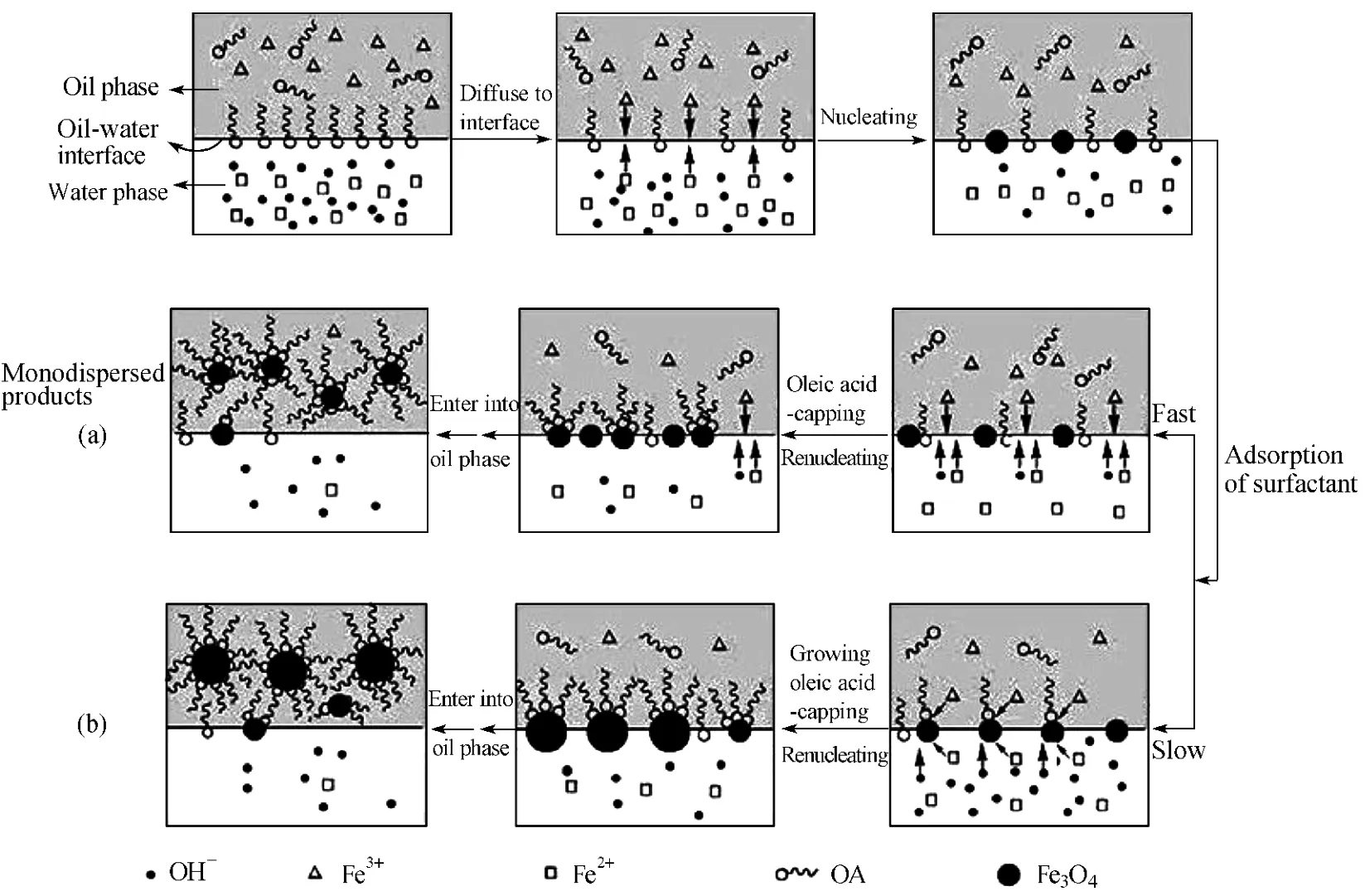
Fig.8 Formation mechanism of Fe3O4 NPs in the oil-water interface
3 Conclusions
Uniform oil-soluble Fe3O4NPs were successfully synthesized by the novel oil-water interface method.The obtained Fe3O4NPs were of an average particle size of 3.9 nm.TEM images and XRD profiles revealed that the most suitable reaction concentration of NH3·H2O,oleic acid/water in volume,reaction temperature and reaction time were 4.5mol/L,50∶1 000,80 ℃and 6 h,respectively.VSM showed that the synthesized Fe3O4NPs were superparamagetic and the saturation magnetization was 0.865 emu·g-1.TGA proved that oleic acid was combined to the surface of Fe3O4NPs closely.The outer loose capping and the inner close capping of oleic acid were 11% and 6%,respectively.The formation mechanism of the nearly monodispersed Fe3O4NPs by the oil-water interface method was proposed to be that the pre-formed Fe3O4nuclei were capped by oleic acid as early as the nucleation occurred and subsequently entered into oil phase to stop growing.Compared with the modification of Fe3O4NPs after aqueousphase synthesis and the other methods,the oil-water interface method exhibits great advantages in low cost,cheap raw materials and good oil-solubility products despite of the defect of a relatively weak ferromagnetic behavior.
[1]Park H Y,Schadt M J,Wang L Y,et al.Fabrication of Magnetic Core@Shell Fe Oxide@ Au Nanoparticles for Interfacial Bioactivity and Bio-separation [J].Langmuir,2007,23(17):9050-9056.
[2]Wang X Q,Tu Q,Zhao B,et al.Effects of Poly(l-lysine)-modified Fe3O4Nanoparticles on Endogenous Reactive Oxygen Species in Cancer Stem Cells[J].Biomaterials,2013,34(4):1155-1169.
[3]Yang X W,Jiang W,Li L,et al.One-Step Hydrothermal Synthesis of Highly Water-Soluble Secondary Structural Fe3O4Nanoparticles [J].Journal of Magnetism and Magnetic Materials,2012,324(14):2249-2257.
[4]Wang Y M,Cao X,Liu G H,et al.Synthesis of Fe3O4Magnetic Fluid Used for Magnetic Resonance Imaging and Hyperthermia [J].Journal of Magnetism and Magnetic Materials,2011,323(23):2953-2959.
[5]Zhao D L,Zeng X W,Xia Q S,et al.Inductive Heat Property of Fe3O4Nanoparticles in AC Magnetic Field for Local Hyperthermia[J].Rare Metals,2006,25(6):621-625.
[6]Joseph J,Nishad K K,Sharma M,et al.Fe3O4and CdS Based Bifunctional Core-Shell Nanostructure[J].Materials Research Bulletin,2012,47(6):1471-1477.
[7]Gunay M,Kavas H,Baykal A.Simple Polyol Route to Synthesize Heptanoic Acid Coated Magnetite (Fe3O4)Nanoparticles[J].Materials Research Bulletin,2013,48(3):1296-1303.
[8]Hong R Y,Pan T T,Li H Z.Microwave Synthesis of Magnetic Fe3O4Nanoparticles Used as a Precursor of Nanocomposites and Ferrofluids[J].Journal of Magnetism and Magnetic Materials,2006,303(1):60-68.
[9]Chiu W S,Radiman S,Abdullah M H.One Pot Synthesis of Monodisperse Fe3O4Nanocrystals by Pyrolysis Reaction of Organometallic Compound [J].Materials Chemistry and Physics,2007,106(2/3):231-235.
[10]Liu Y S,Liu P,Su Z X,et al.Attapulgite-Fe3O4Magnetic Nanoparticles via Co-precipitation Technique [J].Applied Surface Science,2008,255(5):2020-2025.
[11]Lemine O M,Omri K,Zhang B,et al.Sol-Gel Synthesis of 8 nm Magnetite (Fe3O4)Nanoparticles and Their Magnetic Properties[J].Superlattices and Microstructures,2012,52(4):793-799.
[12]Lu T, Wang J H,Yin J.Surfactant Effects on the Microstructures of Fe3O4Nanoparticles Synthesized by Microemulsion Method [J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2013,436(5):675-683.
[13]Alexander N K,Svetlana A V,Anatoly I L,et al.Optical Properties of Cadmium Sulfide Colloidal Dispersions Prepared by Interphase Synthesis[J].Optical Materials,2008,30(8):1304-1309.
[14]Kasuya A,Sivamohan R,Barnakov Y A,et al.Ultra-stable Nanoparticles of CdSe Revealed from Mass Spectrometry[J].Nature Materials,2004,3(2):99-102.
[15]Huang X Y,Liu Y G,Zhou X P,et al.Formation of Oil-Soluble Uniform Anatase Titania Nanoparticles and Their Characterization [J ].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2013,423(20):115-123.
[16]Du Y X,Zhou X P,Liu Y,et al.Synthesis and Properties of ZnS Quantum Dots by an Oil-Water Interface Method [J].Journal of Nanoscience and Nanotechnology,2012,12(11):8487-8493.
[17]Fan Y H,Ma C H,Li W G,et al.Synthesis and Properties of Fe3O4/SiO2/TiO2Nanocomposites by Hydrothermal Synthetic Method[J].Materials Science in Semiconductor Processing,2012,15(5):582-585.
[18]Ren L L,Huang S,Fan W,et al.One-Step Preparation of Hierarchical Superparamagnetic Iron Oxide/Graphene Composites via Hydrothermal Method [J].Applied Surface Science,2011,258(3):1132-1138.
[19]Liu Y S,Liu P,Su Z X,et al.Attapulgite-Fe3O4Magnetic Nanoparticles via Co-precipitation Technique [J].Applied Surface Science,2008,255(5):2020-2025.
[20]Meng J H,Yang G Q,Yan L M,et al.Synthesis and Characterization of Magnetic Nanometer Pigment Fe3O4[J].Dyes and Pigments,2005,66(2):109-113.
[21]Shen Y F,Tang J,Nie Z H,et al.Tailoring Size and Structural Distortion of Fe3O4Nanoparticles for the Purification of Contaminated Water [J].Bioresource Technology,2009,100(18):4139-4146.
[22]Lemine O M,Omri K,Zhang B,et al.Sol-Gel Synthesis of 8 nm Magnetite (Fe3O4)Nanoparticles and Their Magnetic Properties[J].Superlattices and Microstructures,2012,52(4):793-799.
[23]Tang N J,Zhong W,Jiang H Y,et al.Nanostructured Magnetite(Fe3O4)Thin Films Prepared by Sol-Gel Method[J].Journal of Magnetism and Magnetic Materials,2004,282:92-95.
猜你喜欢
杂志排行
Journal of Donghua University(English Edition)的其它文章
- A Motivation Framework to Promote Knowledge Translation in Healthcare
- Measurement and Evaluation of Pilot Mental Workload Based on Flight Simulation System
- A Dependent-Chance ProgrammingModel for Proactive Scheduling
- Analysis of Co3O4/ Mildly Oxidized Graphite Oxide (mGO )Nanocomposites of Mild Oxidation Degree for the Removal of Acid Orange 7
- Two Types of Adaptive Generalized Synchronization of Chaotic Systems
- Ontology-Based Semantic Multi-agent Framework for Micro-grids with Cyber Physical Concept
