Mapping of QTLs for Dehiscence Length at Basal Part of Thecae in Rice Based on TD70/Kasalath RIL Population
2015-12-14LingZHAOYadongZHANGChunfangZHAOLihuiZHOUShuYAOXinYUDanDINGTsutomuMATSUICailinWANG
Ling ZHAO, Yadong ZHANG, Chunfang ZHAO, Lihui ZHOU, Shu YAO, Xin YU, Dan DING,Tsutomu MATSUI, Cailin WANG*
1. Institute of Food Crops, Jiangsu Academy of Agricultural Sciences/Jiangsu High Quality Rice R&D Center/Nanjing Branch of China National Center for Rice Improvement, Nanjing 210014, China;
2. Faculty of Applied Biological Science, Gifu University, Gifu 501-2354, Japan
In recent years, the frequent occurrence of disasters caused by global warming and high temperature has resulted in serous loss to rice production in China, even all over the world. Particularly high temperature at booting and flowering stages significantly decreases rice yield and quality. In-depth study on rice tolerance to high temperature will provide theoretical basis for the development of rice cultivars with high-temperature tolerance at flowering stage.High-temperature tolerance is a quantitative trait which is difficult to be identified directly or indirectly. Direct identification of rice tolerance in field under natural high-temperature conditions is affected by various factors and hardly repeatable. The indirect method identified rice tolerance under simulated high-temperature conditions in greenhouse or artificial climate chamber, is accurate and repeatable, but expensive equipment was needed.Appropriate indices for evaluating rice tolerance to high temperature are important in both direct and indirect approaches. Among the various indices proposed in previous studies to determine high-temperature tolerance of rice at flowering stage, seed setting rate is the mostly used one[1]. Since it is difficult to identify seed setting rate under high-temperature conditions,some other indices such as the stress index of seed setting rate, percentage of unfilled grains, weight of filled grains, percentage of whole-kernel milled rice, chalkiness, the stress index of protein content, as well as the chlorophyll content, the free proline content, the ABA content and the pollen viability under high temperatureconditions were then proposed[2-3].Many QTL loci related to rice tolerance to high temperature have been identified based on different indices[4-11], but it is difficult to apply these indices directly in rice breeding. Therefore, it is urgent to find some indexes which closely related to high-temperature tolerance and easy to be identified at normal temperature for rice breeding.
The correlation between the length of dehiscence at the basal part of thecae (LDBT) and high-temperature tolerance was confirmed by Matsui et al.[12-13],and the studies revealed that a large LDBT improved the seed setting rate of rice under high-temperature conditions (Fig.1). Subsequent studies showed that LDBT was a stable trait at both normal and high temperatures, and thus could be used as an index to identify rice tolerance to high temperature at flowering stage under normal temperature conditions[14-16].Three traits LDBT,flowering period and panicle temperature were found probably to be related to rice fertility at high temperature by Zhao et al.[17]seedling setting under high-temperature conditions had no significant correlation with flowering period and panicle temperature, but significant positive correlation with LDBT. The study of Jagadish et al.[6]also confirmed the correlation between LDBT and high-temperature tolerance of rice at flowering stage.
In the present study, a population of recombinant inbred lines (RIL)with different LDBT was generated and their linkage map including 141 SSR markers was used to locate the QTLs controlling LDBT, with an attempt to reveal the correlations between LDBT and other agronomic traits and thus provide a theoretical basis for the study of high-temperature tolerance of rice at flowering stage.
Materials and Methods
Materials
A combination was made between an Indica cultivar Kasalath and a Japonica cultivar TD70 which was derived from Swan Valley///9520//(72-496/Suyunuo).In the summer of 2005,a population of 240 recombinant inbred lines(RIL)was obtained from the F1generation by single seed descent breeding. In 2012, the 240 F6:7RILs and their parents were sown in the experimental field of Institute of Food Crops, Jiangsu Academy of Agricultural Sciences. 30-day-old seedlings were transplanted into plots. One cultivar was planted in four rows with 10 seedlings in each row, in a plot.Seedling spacing was 26.7 cm between rows and 16.7 cm within rows.The plots were ranged at random with two repetitions. After maturity, five plants randomly selected from each plot were separately harvested.
Temperature measurement
The temperature at rice canopy was measured using a temperature and humidity monitor (HOBO U23-001) once every hour throughout the whole growth period.
Measurement items and methods
Measurement of LDBT of RILs Five representative plants of each line were selected for the measurement of LDBT using a digital microscope(VHX-500,Keyence Corporation,Osaka,Japan).The LDBT of five anthers from each floret was measured and the average was calculated as the LDBT of the floret. The average LDBT of five florets from a plant was considered as LDBT of the plant.In the same way, the average LDBT of five plants of a line was taken as the LDBT of the line.
Measurement of heading date of RILs The day when 50% plants in a plot already headed was considered as the heading date of the plot.For the calculation of correlation coefficients,this index was considered as the days from seed sowing to heading date.Heading period was defined as a period of 7 d from three days before heading date to three days after heading date.
Determination of agronomic traits of RILs The plant height, tiller number, flag leaf length, flag leaf width,seeding setting rate, kernel number,1 000-grain weight, grain width, grain length,grain thickness and awn length of five plants randomly selected from each plot were measured and the averages were taken for later analysis.
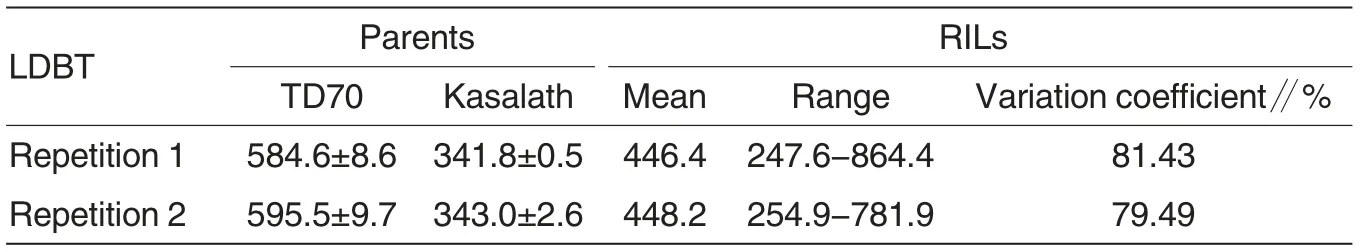
Table 1 Phenotypic variation of LDBT among RILs and two parents
Statistical analysis
Molecular linkage map of the 240 recombinant inbred lines (RIL) were derived from the cross between TD70 and Kasalath included 141 markers[18].QTL IciMapping3.4 software was adopted to scan the QTLs controlling LDBT within whole genome sequences by inclusive composite interval mapping (ICIM)[19]. The QTLs having a LOD value above 2.5 were recorded and named after the nomenclature of McCouch et al[20].
SPSS17.0 software was adopted for variance analysis and correlation analysis.
Results and Analysis
LDBT phenotypic variation among RILs and their parents
As shown in Table 1, the LDBTs of TD70 and Kasalath were 590.5 and 342.4 μm on average, exhibiting extremely significant difference between them. The LDBT of the 240 RILs in repetition ranged from 247.6 to 864.4 μm, with an average of 446.4 μm, and a coefficient of variation of 81.43%,while that in repletion 2 ranged from 254.9 to 781.9 μm, with an average of 448.2 μm and a coefficient of variation of 79.49% (Table 1). There was no significant difference between the two repetitions.
The results proved transgressive inheritance of LDBT in the RIL population.The LDBT showed a normal dis-tribution in the RILs, consistent with the inheritance of a quantitative trait(Fig.2).
QTLs controlling LDBT of rice
Two QTLs controlling LDBT were detected among the RIL population and fine mapped on chromosome 6 and 8, respectively (Fig.3). qLDBT6 was mapped within a 15.8-cM interval between RM3 and RM3628 markers on chromosome 6 with a LOD value of 3.23, contribution rate of 6.18% and additive effect of 17.74% . qLDBT8 was mapped within a 11.1-cM interval between RM1376 and RM4085 markers on chromosome 8 with a LOD value 2.95,contribution rate of 8.00%and additive effect of 20.09% . The increasing alleles at both QTL loci were from the female parent TD70 (Table 2).
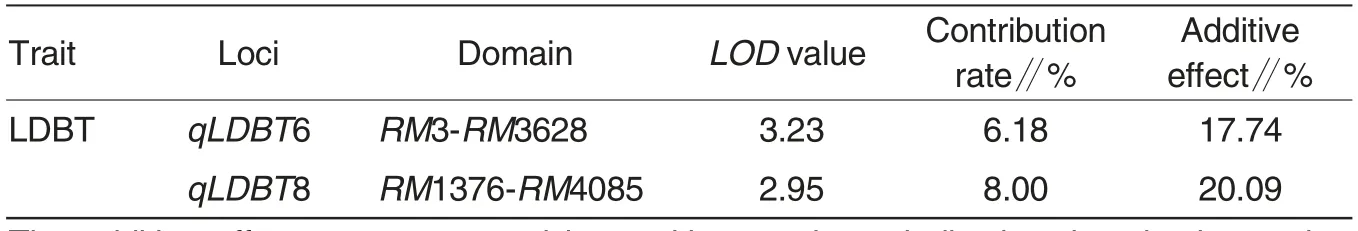
Table 2 Identification of QTLs for LDBT in RIL population
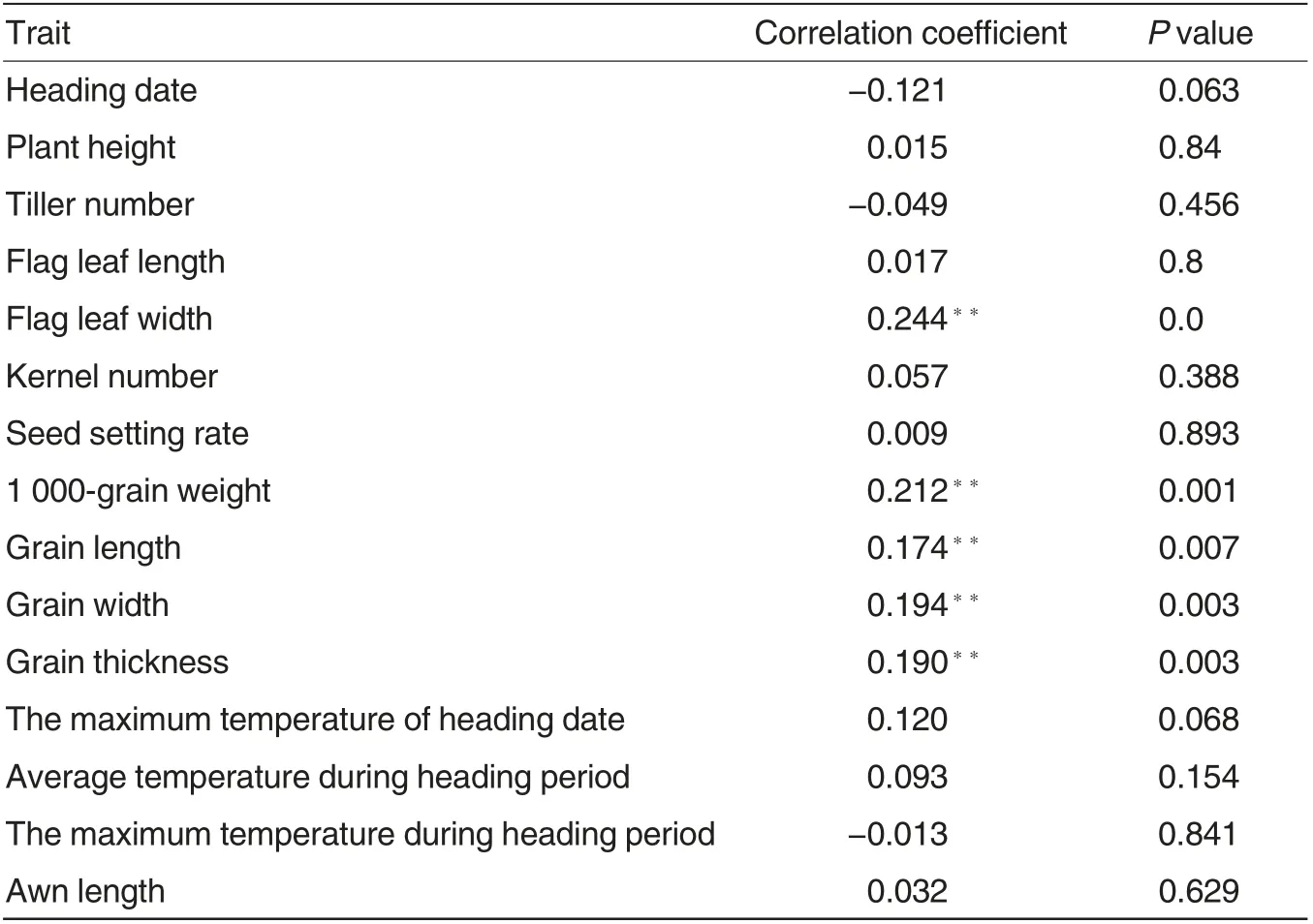
Table 3 Correlation coefficients between LDBT and agronomic trait
Temperature at late botting stage and flowering stage of RIL population
August 9 and 11 were the heading dates of two parents Kasalath and TD70,while the heading dates of RILs distributed from July 25 and September 9.The daily maximum temperature and the daily average temperature from July 22 to September 9, 2012 were shown in Fig.4. The daily maximum temperature and the daily average temperature during this period peaked on July 30, up to 32.34 and 37.18 ℃; they reduced to the lowest level on September 9, only 20.39 and 21.08 ℃, respectively. The daily average temperature was above 30 ℃in 14 d from July 19 to September 12,even exceeded 32 ℃in 3 d, and the daily maximum temperature exceeded 37 ℃in only one day.
Correlations between LDBT and other agronomic traits
The LDBT of RIL population shared extremely significant positive correlations with flag leaf width,1 000-grain weight, grain length, grain width and grain thickness, but no significant correlations with heading date, plant height, tiller number, flag leaf length,kernel number, seed setting rate, the maximum temperature of heading date, average temperature during heading period,the maximum temperature during heading period and awn length(Table 3).
Discussion
Heat stress has become one of the main factors affecting rice production now, it is important to learn rice tolerance on high temperature. Previous studies revealed that LDBT was correlated to high-temperature tolerance of rice,and proposed that it could be used as an index for evaluating rice tolerance to high-temperature. Indepth study on LDBT of rice will provide more theoretical basis for this hypothesis.
In the present study,qLDBT8 was fine mapped to a 1.28 -Mb domain between RM1376 and RM4085 markers on chromosome 8. According to the data from Graneme website, only four QTLs of rice had been mapped to this domain till January, 2014 (Fig.5).Among them, S8, a QTL locus related to rice sterility was detected in this domain using a F2 population of 240 lines generated from the cross between Peiai 64S and 8902S[21].S8 was fine mapped to a 0.57-Mb domain between RM885 and RM333s on chromosome 8, and it could be detected under natural long-day and short-day conditions, artificially simulated longday and high-temperature conditions,artificially simulated long-day and lowtemperature conditions,artificially simulated short-day and high-temperature conditions, indicating it was a stable QTL locus controlling rice sterility[22].As LDBT was proven to be related to rice fertility at high temperature, we speculated that the 1.28-Mb domain where qLDBT8 was located might closely related to rice sterility, which is expected to be studied in future.
A major QTL Ghd8 which controls rice heading period also locates in the same domain with qLDBT8 on chromosome 8, from 4 331 109 to 4 333 832 bp, encodes the HAP3H subunit of a protein binding to CCAAT box transcription factor[23]. Ghd8, allelic to Hd5, can regulate the heading period,yield and plant height of rice[24-25].In addition, there are a QTL for rice biomass production (qpw)and a QTL for quantitative resistance to Pyricularia grisea(rbr8)locating in a 2.7-Mb domain between RG333 and RM25[26-27].
Our early work proved that LDBT had certain correlation with seed setting rate of rice under high-temperature conditions. The existing studies about rice LDBT mostly focused on its correlations with high-temperature tolerance. The correlations between LDBT and other agronomic traits were analyzed for the first time in the present study. TD70 is a large-grain Japonica rice cultivar,with 1 000-grain weight up to 70 g.It has long and wide grains and large LDBT, and thus was considered as an perfect material for the study on rice LDBT. The study showed that there was no significant correlation between rice LDBT and seed setting rate, which may be related to the air temperature during heading period in the summer of 2012. Shi et al.[28]found that air temperature below 33 ℃had no significant influence on the seed setting rate of Huajing 1 and Teyou 559. By studying the effects of high temperature at flowering stage on rice yield, Shi et al.[29]discovered that compared with air temperature of 35 ℃, high temperature between 35 and 39 ℃had no significant influence on rice yield, while extreme temperature above 39 ℃significantly reduced rice yield by 13%. The study of Wang et al.[30]revealed that pollination of rice was affected if the daily average temperature at flowering stage exceeded 32 ℃. From July 25 to September 9, 2012, the daily average temperature exceeded 32 ℃in only 3 d,and the daily maximum temperature exceeded 37 ℃in only one day (July 30). Among the 240 RILs we tested,the heading dates of 16 lines were before August 5, and the maximum temperature in following days was below 37 ℃.The air temperature at flowering stage was not too high,which might be the reason why no significant correlation between LDBT and seed setting rate was observed among the RILs we tested.
[1]YANG YJ (杨永杰), FU GF (符冠富),XIONG J (熊杰), et al. Effects of high temperature on rice and evaluation on rice tolerance to high temperature(高温对水稻的影响及水稻耐热性测评方法研究)[J].China Rice(中国稻米),2012,18(1):39-40.
[2]HUANG YJ(黄英金),YANG ZY(杨芝燕),RAO ZM (饶志明),et al.Active oxygen damage effect and regulation of chlorophyll degradation in rice leaves at filling stage under high temperature stress(灌浆期高温胁迫下水稻叶片叶绿素降解的活性氧损伤及调控研究)[J].Acta Agriculturae Universitatis Jiangxiensis(江西农业大学学报),2000,22(5):1-6.
[3]LEI DY(雷东阳), CHEN LY(陈立云), LI WX (李稳香),et al. Effect of high temperature on physiology difference of flowering among different hybrid rice(高温对不同杂交稻开花期影响的生理差异) [J]. Research of Agricultural Modernization (农业现代化研究), 2005, 26(5):397-400.
[4]YANG TF (杨梯丰),ZHANG SH (张少红),WANG XF (王晓飞),et al.Screening for germplasm with heat tolerance at flowering stage in Oryza sativa (水稻抽穗开花期耐热种质资源的筛选鉴定)[J].Journal of South China Agricultural University(华南农业大学学报),2012,33(4):585-589.
[5]ZHANG GL, CHEN LY, XIAO GY, et al.Bulked segregant analysis to detect QTL related to heat tolerance in rice(Oryza sativa L.) using SSR markers[J]. Agricultural Sciences in China,2009,8(4):482-487.
[6]JAGADISH S, CAIRNS J, LAFITTE R,et al.Genetic analysis of heat tolerance at anthesis in rice [J]. Crop Science,2010,50:1633-1641.
[7]PAN Y(盘毅), LUO LH(罗丽华), DENG HB(邓化冰),et al.Quantitative trait Loci associated with pollen fertility under high temperature stress at flowering stage in rice (水稻开花期高温胁迫下的花粉育性QTL 定位)[J].Chinese Journal of Rice Science (中国水稻科学),2011,25(1):99-102.
[8]XIAO YH, PAN Y, LUO L. Quantitative trait loci associated with seed set under high temperature stress at the flowering stage in rice [J]. Euphytica, 2011, 178:331-338.
[9]YE CR, ARGAYOSO MA, REDONA ED, et al. Mapping QTL for heat tolerance at flowering stage in rice using SNP markers [J]. Plant Breeding, 2012,131:33-41.
[10]CHENG L, WANG JM, VERONICA U,et al.Genetic analysis of cold anthesis in rice tolerance at seedling stage and heat tolerance at anthesis in rice(Oryza sativa L.) [J]. Journal of Integrative Agriculture,2012,1(3):359-367.
[11]LEI DY,TAN LB,LIU FX,et al.Identification of heat-sensitive QTL derived from common wild rice (Oryza rufipogon Griff.) [J].Plant Science,2013,201-202:121-127.
[12]MATSUI T,OMASA K,HORIE T.High temperature at flowering inhibits swelling of pollen grains,a driving force for thecae dehiscence in rice(Oryza sativa L.)[J]. Plant Production Science,2000,3:430-434.
[13]MATSUI T,KOBAZSAI K,KAGATA H,et al. Correlation between viability of pollination and length of basal dehiscence of the theca in rice under a hot and humid condition[J].Plant Production Science,2005,8(2):109-114.
[14]MATSUI T. Function of long basal dehiscence of the thecae in rice(Oryza sativa L.)pollination under hot and humid condition [J]. Phyton, 2005, 45:401-407.
[15]MATSUI T, OMASA K, HORIE T. The difference in sterility due to high temperature during the flowering period among japonica-rice varieties [J].Plant Production Science,2001,4:90-93.
[16]TIAN X,MATSUI T,LI S,et al.Heat induced floret sterility of hybrid rice(Oryza sativa L.)cultivars under humid and low wind conditions in the field of Jianghan Basin, China [J]. Plant Production Science,2010,13:243-251.
[17]ZHAO L, KOBAYASI K, HASEGAWA T,et al.Traits responsible for variation in pollination and seed set among six rice cultivars grown in a miniature paddy field with free air at a hot, humid spot in China [J]. Agriculture, Ecosystems and Environment, 2010, 139:110-115.
[18]DONG SL(董少玲), ZHANG YH(张颖慧),ZHANG YD (张亚东),et al.Construction of molecular genetic linkage map based on a rice RIL population and detection of QTL for tiller angle(水稻重组自交系分子遗传图谱构建及分蘖角的QTL 检测)[J].Jiangsu Journal of Agricultural Sciences (江苏农业学报),2012,28(2):236-242.
[19]WANG JK (王建康).Inclusive composite interval mapping of quantitative trait genes (数量性状基因的完备区间作图方法) [J]. Acta Agronomica Sinica(作物学报),2009,35(2):239-245.
[20]MCCOUCH SR, CHO YG, YANO M,et al. Report on QTL nomenclature[J].Rice Genet Newsl,1997,14:11-13.
[21]HE Y Q, YANG J, XU CG, et al. Genetic bases of instability of male sterility and fertility reversibility in photoperiod-sensitive genic male-sterile rice[J].Theoretical and Applied Genetics,1999,99:683-693.
[22]WEI X J, XU J F, GUO H N, et al.DTH8 suppresses flowering in rice,influencing plant height and yield potential simultaneously [J]. Plant Physiology,2010,153(4):1747-1758.
[23]YAN W H,WANG P,CHEN H X, et al.A major QTL, Ghd8, plays pleiotropic roles in regulating grain productivity,plant height, and heading date in rice[J].Molecular Plant,2011,4 (2):319-330.
[24]DAI XD, DING YN, TAN L B, et al.LHD1, an allele of DTH8/Ghd8, controls late heading date in common wild rice(Oryza rufipogon)[J].Journal of Integrative Plant Biology, 2012, 54(10):790-799.
[25]SHIBAYA T, NONOUE Y, ONO N, et al. Genetic interactions involved in the inhibition of heading by heading date QTL,Hd2 in rice under long-day conditions [J]. Theoretical and Applied Genetics,2011,123:1133-1143.
[26]LIAN X, XING Y, YAN H, et al. QTLs for low nitrogen tolerance at seedling stage identified using a recombinant inbred line population derived from an elite rice hybrid [J]. Theoretical and Applied Genetics,2005,112:85-96.
[27]CHEN H, WANG S, XING Y, et al.Comparative analyses of genomic locations and race specificities of loci for quantitative resistance to Pyricularia grisea in rice and barley [J].Proceedings of the National Academy of Sciences of the United States of America,2003,100(5):2544-2549.
[28]SHI CL (石春林), JIN ZQ (金之庆),ZHENG JC(郑建初),et al.Quantitative analysis on the effects of high temperature at meiosis stage on seed-setting rate of rice florets (减数分裂期高温对水稻颖花结实率影响的定量分析) [J].Acta Agronomica Sinica (作物学报),2008,34(4):627-631.
[29]SHI KY(时宽玉),CUI YW(崔永伟),HU RF (胡瑞法).Impact of high temperature at flowering on midseason rice yield(水稻花期高温对产量的影响研究)[J].Journal of Agricultural Science and Technology(中国农业科技导报),2009,2:78-83.
[30]WANG CL(王才林),ZHONG WG(仲维功). Effects of high temperature on seed setting rate of rice and its prevention(高温对水稻结实率的影响及其防御对策) [J]. Jiangsu Agricultural Sciences(江苏农业科学),2004,1:15-18.
猜你喜欢
杂志排行
Agricultural Science & Technology的其它文章
- Effects of Specific Gravity-based Seed Grading on Seed Germination,Seedling Emergence and Grain Yield of Hybrid Rice
- Effects of NaCl Stress on Seed Germination of Four Canavium album Raeuseh Cultivars
- Application Effects of Ultra-fine Powder Shaped Maize Seed Coating Agent in Spring Sowing areas in northeast China
- Breeding and Application of a Japonic Rice Cytoplasmic Male Sterility Line,E-Jing A
- Effect of Low Temperature and Sparse Light Conditions on Cold Tolerance of Different Rice Lines at Seedling Stage
- Molecular Marker Assisted Selection for Fusarium Wilt Resistance Breeding in Watermelon(Citrullus lanatus)
