Breeding of Mycoplasmal pneumonia-negative Swine Population Using Combination Therapy,Segregated Early Weaning(SEW)and Three-point Production System
2015-12-13YuanGANLizhongHUAZhixinFENGQiyanXIONGMaojunLIUGaimeiDUQinghongMAAiquanWANGYannaWEIPingJIANGGuoqingSHAO
Yuan GAN, Lizhong HUA, Zhixin FENG, Qiyan XIONG, Maojun LIU, Gaimei DU,3, Qinghong MA, Aiquan WANG, Yanna WEI, Ping JIANG, Guoqing SHAO*
1. Institute of Veterinary Medicine, Jiangsu Academy of Agricultural Sciences/Key Laboratory of Veterinary Biological Engineering and Technology, Ministry of Agriculture/National Center for Engineering Research of Veterinary Bio-products, Nanjing 210014, China;
2. College of Veterinary Medicine, Nanjing Agricultural University, Nanjing 210095, China;
3. Department of Animal Science and Technology, Jinling Institute of Technology, Nanjing 210038, China;
4. Luhe Base of Animal Science, Jiangsu Academy of Agricultural Sciences, Nangjing 211501, China;
Mycoplasmal pneumonia of swine (MPS) is also known as swine enzootic pneumonia and pig asthma.It is a chronic respiratory disease caused by infection of Mycoplasma hyopneumoniae (Mhp).Mycoplasmal pneumonia is one of the world’s most important diseases in swine[1]. The main clinical features of Mycoplasmal pneumonia include cough, breathing and growth retarda tion[2]. Mhp can be spread through di rect contact or aerosol[3-4], and its spreading distance is up to 4.7 km[5],so Mhp is extremely difficult to prevent and control. In US farms, although 45%of the vaccination costs are used to buy inactivated mycoplasma pneumonia vaccine, the mycoplasma pneumonia of swine still cannot be controlled completely. In recent years,the intensive and large-scale pig industry has developed rapidly in China.Due to the absence of management,insufficient emphasis on biosafety,high farm density, high breeding density and frequent transportation of pigs, the risks of spreading and infection of Mhp are greatly increased, reducing feed conversion, extending the growth period of pigs, leading to the abuse of antibiotics, increasing the costs of pig farming and threatening food safety, thereby resulting in huge losses in pig industry. How to effectively control Mycoplasmal pneumonia of swine has troubled the global pig industry. Studies have shown that thebest way to control the disease is to purify thoroughly the pathogens at the sources.
As early in 1970s, Juhr et al. reported the control method of Mycoplasmal pneumonia in experimental animals[6]. During late 1970s to early 1980s, Alerander also developed MEW technology, a new therapeutic weaning technology to control pig diseases. In the early 1990s, Dritz developed the segregated early weaning(SEW) technology for US pig industry.Currently, SEW has been promoted in 60%of US herds,and it has also been primarily applied in Japan and Taiwan and Guangdong Provinces of China.Ji et al.found that SEW reduced the positive rate of Mycoplasma hyopneumoniae by 68%[7]. It suggests that SEW technology has certain control effect on Mycoplasmal pneumonia of swine,but it cannot completely purify the pathogen, and it requires the auxiliary of other techniques. In later domestic and foreign researches, a number of new purification methods were developed for Mycoplasma hyopneumoniae, including Swiss populationreducing method, programmed therapy, segregated early weaning and population isolation[8].In many farms of Western Europe, tiamulin hydrogen famarate,along with Swiss populationreducing method, is often used to control Mycoplasmal pneumonia of swine. Alfonso et al. successfully purify the Mycoplasma hyopneumoniae in a farm containing 1 700 sows using SEW and multi-point production model[9].For the control methods mentioned above,the most critical key is to develop scientific and rational purification measures.
Aiming at the increasingly grim situation of the control of Mycoplasma hyopneumoniae and referring to previous studies, the control methods of combination therapy, SEW and threepoint production system were combined based on the study results about Mycoplasma hyopneumoniae control and its pathogenic mechanism in this study so as to breed Mycoplasmal pneumonia-negative swine population.The aim of this study was to control and purify Mycoplasmal pneumonia of swine at the sources through providing Mycoplasmal pneumonia-negative breeding pigs for healthy nuclear breeding population, thereby providing theoretical basis and practical experience for domestic control and purification of Mycoplasmal pneumonia.
Materials and Methods
Materials
Main instruments and equipment The barrier system consisted of positive-pressure animal room and isolation feeder (Laboratory Animal Center of Jiangsu Academy of Agricultural Sciences). The other instruments and equipment included 7 500 real-time fluorescent quantitative PCR (Life Technologies Inc., US), sterile milk tank, excreta disposal system and suckling sterile transportation boxes.
Main reagents The Classical Swine Fever Virus Antibody ELISA Kit,PPRS ELISA Kit,Porcine Pseudorabies Virus gE Antibody ELISA Kit and Mycoplasma hyopneumoniae ELISA Kit were purchased from the IDEXX (US)Biotechnology Company. The Porcine Circovirus Type 2 Antibody Test Kit(ELISA) was purchased from the Wuhan Keqian Animal Biological Products Co., Ltd. The FMDV Antibody Detection ELISA Kit was purchased from the JBT Corporation(Korea). The Premix Ex TaqTMwas purchased from the TaKaRa Biotechnology(Dalian)Co.,Ltd.
Experimental animal Several sows carrying antigen and antibody of Mycoplasma hyopneumoniae at the fourth (or more) pregnancy and their piglets were selected from a farm in Changzhou. A total of five batches of sows and piglets were selected.
Strains and plasmid vector The Mhp strains were preserved by the Major Livestock Diseases Research Team of Institute of Veterinary Medicine, Jiangsu Academy of Agricultural Sciences. The recombinant plasmid pMD18-T/P97 was also prepared by the Major Livestock Diseases Research Team of Institute of Veterinary Medicine, Jiangsu Academy of Agricultural Sciences.
Main feed and drugs The commercial formula and creep feed were purchased from the Anyou(Jiangsu)Tech Feed Co., Ltd. The 80% tiamulin hydrogen famarate and doxycycline hydrochloride were purchased from the Shanghai Novartis Animal Health Co.,Ltd. The compound multiplex vitamin of electrolyte, formaldehyde, povidone iodine, 2% chloroacetic acid, lime and anhydrous dextrose were all conventional drugs and reagents.
Methods
Site selection and biosafety management of three-point production model The three-point production system[10-12]and some SPF management techniques[13]were adopted in this study. The A location was Mycoplasmal pneumonia-positive farm, B location was the positive-pressure sterile animal house and isolation feeder, and C location was Mycoplasmal pneumonia-negative farm with excellent geographic isolation. The latest study has shown that the spreading distance of Mycoplasma hyopneumoniae is up to 4.7 km[5]. So the straightline distances among the three locations should be not less than 5 km.Moreover, there were no other breeding farms or slaughterhouses 5 km from the selected locations, and the three farms were all farm from the traffic routes and high-mobility regions,avoiding introducing new pathogens,thereby establishing an integrated biosecurity system for feeding and management(Fig.1).
Screening of sows from Mycoplasmal pneumonia-positive A farm and prenatal pre-treatment The blood and nose swab samples of pregnant sows were collected from certain farms. Then the Mycoplasma hyopneumoniae antigen and antibody-positive sow population was screened by fluorescent quantitative PCR and ELISA. The source of experimental animal was determined. The Mycoplasmal pneumonia-positive farm was defined as A farm. The sows at late pregnancy were selected, and 20 d before their delivery, the 80% tiamulin hydrogen famarate (125 g/t),doxycycline hydrochloride (150 g/t)and amoxicillin (200 g/t) were started to be fed to the selected sows. After two weeks, the drug application was stopped.The delivery room was disinfected with povidone iodine with a dilution of 500 times three times a day.Before transferred to the disinfected delivery room,the sows were also disinfected. The newly-born piglets werecared more carefully. In the A farm, a total of 10 piglets, delivered by the sows that had not been treated by foregoing procedures, were selected randomly from each of the batches to be treated as control.For the pigs from the treatment and control groups, the labeling,sample collection and antigen and antibody detection (monitoring)were simultaneous.
Breeding and management of piglets obtained from SEW in barrier isolation system of B farm The 7-d-old healthy piglets were transferred with the sterile transportation box from the A farm to the B location which was farm from the mother source. The breeding environment of B location was barrier room installed with air filtration device. The temperature and humidity in the barrier room were also strictly controlled. In addition, the daily management for isolation breeding of piglets was also strictly implemented. During the population transfer and breeding,disinfection was strictly carried out and biosecurity should be taken good care of. According to feeding and management procedures, the daily care, vaccination, iron supplementary, zinc supplementary and ear number were carried out.During the feeding, Mycoplasma?hyopneumoniae-sensitive antibiotics,Nanny milk (1 L, Anyou) or creep feed (1 kg) mixed with 80% tiamulin hydrogen famarate (1 g) and compound multiplex vitamin of electrolyte (4 g), were used for purification. All the piglets were transferred to the C location 28 d later.
Isolation breeding of piglets in C farm Based on the antigen and antibody detection (monitoring) results,the 35-d-old healthy piglets were selected from the B location and transferred to the C location with sterile transportation box. In the C location,the piglets were bred by experienced staff isolatedly. During the population transfer and breeding,disinfection was strictly carried out and biosecurity should be taken good care of. According to feeding and management procedures, the daily care and vaccination were better carried out.
Development and implementation of purification program for specific drugs For all the breeding pigs(breeding boars and breeding sows)in the A farm, the feed (1 t) was mixed with 80% tiamulin hydrogen famarate(125 g), 15% chlortetracycline (2 000 g) and compound multiplex vitamin of electrolyte (400 g). The drugs were started to be feed to the pregnant sows 20 d before their delivery, and the drug application lasted for 2 weeks. In the B location, the piglets were fed Daimuru (1 L) or creep feed(1 kg) mixed with 80% tiamulin hydrogen famarate (1 g) and compound multiplex vitamin of electrolyte (4 g)until their transferring to the C location.For all the piglets in the C location,the feed(1 t)was mixed with 80%tiamulin hydrogen famarate (125 g) and compound multiplex vitamin of electrolyte(400 g) till 7 d after their transferring,and then the drugs were fed in the first 7 d of each month. For the propagation-using breeding pigs, the drug application was interrupted one week before their delivery or mating.
Serum antibody detection of Mycoplasma hyopneumoniae The blood samples of pigs in the control group of A farm, B farm and C farm were collected at the ages of 21, 35,75 and 120 d. The Mycoplasma?hyopneumoniae-specific antibody in the serum was detected using ELISA(IDEXX,US).The Mycoplasma?hyopneumoniae antibody-positive swine population was eliminated. The Mycoplasma hyopneumoniae-specific antibody was detected every two months. If one Mycoplasma hyopneumoniae antibody-positive pig was found, the entire nest would be eliminated.In addition,the pigs were strictly vaccinated against CSFV,PPRSV and PRV. If pigs were found not to meet the requirements, their vaccination should be enhanced or they were eliminated directly.
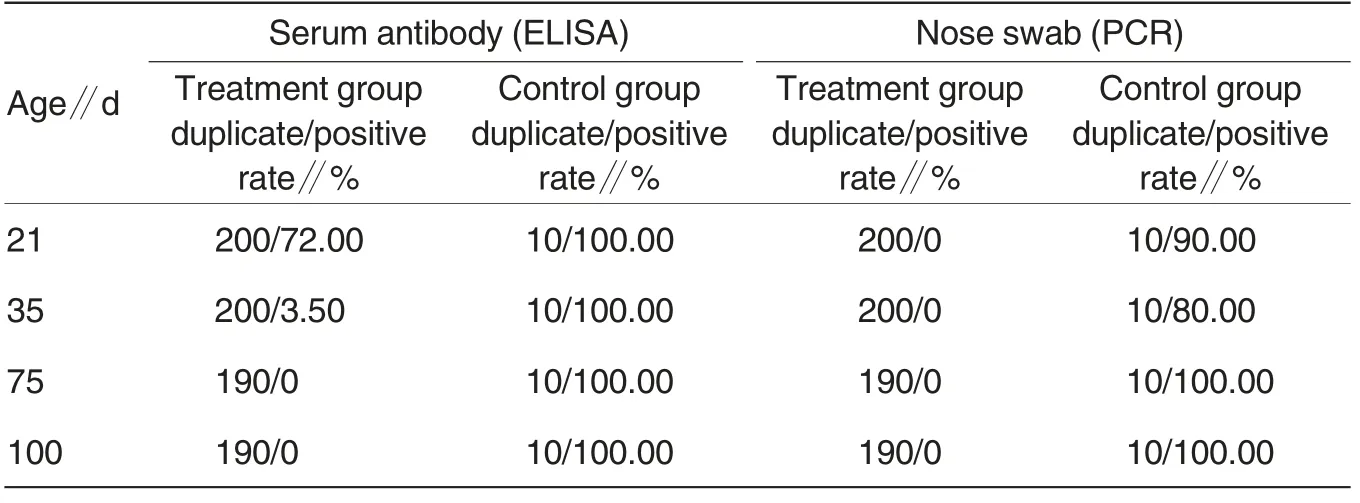
Table 1 Statistical results of Mhp antigen and antibody detection (monitoring) in swine populations of treatment and control groups
Detection of Mycoplasma hyopneumoniae in nose swab samples The nose swab samples of pigs were collected at the ages of 21, 35, 75 and 120 d. They were first pre-treated[14],and the Mycoplasma hyopneumoniae in the collected nose swab samples was detected by fluorescence quantitative PCR[15]. The Mycoplasma hyopneumoniae-positive swine population was eliminated. In the future, the Mycoplasma hyopneumoniae was detected every two months. If one Mycoplasma hyopneumoniae-positive pig was found, the entire nest would be eliminated.
Daily management The breeding and management parameters of pigs were recorded every day. The serum antibody and nose swan antigen detection results were processed regularly. The purification record was established.For every time of population transfer, the pigs were all in all out.The breeding environment was thoroughly cleaned and disinfected regularly every week, and the three kinds of disinfectant were used alternatively.The rodent, mosquitos and pests in swine populations were controlled regularly.
Purification method of Mycoplasma hyopneumoniae The purification method of Mycoplasma hyopneumoniae in swine population was shown in Fig.1.
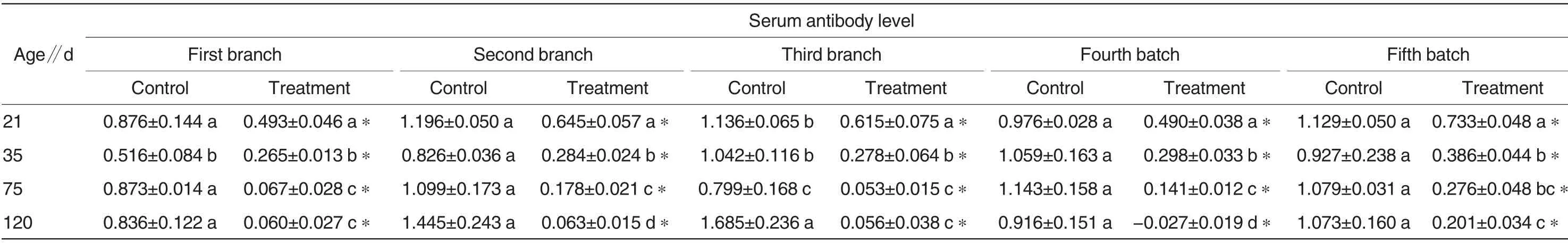
Table 2 Effect of local purification method on serum Mhp antibody level in five-batch swine populations in treatment and control groups
Results and Analysis
Antigen detection results of nose swab samples in treatment and control groups
As shown in Table 1, the antigen detection results showed that all the nose swab samples in the treatment group were antigen (Mycoplasma hyopneumoniae)-negative, but the nose swan samples in the control group were all antigen-positive. It indicated that the purification method can effectively cut off the horizontal and vertical transmission of mycoplasma pathogen.
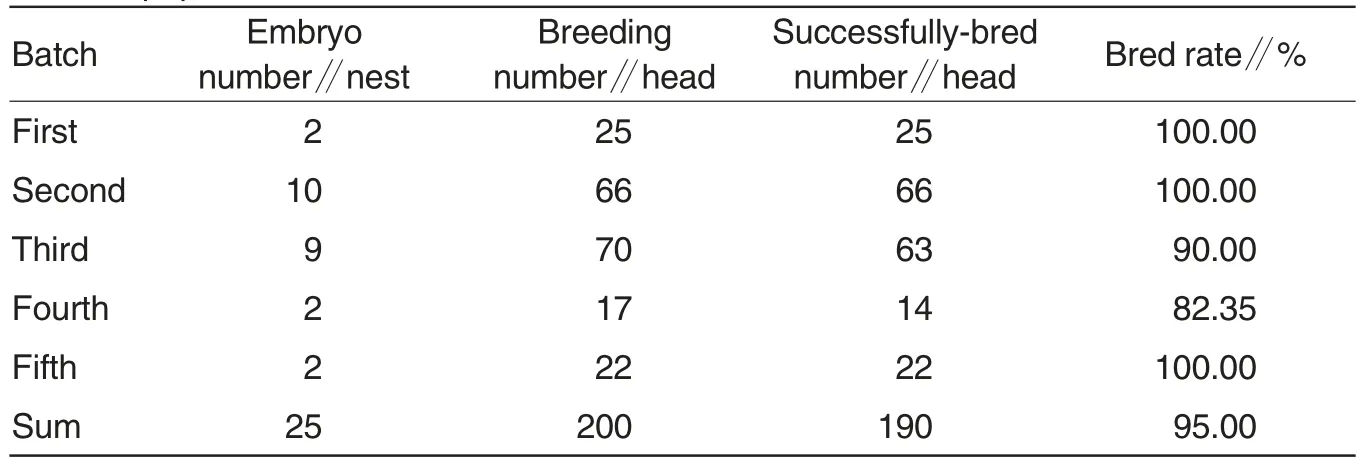
Table 3 Production statistics of newly-bred Mycoplasma hyopneumoniae-negative swine population
Antibody detection results of serum samples in treatment and control groups
The serum Mhp antibody levels in the five-batch pigs in the treatment and control groups were determined(Table 2). There were basically no significant differences in serum Mhp antibody level among differently aged pigs in the control group within the same batch (P<0.05), and at the same age within the same batch,the serum Mhp antibody level in the treatment group was significantly lower than that in the control group (P <0.05). It suggested that Mhp infection always existed inpigs in the control groups. In addition,the serum antibody levels in the 21-dold pigs were significantly higher than those in the other pigs aging 35, 75 and 120 d, which might be due to the higher maternal antibody levels in the 21-d-old pigs.
The variation of average Mhp antibody level at different ages in the fivebatch pigs in the treatment and control groups was shown Fig.2.The Mhp antibody levels in the pigs of treatment groups were all lower than those of control groups. In the treatment groups, the serum antibody levels in the five-batch pigs were all trended to be decreased with the increase of age,meeting variation laws of antibody;when the pigs were older than 35 d,the serum antibody levels were almost decreased to zero, indicating that the pigs in the treatment groups had not be infected with Mhp during their growth.In the control groups,maternal antibody exited in all the 21-d-old pigs;when the pigs were older than 35 d,the serum maternal antibody levels were basically decreased; but due to Mhp infection,the serum Mhp antibody levels were increased again when the pigs were older than 75 d.
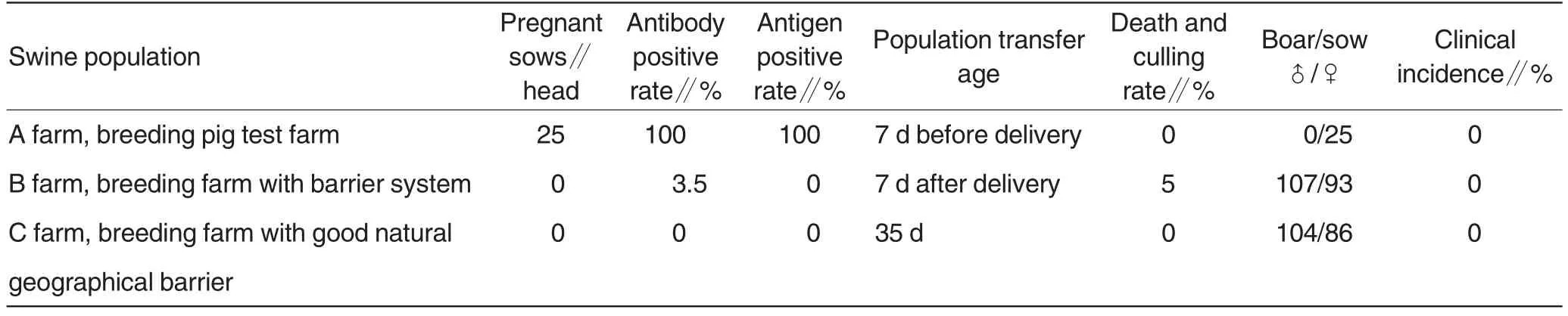
Table 4 Performance analysis of newly-bred Mycoplasma hyopneumoniae-negative swine populations in the production systems
Production statistics of newly-bred Mycoplasma hyopneumoniae-negative swine population
Table 3 showed that the fivebatch Mhp-negative swine populations were successfully bred through programmed therapy, SEW and threepoint production system,with bred rate up to 95%. Table 4 showed that various functional indicators all met requirements by normal management and health status. All the pigs bred in this study, except one eliminated nest(7 pigs)and three dead pigs for stress,showed good production performance.The Mycoplasmal pneumonial pneumonia-negative swine population was used for propagation and researches.
Discussion
Effects of drug and weaning age on purification effect of Mycoplasma hyopneumoniae in swine population
Based on the infection background and prevalence characteristics of Mycoplasma hyopneumoniae in swine populations in China, it is concluded that the beast control method for Mycoplasmal pneumonia of swine is to control Mycoplasma hyopneumoniae at the sources. Referring to study results and control methods of Mycoplasmal pneumonia of swine in previous researches, a more complete purification system was established for Mycoplasma hyopneumoniae in this study. With the development of economy and pharmaceutical industry, application of drugs in Mhp purification has been a trend. In this study, the 80% tiamulin hydrogen famarate and 15% chlortetracycline were selected,and they could reduce the application amount and dosing time of drugs, reduce production costs and reduce the generation risk of drug resistance. It was reported that if the SEW was adopted for purifying Mhp, the piglets should be better weaned at the age of 10-20 d.In this study,the piglets were weaned at the age of 7 d,but the bred rate was higher. It is indicated that under the protection of certain amount of maternal antibody, the earlier the weaning is, the lower the risk of pathogen vertical transmission is.
Effect of technical specification on purification effect of Mycoplasma hyopneumoniae in swine population
In 1970, Juhr NC reported the control method of pathogens in experimental animal[6]. During late 1970s to early 1980s,Alerander also developed MEW technology, a new therapeutic weaning technology to control pig diseases. In the early 1990s, Dritz developed the segregated early weaning(SEW) technology for US pig industry.According to the characteristics of aforementioned control methods and based on the study results about Mycoplasmal pneumonia of swine, a more complete purification system was developed for Mycoplasma hyopneumoniae in this study.
Currently, the domestic and foreign study results about purification of various pathogens in swine populations show that the purification tests all have following characteristics. First is long term. The test period is always long, and the purification tests always require several years or longer time to conclude purification effect. Second is continuity. The maintaining of Mhpnegative swine population requires continued programmed therapy and regular antigen and antibody detection(monitoring). Third is conditionality.The purification tests usually require careful planning, perfect daily management and production system in farm, good laboratory testing platform and purification site that meets requirements or test site with natural geographical barrier environment.
Key points of breeding Mycoplasmal pneumonia-negative swine population using programmed therapy, SEW and three-point production system
In this study, the piglets were weaned segregatedly at the age of 7 d.They were then transferred to the physical isolator in positive-pressure sterile animal house. The pressure in the three-level air purification barrier system is positive. Before entering the isolator, the air will be passes though the 0.2 μm filter high efficiently, thus the aerosol carrying Mhp pathogen cannot enter the isolator. The barrier system is characterized by better control of temperature and humidity and easy feeding, feces collection, disin-fection and cleaning. It has good purification effect on Mycoplasma hyopneumoniae,greatly reducing the risk of Mhp infection in swine populations through aerosol carrying Mhp pathogen. However, in the actual production,the ideal clean condition is difficult to achieve.In this study,the barrier system was simplified step by step according to requirements by actual production.
After the successful breeding of Mycoplasmal pneumonia-negative swine population, its maintenance is extremely important.The technical difficulty and failure reason of Mhp purification are not to breed mycoplasmal pneumonia-negative swine population but to maintain Mycoplasmal pneumonia-negative swine population[16]. The maintenance of Mycoplasmal pneumonia-negative swine population is a complex, long-term and arduous task.It requires scientific breeding and management system, drug application and vaccination. At the same time,good geographical barrier environment and biosecurity are also important factors for the successful purification of Mycoplasma hyopneumoniae.
Many domestic scholars have studied the methods of controlling or reducing horizontal and vertical transmission of pathogens and diseases from positive sows to their offspring.Yuan et al.found that the spreading of Mycoplasma hyopneumoniae by natural delivery[17]. The successful control of pathogens and diseases mentioned above all depends on the passive immunization in piglets provided by maternal antibody from mother sows to certain extent. Only the weaning and isolation breeding are performed during the effective protection of maternal antibodies can the vertical transmission of pathogens be cut off.
Finally, before the establishment of purification method researched in this study, the comprehensive testing program, excellent test conditions and related preparatory work had been done. The study results confirms the practicability of breeding Mycoplasmal pneumonia-negative swine populations using programmed therapy,SEW and three-point production system,providing a new method and some reference for Mhp purification. At the same time of promoting the established purification method, the operation procedures will be simplified and the operating costs will be reduced in the future studies so as to facilitate its promotion and application.
[1]STRAW B E. Hyoiatrics: Mycoplasma Diseases(Ninth Edition)(猪病学: 支原体病(第9 版))[M].UK:Blackwell Publishing(英国: 布莱克威尔出版公司),2006.
[2]CHEN PY(陈溥言).Veterinary Infectious Diseases (兽医传染病学)[M]. Beijing:China Agriculture Press(北京: 中国农业出版社),2006.
[3]CARDONA AC, PIJOAN C, DEE SA.Assessing Mycoplasma hyopneumoniae aerosol movement at several distances[J].Veterinary Record,2005,156(3):91-92.
[4]HERMANN JR, BROCKMEIER SL,YOON KJ,et al.Detection of respiratory pathogens in air samples from acutely infected pigs [J]. Canadian Journal of Veterinary Research,2008,72(4):367-370.
[5]DEE S, OTAKE S, OLIVEIRA S, et al.Evidence of long distance airborne transport of porcine reproductive and respiratory syndrome virus and Mycoplasma hyopneumoniae [J]. Veterinary Research,2009,40(4):39.
[6]JUHR NC,OBI S.Control of SPF-status in laboratory animals:exclusion of specific Mycoplasma infections in rat and mouse[J]. Berliner und Munchener Tierarztliche Wochenschrift, 1970, 83(23):470-472.
[7]JI SR (纪孙瑞), HUA JQ (华坚青),ZHONG TM (钟土木),et al.Breeding of healthy swine population using segregated early weaning technology(采用仔猪早期隔离断乳技术培养健康群的研究)[J].Swine Production(养猪),2005,5:6-8.
[8]VILLARREAL I. Epidemiology of M. hyopneumoniae infections and effect of control measures [D]. Gent, Belgium:Ghent University,2010:28-41.
[9]ALFONSO A, GEIGER JO, FREIXES C, et al. Mycoplasma hyopneumoniae and PRRSV elimination in a 1700 sow multi-site system [C]. IPVS Congress,2004:174.
[10]TURNER M, DUFRESNE L. A MEW program to eliminate PRRS, APP and Mycoplasma hyopneumoniae[C]. Proceedings of the 17thIPVS Congress,Lowa,2002:119.
[11]HAMMER JM. Production improvements in a three site production system by reducing clinical Mycoplasma hyopneumoniae [C]. Proceedings of the 18thIPVS Congress, Hamburg,Germany,2004:231.
[12]EVANS. Elimination of Mycoplasma hyopneumoniae and Actinobacilus pleuropneumonia by “Swiss depopulation” combined with segregated medicated early weaning [C]//NIELSEN JP, JORSAL SE. Proceedings of the 19th IPVS Congress,Copenhagen, Denmark: Narayana Press,2006:316.
[13]LI M(李明), WANG CJ(王朝军), LI JP(李纪平), et al. Cultivation and management of SPF Chinese experimental mini-pig (SPF 小型猪培育和管理)[J].Laboratory Animal Science and Administration (实验动物科学与管理),2005,22(2):18-20.
[14]FENG ZX (冯志新),ZHANG Y (张亚),HUA LZ(华利忠),et al.The standardization of the collection and treatment methods for swine nasal swab sample(猪鼻拭子样品采集及制备方法的标准化)[J]. Chinese Agricultural Science Bulletin (中国农学通报), 2013, 29(5):17-20.
[15]WU YZ(武昱孜),JIN MM(靳蒙蒙),BAI FF (白方方), et al. Development and application of TaqMan-BHO real time PCR assay for detection of Mycoplasma hyopneumoniae (猪肺炎支原体P97 TaqMan-BHQ荧光定量PCR 检测方法的建立及应用)[J]. Veterinary Science in China (中国兽医科学),2012,42(12):1268-1272.
[16]WALLGREN P,SAHLANDER P,HASSLEBACK G, et al. Control of infections with Mycoplasma hyopneumoniae in swine herds by disrupting the chain of infection,disinfection of buildings and strategic medical treatment[J].Journal of Veterinary Medicine:Series B,1993,40(3):157-169.
[17]YUAN GH (袁国华),XU CL (徐翠莲),GONG KM (龚奎满). Discussion on breeding of healthy swine population using natural childbirth movement (自然分娩法培育健康猪群的探讨)[J].Zhejiang Journal of Animal Science and Veterinary Medicine (浙江畜牧兽医),1991(4):12-13.
猜你喜欢
杂志排行
Agricultural Science & Technology的其它文章
- Land Use Situation and Strategies under the Background of New Urbanization in Yunnan Province
- Regeneration Cultivation Technology of Flammulina velutipes in Factories
- Current Status of Rice Production in Burundi and Its Development Strategies
- Effect of Meteorological Factors on Yield of Cotton in Different Years
- Phytoremediation of Contaminated Chemical Plant Sites
- Study on FTIR Spectra of Corn Germs and Endosperms of Three Different Colors Combining with Cluster Analysis
