Cd(Ⅱ)/Zn(Ⅱ)与三(2-巯吡啶基)甲烷配合物的合成、表征及配合物中C-S键的断裂机理
2015-12-05杨红韩新利
杨红 韩新利
(1太原工业学院化学与化工系,太原030008) (2山东新华制药股份有限公司,淄博255086)
Cd(Ⅱ)/Zn(Ⅱ)与三(2-巯吡啶基)甲烷配合物的合成、表征及配合物中C-S键的断裂机理
杨红*,1韩新利2
(1太原工业学院化学与化工系,太原030008) (2山东新华制药股份有限公司,淄博255086)
以三(2-巯吡啶基)甲烷(L)为配体合成了2个Cd(Ⅱ)和Zn(Ⅱ)的配合物:Cd(L)4(NO3)2和Zn(L)4(ClO4)2,发现在配合物中配体的C-S键发生了断裂,通过紫外、质谱手段研究并预测了其反应机理。单晶X-射线衍射的结果显示,配合物1和2中金属原子均处于扭曲的四面体配位环境中,分子间π-π堆积作用将配合物1的分子结构延伸为二维网状结构。
三(2-巯吡啶基)甲烷;C-S键断裂;π-π堆积作用
0Introduction
Sulfur-containingheterocycliccomplexeshave attracted enormous attentions,not only because of their versatile structure topologies,but also because of their potential applications in farm drugs and medicines[1],semiconducting materials,fluorescent,biomimetic and so on[2-4].In this paper,we choose tris(2-mercaptopyridyl)methane(L)as ligand to coordinate to metal ions.It has been found that when it coordinates, the C-S bonds of the ligand in complexes are all cleaving.So we obtained the complexes of 2-thiopyridone coordinating to metal ions.The cleavage of C-S bonds in the tripodal ligand does not depend on thekinds of metal ions,because when various metal salts are employed to prepare the metal complexes,the same result has been obtained.
The desulfurization reactions caused by cleavage of C-S bonds have been found under hydrothermal or solvothemal conditions,even under room temperature[5-8]. But few reports on the cleavage of C-S bonds for metal complexes with tripodal ligand have been found so far.Herein we report the crystal structures of Cd and Zn complexes with 2-thiopyridone which comes from the tripodal ligand L,and studies of the cleavage mechanism by UV-Vis titration and GC-MS.
1Experimental
1.1 Materials and general methods
The ligand L was prepared via a previously reported method[9].The molecular structure of L is shown in Scheme 1.All the other starting materials were of reagent grade and obtained from commercial sourceswithoutfurtherpurification.Elemental analyses were performed in a PE240C elemental analyzer.The infrared spectra were recorded on a VECTOR 22 spectrometer with KBr pellets.The UVVis titration were carried out on a Shimadzu UV-2450 spectrophotometer.The GC-MS were carried out with a TRACE DSQ instrument.

Scheme 1Molecular structure of L
1.2 Synthesis of the complex 1
ThesolutionofCd(NO3)2·4H2O(30.8mg,0.1mmol) in methanol(3 mL)was added dropwise to the solution of L(34.3 mg,0.1 mmol)in chloroform(3 mL),and the resulting mixture was stirred for 0.5h at room temperature.The final mixture was transferred to a test tube which was left to stand for about two weeks, and the yellow single crystals of 1 were obtained. Yield:47.1mg,61%(based on Cd).Anal.Calcd.(%) for C20H16CdN6O6S4:C,35.48;H,2.38;N,12.42.Found (%):C,35.39;H,2.45;N,12.38.IR(KBr,cm-1):3 178s, 3079vs,2901br,1863m,1743m,1611s,1577s,1522 m,1 379br,1 245m,980m,903m,761vs,616m,494s.
1.3 Synthesis of the complex 2
The same procedure was run as that of 1,except Zn(ClO4)2was used instead of Cd(NO3)2·4H2O.Colorless crystals of 2 suitable for X-ray analysis were obtained after two weeks.Yield:39.5 mg,56%(based on Zn).Anal.Calcd.(%)for C20H16Cl2N4O8S4Zn:C, 34.07;H,2.29;N,7.95.Found(%):C,33.84;H,2.46;N, 7.81.IR(KBr,cm-1):3 414w,3 232m,3 056br,1 596 s,1 550vs,1 419s,1 308m,1 208m,1 137s,1 070 br, 920m,757w,707m,681s,626s,522m,483m,438m.
1.4 Crystal structure determination
The crystals with dimensions of 0.24 mm×0.20 mm×0.16 mm for complex 1,and 0.20 mm×0.18 mm× 0.14 mm for complex 2 were carefully selected for structure determination.All the data were collected on a Bruker Smart CCD diffractometer with graphite monochromatized Mo Kα radiation(λ=0.071 073 nm) at room temperature using the φ-ω scan technique. The structure was solved by the direct method and subsequent difference Fourier syntheses and refined on F2by a full-matrix least-squares method.The nonhydrogenatomswererefinedanisotropicallyand hydrogen atoms were included in the structure factor calculation but not refined.All the calculations were carriedoutwithSHELXS-97andSHELXL-97 programs[10-11].Thecrystaldataanddatacollectiondetails for complexes 1 and 2 are summarized in Table1 .
CCDC:627210,1;644051,2.

Table1 Crystallographic data and structure refinements for complexes 1 and 2

Continued Table1
2Results and discussion
2.1 Crystal structure of complexes 1 and 2
X-ray single-crystal diffraction study reveals that complexes 1 and 2 all crystallize with the unbound 2-thiopyridone,not the tripodal ligand tris(2-mercaptopyridyl)methane.Selected bond distances and angles are given in Table2 .The structure of 1 consists of four 2-thiopyridone units and an uncoordinated NO3-anion(Fig.1 ).In this complex,the Cd atom coordinate with S atoms of the four 2-thiopyridone,forming a distorted tetrahedron environment.The bond distance of Cd1-S1 and Cd1-S1A(0.250 70 nm)is slight shorter than that of Cd1-S2 and Cd1-S2A(0.253 98 nm).All of them are normal bonds.In addition,the distances between two pyridine rings from different 2-thiopyridone ligands in adjacent layers are 0.360 9 and 0.364 9 nm,respectively,so there is weak π-π interactions between the ligands,which extend the structure to 2D networks(Fig.2 ).To a certain extent, the π-π interactions can enhance the stability of complex.It is noticed that there is a four-member ring [Cd4]with Cd-Cd separation of 0.737,0.946 6,1.036 3 and 1.119 5 nm,namely a second building unit(SUB)[12](Fig.3 ),which is the basic unit of the 2D networks.

Fig.1 Perspective view of the coordination environment of Cd in 1

Fig.2 2D networks of complex 1
The structure of 2 is different from 1.Complex 2 consists of two asymmetric units(Fig.4 ).The Zn1 and Zn2 are both tetra-coordinated with four S atoms ofunbound 2-mercaptopyridine,Zn1 atom is in a nearperfect tetrahedral coordinated environment with the Zn1-S bond distances of 0.234 53 and 0.235 37 nm, while Zn2 is in a distorted tetrahedron with the Zn2-S bond distances of 0.232 10 and 0.233 57 nm.There is no π-π interaction between pyridine rings.
2.2 Study on the cleavage mechanism of C-S bond in L
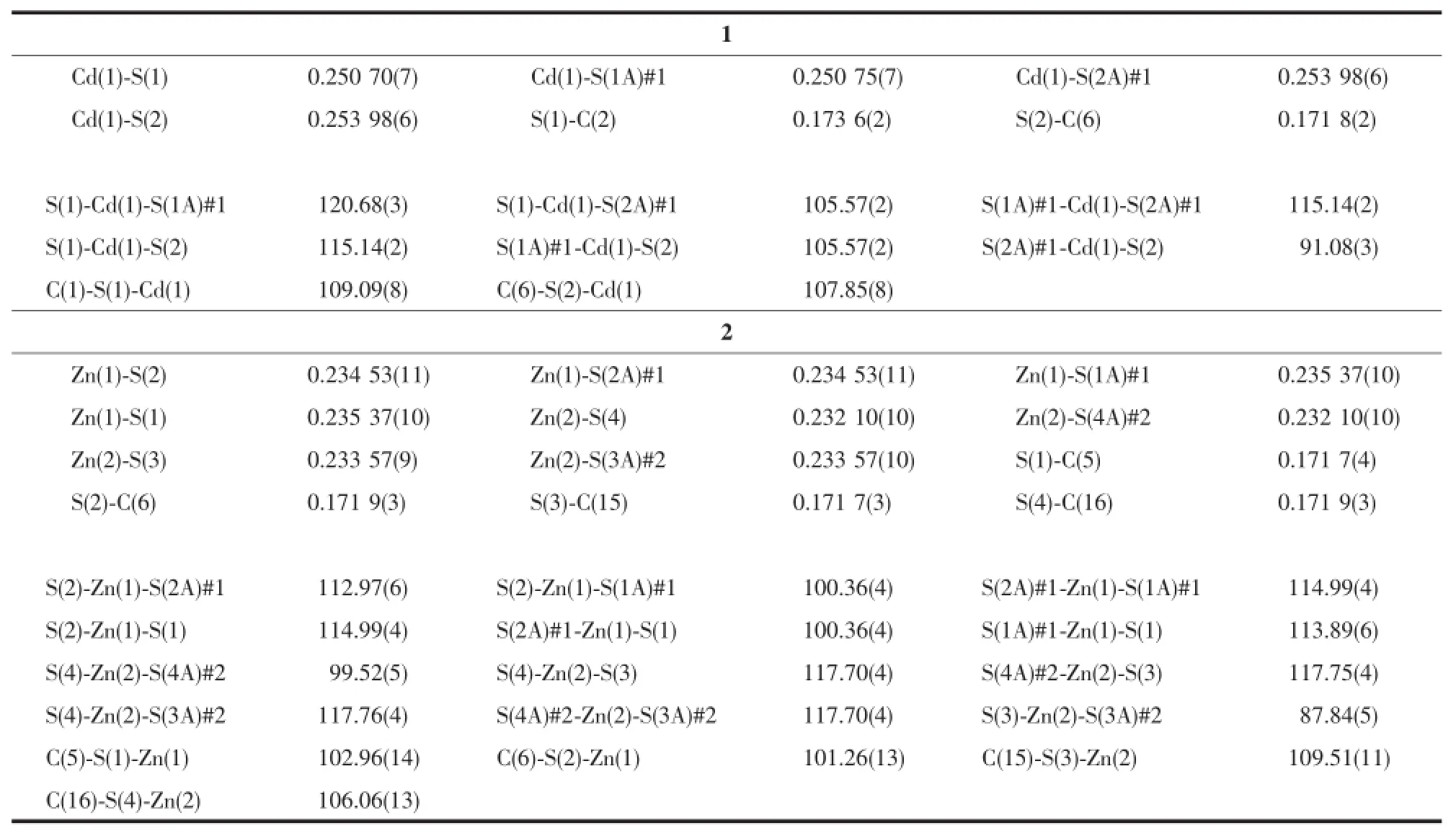
Table2 Select bond lengths(nm)and bond angles(°)for complexes 1 and 2

Fig.3 SBUs in complex 1

Fig.4 Coordination modes of Zn in 2

Fig.5 Structure of ligand L
2.2.1 Structure of ligand L
X-ray single-crystal diffraction study reveals that the ligand L have tripodal structure(Fig.5 ).Thecrystal data and data collection details for ligand L are summarized in Table3 ,and selected bond distances and angles are given in Table4 .

Table3 Crystallographic data and structure refinements for L

Table4 Selected bond lengths(nm)and bond angles(°)for L

Fig.6 UV-Vis absorption curve
2.2.2 UV-Vis analysis
Taking complex 2 as the example,We tried to use the UV-Vis spectral titration method to track the reaction process.The UV-Vis spectra for the solution of tripodal ligand L and Zn(ClO4)2according to the different molar ratios of 4∶1,3∶1,2∶1,1∶1,1∶2,1∶3,1∶4 are summarized in Fig.6 .As can be seen,the UVVis absorption curves of reaction solution are different from that of ligand L and 2-thiopyrine.In the curves of mixture,as L concentration decrease and Zn2+concentration increase,the absorption peaks centered at 254 nm and 287 nm which can be assigned to the ligand-to-metal charge transfer[13]are reduction and increase as shown by the arrows respectively,and the absorption at 260 nm appear isosbestic point,which can be assigned to the 2-thiopyrone,the isomer of 2-thiopyrine[14-15].Compare the isosbestic point with L and 2-thiopyridine,it exhibits red shift and blue shift respectively.Thistransformation suggest thatthe tripodal ligand L cleave into 2-thiopyridine,and the 2-thiopyridine transfer to 2-thiopyrone[16-18].This can also be proved by the bond lengths of C-S in complexes. The bond lengths of C-S in complexes 1 and 2 are 0.173 6,0.171 8 nm and 0.171 7,0.171 9 nm respectively.They are shorter obviously than the length of C-S(0.177~0.180 nm)in the thiol ligand with S deprotonated in complexes.The mechanism detailshave been studied and confirmed further by ESI-MS spectra.
2.2.3 ESI-MS spectra and cleavage mechanism studies
Afterthecomplexwasseparatedfromthe reaction system of tripodal ligand L with the metal salt,the mother solution was taken to carry out ESIMS in order to detect other product molecules.Taking complex 2 as the example,there is a moderate intensity peak of m/z+H at 107.20(intensity:4×105)assigned to CH(OCH3)3in the ESI-MS spectra.A weaker peak of m/z-H at 109.4 assigned to 2-thiopyridone was also observed.The result further proves that the tripodal ligand L cleaved into 2-thiopyridone coordinated to the metal ion.It is consistent with the result of UVVis spectral titration method.
These results indicate that the tripodal ligand L cleaved in the solution contained Zn2+and Cd2+.We speculate that it generated via a SN2-like mechanism. The S from L attack metal ion and the-OCH3groups from methanol attack carbenium ion to generate CH (OCH3)3molecules.The reaction can be described as follows(Scheme 2):
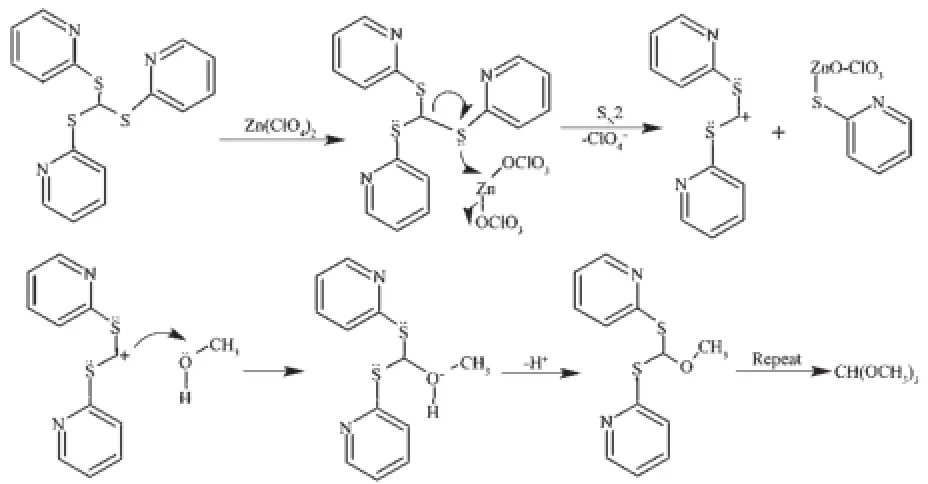
The following process occurs because of the more stability of 2-thio-pyridone[14]:

[1]LI Yong-Qiang(李永强),CHEN Xu(陈煦),ZHANG He(张和),et al.Tianjin Chem.Ind.(天津化工),2000(4):23-24
[2]Hong W S,Weng M J,Cao R,et al.Angew.Chem.Int.Ed., 2000,39:2911-2914
[3]Lobana T S,Sharma R,Sharma R,et al.Inorg.Chem.,2005, 44:1914-1921
[4]Chen X D,Wu H F,Du M.Chem.Commun.,2008,11:1296-1298
[5]Li D,Wu T.Inorg.Chem.,2009,44:1175-1177
[6]Li D,Wu T,Zhou X P,et al.Angew.Chem.Int.Ed.,2005, 44:4175-4178
[7]Song J L,Dong Z C,Zeng H Y,et al.Inorg.Chem.,2003, 42:2136-2139
[8]Cheng J K,Chen Y B,Wu L,et al.Inorg.Chem.,2005,44: 3386-3390
[9]de Castro V D,de Lima G M,Filgueiras C A L,et al.J. Mol.Struct.,2002,609:199-203
[10]Sheldrick G M.SHELXS-97,Program for the Solution of Crystal Structures,University of Göttingen,Germany,1997.
[11]Sheldrick G M.SHELXL-97,Program for Refinement of Crystal Structure,University of Göttingen,Germany,1997.
[12]Ma A Q,Zhu L G.Transition Met.Chem.,2005,30:869-872
[13]SHI Xiu-Min(石秀敏),JIANG Rui(姜锐),SONG Wei(宋薇), et al.Spectrosc.Spectral Anal.(光谱学与光谱分析),2012, 32(6):1588-1591
[14]GUO Xiao-Nan(郭晓楠),DU Rui(杜蕊),ZHAO Yan-Ying (赵彦英),et al.Acta Phys.-Chim.Sin.(物理化学学报),2012, 28(7):1570-1578
[15]Lima M C P,Coutinho K,Canuto S,et al.J.Phys.Chem.A, 2006,110:7253-7261
[16]LI Yan-Rong(李艳荣),GUO Yong-Min(国永敏),LI Bao-Zong(李宝宗).Chem.Res.(化学研究),2006,32(6):71-73
[17]XIAO Ling-Mei(萧岭梅),GAO Hong-He(高红核),GUO Xin(郭欣),et al.J.Capital Normal University(首都师范大学学报),2002,23(4):44-49
[18]Gavin D,Alexander Z,Benjamin W R,et al.Organometallics, 2011,30(2):5844-5850
Syntheses,Crystal Structures and Cleavage Mechanism of C-S Bond in Cd(Ⅱ)and Zn(Ⅱ)Complexes with Tris(2-mercaptopyridyl)methane
YANG Hong*,1HAN Xin-Li2
(1Department of Chemistry and Chemical Engineering,Taiyuan Institute of Technology,Taiyuan 030008,China)
(2Shandong Xinhua Pharmaceutical Co.,Ltd.,Zibo,Shandong 255086,China)
Two complexes of Cd(Ⅱ)or Zn(Ⅱ)with L(L=tris(2-mercaptopyridyl)methane),Cd(L)4(NO3)2(1)and Zn(L)4(ClO4)2(2),have been synthesized and characterized.The cleavage of the C-S bonds of the ligand has been discovered and the cleavage mechanism has been studied by UV-Vis titration and GC-MS.The possible reaction mechanism has been proposed.Single crystal X-ray analyses show that the Cd and Zn atoms are all in a fourcoordinated distorted tetrahedron environment.The intermolecular π-π stacking action extends the complex 1 to a 2D net work.CCDC:627210,1;644051,2.
tris(2-mercaptopyridyl)methane;cleavage of the C-S bonds;π-π stacking action
O614.24+1;O614.24+2
A
1001-4861(2015)08-1597-06
10.11862/CJIC.2015.217
2014-09-18。收修改稿日期:2015-07-02。
*通讯联系人。E-mail:hmily820805@163.com
