Improving beef hamburger quality and fatty acid profiles through dietary manipulation and exploitation of fat depot heterogeneity
2015-11-22CletosMapiyeJenniferAalhusPayamVahmaniDavidRollandTimothyMcAllisterHushtonBlockBethanyUttaroSpencerProctorandMichaelDugan
Cletos Mapiye,Jennifer L Aalhus,Payam Vahmani,David C Rolland,Timothy A McAllister,Hushton C Block,Bethany Uttaro,Spencer D Proctorand Michael E R Dugan*
Improving beef hamburger quality and fatty acid profiles through dietary manipulation and exploitation of fat depot heterogeneity
Cletos Mapiye1,2,Jennifer L Aalhus1,Payam Vahmani1,David C Rolland1,Timothy A McAllister3,Hushton C Block1,Bethany Uttaro1,Spencer D Proctor4and Michael E R Dugan1*
Background:Hamburger is the most consumed beef product in North America,but lacks in nutritional appeal due to its high fat content and high proportion of saturated fatty acids(SFA).Objectives of the present study were to improve the FA profiles of hamburgers made with perirenal fat(PRF)and subcutaneous fat(SCF)when feeding steers different diets along with examining differences in sensory attributes and oxidative stability.Diets included a control diet containing 70∶30 red clover silage∶barley based concentrate,a diet containing sunflower-seed(SS)substituted for barley,and diets containing SS with 15%wheat dried distillers’grain with solubles(DDGS-15)or 30%DDGS(DDGS-30).Hamburgers were made from triceps brachii and either PRF or SCF(80∶20 w/w).
Beef,DDGS,Fat depot,Fatty acids,Sensory attributes,Oxidative stability
Background
Ground beef and its by-products,including hamburger containing up to 30%added fat,are the most commonly purchased beef products in North America[1],probably due to their price and preparatory versatility.Of the 28.5 kg/capita per annum consumption of fresh beef in North America[2],about 52%is ground beef[3].However,the consumer perception of the healthfulness of beef,especially ground beef and hamburgers has been declining[3],largely because it is a rich source of saturated fatty acids(SFA)that have relationships with several diseases from cardiovascular disease(CVD)to cancer[4]. On the other hand,beef contains polyunsaturated fatty acids(PUFA)biohydrogenation products(BHP)including rumenic acid(t9,c11-18:2)and its precursor vaccenic acid(t11-18:1),which may have potential human health benefits[5].In this regard,enriching beef and its further processed products with unsaturated fatty acids(FA)is one strategy that could be used to gain consumer confidence and subsequently improve the image of beef.
Hamburger can be enriched with unsaturated FA through animal nutrition with the strategic use of foragesand dietary lipids[6],and by the selective use of trim fats which can contain different amounts of PUFA-BHP[7]. Feeding cattle high-forage diets with sunflower-seed(i.e.,a rich source of linoleic acid,18:2n-6)is an effective way to promote deposition of t11-18:1 and t9,c11-18:2,with greater accumulations of total PUFA-BHP in perirenal fat(PRF)vs.subcutaneous fat(SCF)[8].With these diets,however,the high forage content(up to 70%DM)negatively affects animal performance and beef quality(i.e.,marbling fat deposition)[9].In a follow-up experiment we examined the effects of substituting red clover silage with a non-forage fibre source(wheat dried distiller’s grains plus solubles(DDGS))along with sunflower seed(SS)addition to the diet and found that it improved beef quality while maintaining or increasing PUFA-BHP proportions in beef[10].Wheat DDGS has been found to have a feeding value comparable to barley grain while maintaining beef quality[11],and has been shown to increase t9,c11-18:2 and t11-18:1[12],while reducing t10-18:1 which has been shown to negatively affect blood lipid profiles in animal models[13].The objectives of the present study were to examine hamburger quality and FA composition from this trial,first comparing hamburgers made with SCF vs.PRF,and then to further investigate specific effects of diet on PRF hamburger.Our focus was on PRF as it may be an underutilized fat depot that is easily accessible during the slaughter process.It has a higher content of total PUFA-BHP,but has a higher SFA content vs.SCF[8].The difference in SFA is,however,almost entirely due to an increased amount of 18:0,which is known to have neutral effect on plasma cholesterol profiles when consumed by humans[14].
Material and methods
Animals and diets
Procedures for the care and handling of animals used in this study were approved by the Lacombe Research Centre Animal Care Committee in compliance with the principles and guidelines established by the Canadian Council on Animal Care[15].Tissues used for the current study were collected from the study reported by Mapiye et al.[10].In summary,64 12-month-old British×Continental crossbred steers with an initial mean body weight(BW)of 362.7±4.50 kg were stratified by weight to four experimental diets(control,SS,DDGS-15 and DDGS-30),with two pens of eight steers/ diet.The control diet was composed of 70%red clover silage,25.8%barley grain and 4.2%vitamin-mineral supplement on a dry matter(DM)basis(Table 1).The SS diet contained 11.4%SS substituted for barley grain,and the DDGS-15 and DDGS-30 diets contained 15 and 30% DDGS substituted for red clover silage and SS to maintain a targeted 5%added oil in the diets from either SS or DDGS(DM basis;Table 1).
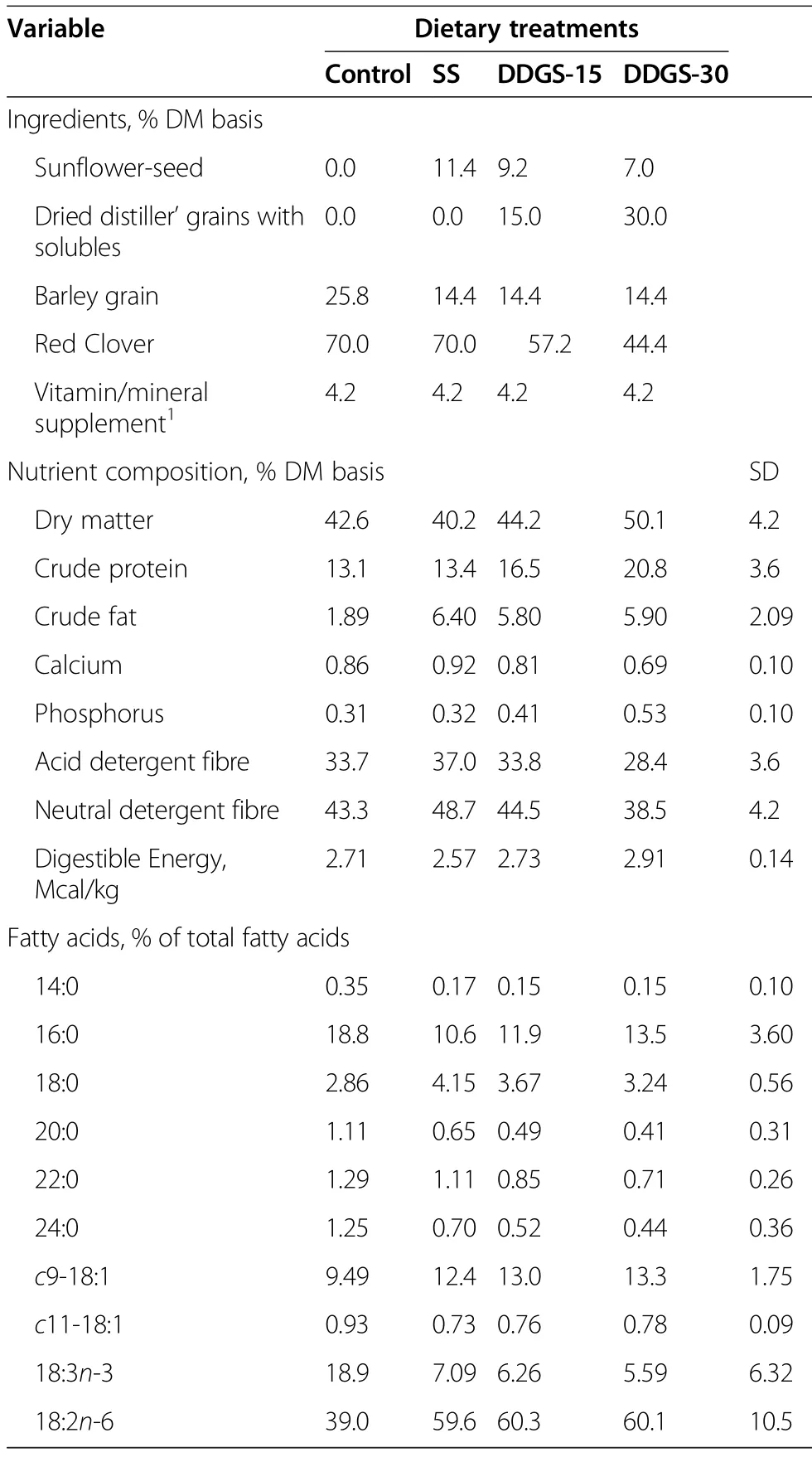
Table 1 Ingredient,nutrient and fatty acid composition of the dietary treatments
Sample collection and preparation
At slaughter,PRF was collected approximately 30 min post-mortem,vacuum packed and held in a 2°C cooler with a wind speed of 0.5 m/s.Carcasses were cooled for 24 h under the same conditions prior to collection and vacuum packing of triceps brachii muscle and SCF from along the dorsal region,over the longissimus thoracis et lumborum.After 6 d storage,80:20(w/w)lean:fat hamburger was prepared with an initial grind through a6 mm plate,then mixed and ground through a 4 mm plate(Butcher Boy meat grinder Model TCA22,Lasar Manufacturing Co,Los Angeles,CA,USA).A 50 g subsample of each grind was collected for an immediate 0 h thiobarbituric acid reactive(TBAR)substance determination[16].A second 50 g sample was blended using a Robot Coupe Blixir BX3 food processor(Robot Coupe USA Inc.,Ridgeland,MS,USA)and frozen at-80°C for subsequent FA analysis.Three 140 g hamburger patties(11.4 cm diameter×0.63 cm thick)were formed from remaining grind using a single hamburger press(Cabelas,Sydney,NE,USA).One patty was placed on a polystyrene tray,over-wrapped with an oxygen permeable polyvinylchloride film (oxygen transmission rate 8,000 mL/m2/ 24 h;Vitafilm Choice Wrap,Goodyear Canada Inc.)and placed into a fan assisted,horizontal retail display case(Hill Refrigeration of Canada Ltd.,Barrie,ON,Canada)with an average temperature of 3.5°C for objective colour measurements at 0 and 4 d.Samples were held under fluorescent room lighting(GE deluxe cool white;General Electric Canada,Oakville,ON,Canada)supplemented with incandescent lighting directly above the display case(GE clear cool beam 150 W/120 V;General Electric Canada)spaced 91.5 cm apart to provide an intensity of 1,076 lux at the meat surface for 12 h per d.After 4 d in retail display,following objective colour measurements,TBAR determination was performed on the patty.The other two remaining patties/animal/depot were vacuum packaged and stored at-20°C for subsequent sensory evaluation.
Colour measurements
Objective colour measurements on hamburger patties were performed at three separate locations over the patty surface using a Minolta CR-300 with Spectra QC-300 Software(Minolta Canada Inc.,Mississauga,ON,CA).Three colour measurements,L*,a*and b*values[17]were used to calculate hue=tan-1(b*/a*);and chroma=(a*2+b*2)0.5.Spectral reflectance readings were also collected at the same time and converted to reflex attenuance[18].Interpolation of isobestic points for 473,525,572,and 700 nm were determined to calculate relative contents of metmyoglobin and oxymyoglobin[19].Retail display effects on L*,hue,chroma,metmyoglobin and oxymyoglobin were calculated as the difference between 0 d and 4 d values.
Fatty acid analysis
Hamburger samples were thawed and lipids extracted with 2:1 chloroform:methanol according to Folch et al.[20]. Separate 1.5 mol/L methanolic HCl and 0.5 mol/L sodium methoxide methylationsofhamburger lipid extracts(10 mg)were performed according to Kramer et al.[21]with the inclusion of 1 mg c10-17:1(Nu-Chek Prep.Inc. Elysian,MN,USA)as an internal standard.Fatty acid methyl esters were analysed by GC using a CP-Sil88 column(100 m,25 μm ID,0.2 μm film thickness,Varian Inc.,Walnut Creek,CA,USA)using complementary temperature programs with 150°C and 175°C plateaus according to Kramer et al.[21].Conjugated linoleic acid(CLA)isomers not separated by GC were further analysed using Ag+-HPLC as described by Cruz-Hernandez et al.[22].Individual peaks were identified using reference standards(GLC-603,Nu-Chek Prep.Inc.,Elysian,MN,USA;BCMix1,Applied Science,State College,PA,USA)and peak order and retention times reported in the literature[21-23].Only groups/families of FA and major FA within groups were reported.
Sensory evaluation
Hamburger patties were placed on a tray in a single layer,and thawed overnight at 4°C.Hamburgers were weighed and then cooked in individual non-stick pans on an electric grill(Garland Grill ED30B,Condon Barr Food Equipment Ltd.,Edmonton,AB)pre-heated to 205°C.Previous testing with surplus prepared patties indicated hamburger patties reached an internal temperature of 71°C with juices running clear after cooking for four min on one side,flipping and then cooking an additional eight min.Following cooking in this manner,hamburger patties were cooled for two min prior to recording final weights and calculating cooking loss.Hamburger patties were divided into eight equal wedges and presented to eight panellists trained according to the American Meat Science Association[24]research guidelines.Panellists evaluated six samples/session and attended four sessions/ d with the experimental treatments randomized amongst these sessions.Attribute ratings were electronically collected(Compusense Inc.,Guelph,On,Canada)using nine point descriptive scale for initial and overall tenderness(9=extremely tender;1=extremely tough),initial and sustainable juiciness(9=extremely juicy;1=extremely dry),flavour and off-flavour intensity(9=extremely intense;1=extremely bland/none),and residual mouth coating(9=no mouth coating;1=extreme mouth coating).
Statistical analysis
Statistical analyses were conducted using Proc Mixed SAS[25].To determine the main effect of fat depot location,the following statistical model was used: Yijk=μ+αi+βj+αβij+εijk;where Yijkis the observation(fatty acids,sensory scores,colour and lipid oxidation values),μ is the overall mean,αiis the effect of the ithfat depot(i=SCF,PRF;df=1),βjis the effect of jthdiet(j=control,SS,DDGS-15,DDGS-30;df=2),αβijis the effect of the interaction between fat depot and diet and εijk(df=2)is the residual error(df=58).Slaughter day and animal within a diet were incorporated asrandom effects.A data sub-set of the PRF hamburgers was then analysed to determine the effects of diet using the following statistical model:Yij=μ+αi+εij,where Yijis the observation,μ is the overall mean,αiis the effect of the ithdiet(df=2)and εijis the residual error(df=61).Slaughter day and animal were included as random effects.Means were generated and separated using the LSMEANS and PDIFF options,respectively. The threshold for significance was set at P<0.05.
Results and discussion
Animal performance and meat quality
Animal performance and meat quality results were detailed by Mapiye et al.[10].In summary,steers fed the DDGS-30 diet tended(P=0.10)to have the highest DMI,followed by steers fed DDGS-15,control and SS diets during the course of the experiment.These results were related to the fibre content reported for the respective diets.Steers fed the DDGS-30 diet had the greatest ADG,final weight and cold carcass weight followed by steers fed the DDGS-15,control and SS diets(P<0.05).These results were related to relatively higher digestible energy and a trend for increasing DMI reported for the DDGS containing diets.Meat from steers fed the SS and control diets had similar(P>0.05)grade fat,rib eye area,lightness and sensory panel meat tenderness.However,meat from steers fed DDGS diets had greater(P<0.05)grade fat,rib eye area,lighter(P<0.05)colour and improved(P<0.05)instrumental and sensory panel meat tenderness relative to steers fed the SS diet. These results are a reflection of the growth performance results reported for the respective diets.
Fatty acid composition of hamburgers made with perirenal and subcutaneous fats Fatty acids of endogenous or dietary origin
Perirenal fat hamburgers had higher(P<0.05)total FA content than SCF hamburgers(Table 2).These findings are likely associated with differences in moisture between PRF and SCF,with SCF typically containing more water than PRF[26].
Of the FA of dietary or endogenous origin,SFA and cismonounsaturated FA(c-MUFA)were the major families(Table 2).Relative to hamburgers made with SCF,those made with PRF had greater(P<0.05)proportions of total and major SFA,with differences relating mostly to the higher proportions of 18:0(Table 2).In support of the current findings,previous studies in beef cattle have shown that mature fat depots located internally such as PRF are more saturated than less mature fat depots located externally such as SCF[7,27].This has been attributed to a lower Δ-9 desaturase activity index in internal vs.external depots,and replacement of 18:0 with c9-18:1 in external fat depots.
Fat depot location had a significant effect on total c-MUFA,c9-14:1,c9-16:1 and c9-18:1,with SCF hamburgers having larger(P>0.05)proportions compared to PRF(Table 2).Current results agree with differences in Δ-9 desaturase gene expression(mRNA)reported by Lee et al.[27].
Fat depot location had no effect on total n-3 PUFA of hamburgers,however,there was a small but significant(P<0.05)increase in the proportion(0.01%)of docosanpentaenoic acid(22:5n-3)in SCF vs.PRF hamburgers(0.01%,P<0.05;Table 2).Total PUFA,total and major n-6 PUFA were influenced(P<0.05)by fat depot location,with SCF hamburgers having greater(P<0.05)proportions than PRF hamburgers.
Fatty acids of microbial origin
Fatty acids of microbial origin include branched-chain FA(BCFA)and PUFA-BHP which includes trans-monounsaturated FA(t-MUFA),CLA,non-conjugated 18:2 biohydrogenation products(i.e.,atypical dienes,AD)and conjugated linolenic acids(CLNA).Hamburgers made with PRF vs. SCF had slightly greater(P<0.05)proportions of total BCFA,iso-17:0 and lower(P<0.05)proportions of anteiso-17:0.The reasons for these differences are not clear but could be linked to depot-specific differences in incorporation of individual FA[28].
Within PUFA-BHP,fat depot location influenced total and major t-18:1(t11-and t13-/t14-18:1)isomers with proportions being greater(P<0.05)in PRF vs.SCF hamburgers(Table 2).In opposition,the proportions of total CLA,t7,c9-18:2 and c9,t11-18:2 were greater(P<0.05)in hamburgers made with SCF as opposed to PRF.Given t7,c9-18:2 and c9,t11-18:2 are known to be Δ-9 desaturase products of their respective t-18:1 precursors,these findings are consistent with the lower Δ-9 desaturase activity index reported for PRF compared to SCF[7,27].
Total and major AD isomers were affected by fat depot location,with SCF having greater(P>0.05)proportions than PRF(Table 2).Fat depot location had no effect on total CLNA,but c9,t11,c15-18:3 proportions were slightly greater(P<0.05)in PRF than in SCF(Table 3).These findings could reflect differences between SCF and PRF in uptake or rate of metabolism of these FA[29].
Effects of animal diet on fatty acid composition of perirenal hamburgers Fatty acids of endogenous or dietary origin
Substitution of SS for barley in the control diet reduced(P<0.05)the total FA content in hamburgers but feeding DDGS-15 and DDGS-30 led to successive increases(P<0.05;Table 3).These findings are consistent with differences in dietary energy levels,DMI,and potential differences in intramuscular fat contents[10].Compared to control,feeding the SS diet reduced(P<0.05)total SFA,14:0 and 16:0(Table 3).Feeding the DDGS-15 diet led to no further change in total SFA(P>0.05)but further reduced(P>0.05)14:0 and 16:0.Feeding the DDGS-30 diet further reduced(P<0.05)total SFA and increased(P<0.05)14:0 and 16:0 back up to proportions equal to the SS diet.On the contrary,substitution of SS in thecontrol diet increased(P<0.05)hamburger proportions of 18:0,feeding the DDGS-15 diet led to no further change(P>0.05),but feeding the DDGS-30 diet reduced 18:0 back down to proportions slightly above those found when feeding the control diet.These results resemble patterns of dietary proportions of the individual SFA observed in the current study,and may also relate to influences of both rates of complete biohydrogenation of PUFA to 18:0,and effects of higher proportions of 18:2n-6 in adipose tissues on de novo FA synthesis[30].
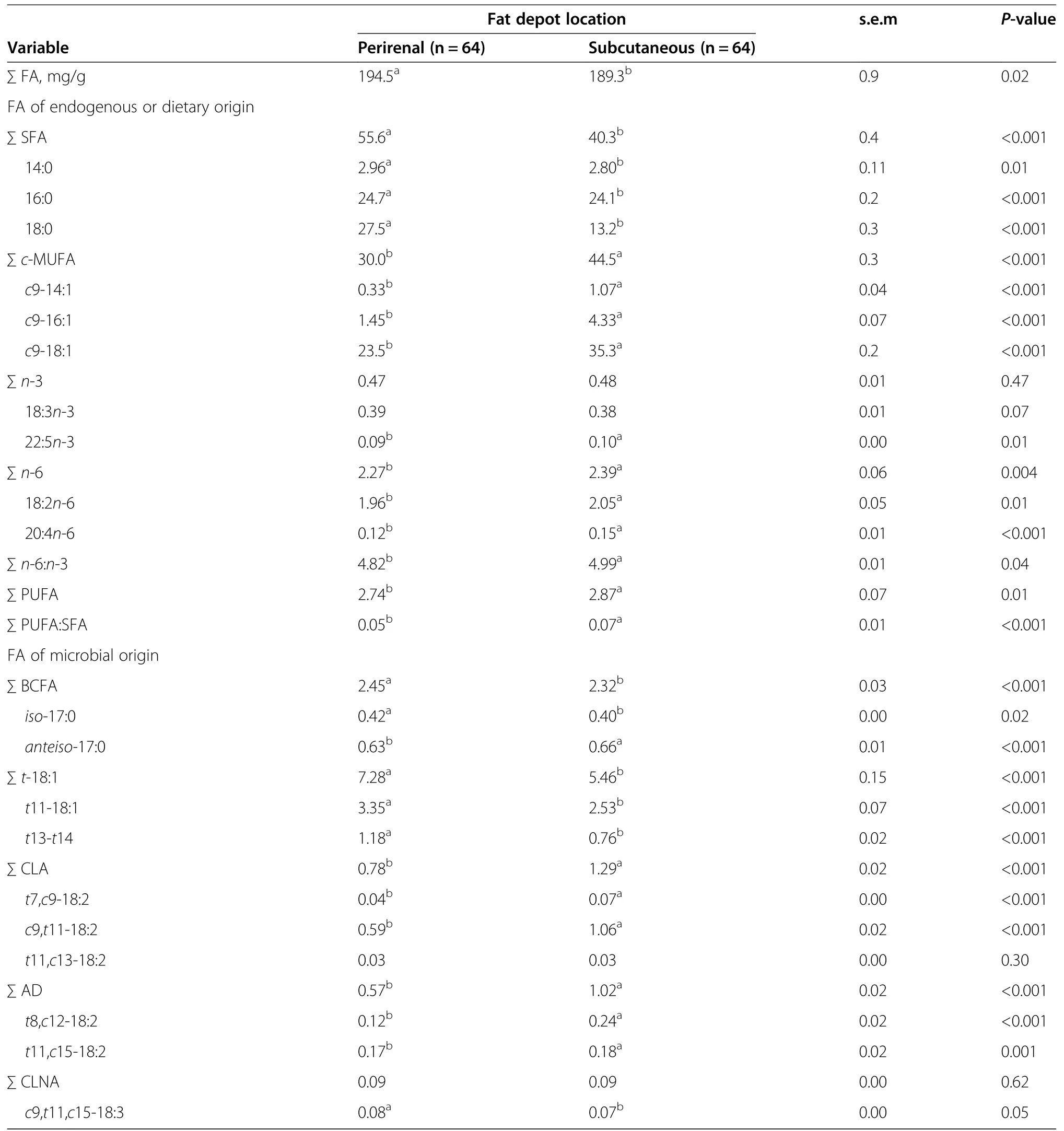
Table 2 Fatty acid composition of hamburgers(%of total fatty acids)made with perirenal or subcutaneous fat across all diets
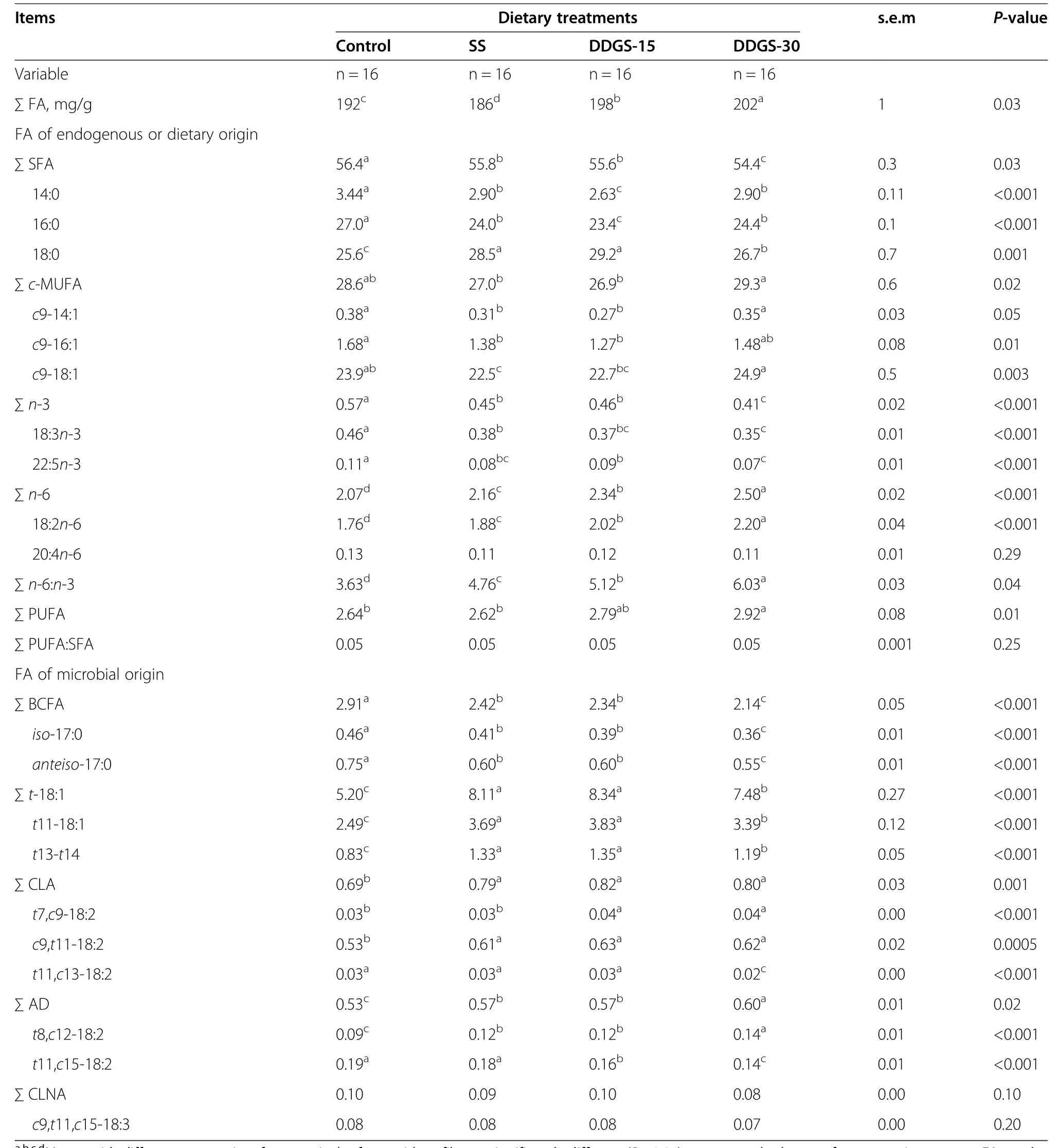
Table 3 Fatty acid profiles of hamburgers made with perirenal fat from steers fed a high forage diet containing sunflower-seed(SS)and 15 or 30%wheat dried distillers’grains with solubles(DDGS)
The proportions of total and individual c-MUFA declined(P<0.05)with substitution of SS in the control diet(Table 3).Feeding the DDGS-15 diet led to no further changes but feeding the DDGS-30 diet brought the proportions of these FA back up to proportions equal to the control diet(P>0.05).Differences in c-MUFA are difficult to interpret,as they did not follow trends in dietary proportions,and c-MUFA can originate from both diet and endogenous synthesis.
Substitution of SS in the control diet led to reductions(P<0.05)in the proportions of total and major n-3 PUFA in beef hamburgers and feeding the DDGS-15 and DDGS-30 diets led to further reductions(P<0.05;Table 3).This is consistent with the dietary proportions of 18:3n-3.Thus,these results could be related to the substitution of barley grain and red clover silage with SS and DDGS that have relatively lower concentrations of 18:3n-3.There is also a possibility that higher proportions of 18:2n-6 in the PRF of steers fed SS and DDGS diets might have reduced the elongation of 18:3n-3 to 22:5n-3 and/or 22:6n-3 due to competition for the Δ-6 desaturase[31].The n-3 PUFA provide a wide range of benefits ranging from general improvements in health to protection against inflammation and disease[32]but the slight differences related to diet(>0.1%)may not be of practical significance.
Substitution of SS in the control diet increased the proportions of total PUFA,total n-6 PUFA and 18:2n-6,and feeding the DDGS-15 and DDGS-30 diets led to further increases(P<0.05;Table 3).These results reflect higher dietary proportions of 18:2n-6 observed in diets containing SS and potentially greater ruminal bypass of 18:2n-6 when feeding DDGS.On a practical basis,however,a difference between diets of<0.5%n-6 PUFA may not influence CVD risk.
Inclusion of SS and DDGS in the diet had no effect(P>0.05)on the PUFA:SFA ratio.The PUFA:SFA ratios for the hamburgers recorded in the current study were less than the lower limit recommended to improve human health[33],but again it can be difficult to recommend a ratio when individual FA within groups/families can have decidedly different biological effects.
Fatty acids of microbial origin
Substitution of SS in the control diet led to reductions(P>0.05)in the proportions of total and major BCFA,feeding the DDGS-15 diet resulted in no further changes(P<0.05),but feeding DDGS-30 resulted in further reductions(P>0.05).Since the majority of BCFA in animal tissue are synthesised de novo by rumen microbes[34],the high levels of 18:2n-6 in SS containing diets might have inhibited the rumen microbes responsible for BCFA production[35].Further reductions observed when feeding the DDGS-30 diet could be a result of decreased ruminal propionate production from readily fermentable starch[36].Propionate is a precursor of methylmalonate,which is utilised as a primer for the biosynthesis of BCFA[34].In vitro and in vivo trials have shown that BCFA inhibit the growth of various cancer cell lines[37,38])and have demonstrated potential to reduce necrotizing enterocolitis in a neonatal rat model[39].It would be of interest to determine if these limited changes(<0.8%)in BCFA would have any practical implications for human health.
Compared to control,proportions of total and major t-18:1 isomers were increased(P<0.05)by feeding the SS diet and feeding the DDGS-15 diet led to no further changes(P>0.05),while the increases were attenuated(P<0.05)when feeding the DDGS-30 diet(Table 3).This could be a result of a combination of factors including higher dietary 18:2n-6 observed for the diets containing SS,greater bypass of 18:2n-6 when feeding DDGS diets,and potential differences in ruminal pH which could have influenced PUFA biohydrogenation patterns[40,41].With t11-18:1 being associated with reductions in plasma triglycerides,total cholesterol and LDL-C in animal models[42,43],and reductions in pro-inflammatory cytokines[44,45]and platelet aggregation in humans[45]there has been a growing interest to increase its concentrations in beef.On the contrary,total t-18:1 FA and individual t-18:1 isomers other than t11-18:1(i.e.,t9-and t10-18:1)have been associated with increases in serum LDL-C and decreases in HDL-C in animal models[13,46]but the effect of the remaining individual t-18:1 isomers on human health have not been investigated.Present results indicate that hamburgers made with PRF from steers fed SS and DDGS-15 diets could be enriched sources of potentially healthy t11-18:1.The increase in total t-MUFA was not,however,completely attributed to t11-18:1,and it would be important to evaluate the health effects of individual t-18:1 isomers,determine the levels regarded as beneficial or detrimental to human health,and develop feeding strategies that promote a healthier balance between isomers without negatively affecting animal performance and meat quality.
The proportions of total and major CLA isomers in beef hamburgers were increased(P<0.05)when substitutingSS in the control diet but feeding the DDGS-15 and DDGS-30 diets led to no further changes(P>0.05;Table 3).Again,these findings could largely be ascribed to dissimilarities in dietary fibre content,18:2n-6 proportions and bypass rates across diets.A minor diet effect was seen for t11,c13-18:2,with feeding the DDGS-30 diet yielding slightly lower(P<0.05)proportions compared to the other diets.The interest in raising the proportions of c9,t11-18:2 in beef is associated with its potential positive effects on human health[5].In the present experiment,however,the increases in c9,t11-18:2 when feeding the SS,DDGS-15 and DDGS-30 diets were just over 0.1%,but it must be remembered effects on c9,t11-18:2 and t11-18:1 should be considered together given t11-18:1 is the precursor for c9,t11-18:2 in animals[5].In addition,there is much to be understood regarding production of consistent amounts of t11-18:1 and c9,t11-18:2 across production cycles,as when feeding a diet very similar to the SS diet in the present experiment[8],the proportion of t11-18:1 in hamburger made with PRF would have been greater(5.9%)with a similar amount of c9,t11-18:2.
Compared to control,feeding the SS diet increased(P<0.05)proportions of total AD and t8,c12-18:2,the major AD likely largely derived from 18:2n-6,and no further changes were noted when feeding DDGS-15(P<0.05)but feeding DDGS-30 led to further increases(P<0.05;Table 3).Overall,these findings reflect greater proportions of 18:2n-6 when feeding SS and DDGS diets. Substituting SS into the control diet had no effect on t11,c15-18:2,but feeding DDGS-15 and DDGS-30 led to reductions(P<0.05).This may be related to dietary proportions of n-3 PUFA which declined with additions of SS and DDGS to the diet.During biohydrogenation,18:3n-3 is isomerised to CLNA,which is in turn hydrogenated to t11,c15-18:2[47].Feeding the SS,DDGS-15 and DDGS-30 diets had no effect(P<0.05)on total CLNA and c9,t11,c15-18:3,the major CLNA isomer(Table 3).
Sensory attributes
Hamburgers made with perirenal vs.subcutaneous fat
Compared to SCF hamburgers,PRF hamburgers had higher(P<0.05)scores for initial and sustainable juiciness,tended to have higher scores for initial tenderness(P<0.09),but had lower(P<0.05)scores for beef flavour intensity(Table 4).Hamburgers made with PRF vs.SCF may have been juicier and tenderer due to their higher FA content and lower(P<0.05)cook loss(Table 4).The disparities in beef flavour intensity scores reported for PRF as opposed to SCF could be related to the differences in the proportions of n-6 and n-3 PUFA reported for these depots.Overall,oxidation of PUFA produces volatile compounds that may contribute to desirable or undesirable meat flavour depending on type,amounts and proportions in meat[48].
Hamburgers made with PRF as opposed to SCF had lower(P<0.05)ratings for residual mouth coating,indicating greater coating(Table 4).This may be related to greater proportions of SFA,and 18:0 in particular,observed for the PRF depot.Higher proportions of 18:0 in meat fat would increase its melting point and consequently,the undesirable mouth-coating properties[49]. Although fat depot location influenced several sensory attributes of hamburgers,absolute differences were all less than one sensory panel unit,and would not likely be detected by untrained consumers who rarely consume plain hamburgers without added seasonings and/or condiments.
Dietary influence on hamburgers made with perirenal fat
Diet had no effect on the sensory attributes of hamburgers made with PRF except for residual mouth coating(Table 5).Feeding SS and DDGS-15 diets did notaffect ratings for mouth-coating but slightly higher(P <0.05)ratings were observed when feeding the DDGS-30 diet(Table 5).The lower residual mouth coating reported for the DDGS-30 diet could be partly associated with lower SFA proportions observed for the same diet.
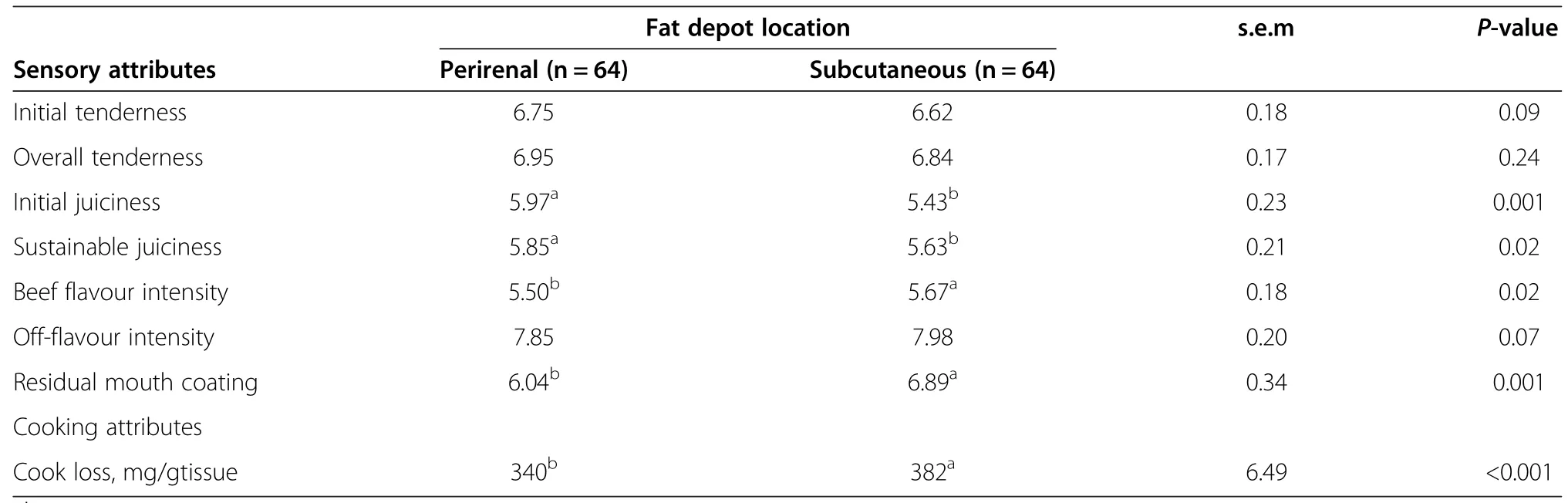
Table 4 Sensory attributes of hamburgers made with perirenal or subcutaneous fat

Table 5 Sensory attributes of hamburgers made with perirenal fat from steers fed a high forage diet containing sunflower-seed(SS)and 15 or 30%wheat dried distillers’grains with solubles(DDGS)
Changes in oxidative stability of hamburgers during retail display
Perirenal vs.subcutaneous hamburgers
Over four days of retail display,hamburgers made with either PRF or SCF had substantial increases(P<0.05)in hue angle(yellowing)combined with reductions(P<0.05)in chroma(colour intensity)and L*(lightness).In addition,during retail display metmyoglobin and malonaldehyde increased(P<0.05)while oxymyoglobin decreased(P<0.05).Effects of fat depot location on hamburger changes during retail display were,however,limited to a slight but greater reduction(P<0.05)in L*and a trend for less of a reduction(P=0.07)in chroma when making hamburgers with SCF(Table 6).This may be due to the fact that SCF contain more water than PRF[26].
Dietary influence on perirenal hamburgers
Feeding SS or DGGS diets neither changed(P<0.05)colour(L*,hue or chroma)nor concentrations of oxymyoglobin,metmyoglobin and malonaldehyde of PRF hamburgers during retail display.
Conclusions
The most notable differences between the two fat depots were those related to the greater proportions of SFA,chiefly 18:0,t-18:1 isomers,primarily t11-18:1 and BCFA in PRF.Feeding SS and DDGS-15 diets compared to the control diet led to increases in proportions of t11-18:1 and c9,t11-18:2 in PRF,but feeding DDGS-30 was less effective.Feeding the DDGS-15 diet might,therefore,be a way to improve the healthfulness of perirenal FA profiles,while improving overall animal performance and meat quality.
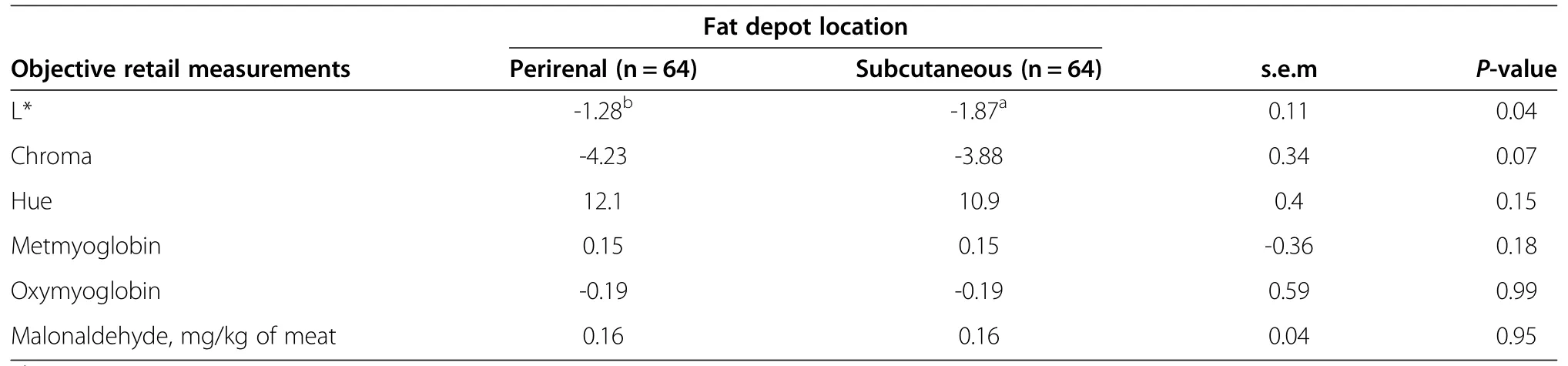
Table 6 Change in retail colour and oxidative stability of hamburgers made with perirenal or subcutaneous fat over 4 d
Competing interests
The authors declare that they have no competing interests.
Authors’contributions
CM and MERD participated in the acquisition of data,analyses of feed and meat fatty acids,statistical analysis of data and drafted the manuscript.MERD conceived the study and acquired funds.DCR analysed feed and meat fatty acids.JLA,PV,TAM,HCB and SDP contributed to conception and design of the study and writing of the manuscript.All authors read and approved the final manuscript.
Acknowledgements
This research was funded by the Alberta Meat and Livestock Agency(ALMA). Drs.C.Mapiye and P.Vahmani acknowledge the receipt of NSERC Fellowships funded through ALMA.Special thanks are extended to staff at the Lacombe Research Centre(LRC)Beef Unit of AAFC for animal care,animal management and feed sample collection.The slaughter and processing of the cattle by the LRC abattoir staff is gratefully acknowledged. Technical contributions of the meat grading and quality staff at the LRC to the results are appreciated.Ms.I.L.Larsen is acknowledged for her valuable assistance in statistical analysis.
Author details
1Agriculture and Agri-Food Canada,Lacombe Research Centre,6000 C and E Trail,Lacombe,Alberta T4L 1 W1,Canada.2Department of Animal Sciences,Faculty of AgriSciences,Stellenbosch University,P.Bag X1,Matieland 7602,South Africa.3Agriculture and Agri-Food Canada,Lethbridge Research Centre,1st Avenue South 5403,PO Box 3000,Lethbridge,Alberta T1J 4B1,Canada.4Metabolic and Cardiovascular Diseases Laboratory,Diabetes and Mazankowski Institutes,Li Ka Shing Centre for Health Research Innovation,University of Alberta,Edmonton,Alberta T6G 2E1,Canada.
Received:20 June 2014 Accepted:18 November 2014
Published:24 November 2014
1. Brewer MS∶Reducing the fat content in ground beef without sacrificing quality:A review.Meat Sci 2012,91(4)∶385-95.
2. Brooks EJ∶Canadian beef's Wilting Trade Position:An eFeedLink Hot Topic,September 2013.2013.http∶//www.efeedlink.com/contents/09-12-2013/ 39fe00c2-fbba-44cf-b342-c08a0e3b88c8-c082.html.Accessed October 2013.
3. CAPI∶Canada’s Beef Food System:A Roadmap for Dialogue on Strategy.,September 2012.2012.http∶//www.capi-icpa.ca/pdfs/2012/CAPI_Beef-Food-System_2012.pdf.Accessed October 2013.
4. Mapiye C,Aldai N,Turner TD,Aalhus JL,Rolland DC,Kramer JKG,Dugan MER∶The labile lipid fraction of meat:from perceived disease and waste to health and opportunity.Meat Sci 2012,92(3)∶210-20.
5. Dilzer A,Park Y∶Implication of conjugated linoleic acid(CLA)in human health.Crit Rev Food Sci Nutr 2012,52(6)∶488-513.
6. Raes K,De Smet S,Demeyer D∶Effect of dietary fatty acids on incorporation of long chain polyunsaturated fatty acids and conjugated linoleic acid in lamb,beef and pork meat:A review.Animal Feed Sci Tech 2004,113(1-4)∶199-221.
7. Jiang T,Mueller CJ,Busboom JR,Nelson ML,O'Fallon J,Tschida G∶Fatty acid composition of adipose tissue and muscle from Jersey steers was affected by finishing diet and tissue location.Meat Sci 2013,93(2)∶153-61.
8. Mapiye C,Aalhus JL,Turner TD,Rolland DC,Basarab JA,Baron VS,McAllister TA,Block HC,Proctor SD,Dugan MER in press∶Types of oilseed and adipose tissue influence the composition and relationships of polyunsaturated fatty acid biohydrogenation products in steers Fed a grass Hay diet.Lipids.49(3)∶275-286.10.1007/s11745-013-3876-1.
9. Mapiye C,Aalhus JL,Turner TD,Rolland DC,Basarab JA,Baron VS,McAllister TA,Block HC,Uttaro B,Lopez-Campos O,Proctor SD,Dugan MER∶Effects of feeding flaxseed or sunflower-seed in high-forage diets on beef production,quality and fatty acid composition.Meat Sci 2013,95(1)∶98-109.
10.Mapiye C,Aalhus JL,Turner TD,Basarab JA,Baron VS,McAllister TA,Block HC,Uttaro B,Proctor SD,Dugan MER submitted∶Inclusion of sunflowerseed and wheat dried distillers’grains with solubles in a red clover silage-based diet enhances steers performance,meat quality and fatty acid profiles Animal,submitted.Animal 2014,8:1999-2010.
11.Gibb DJ,Hao X,McAllister TA∶Effect of dried distillers'grains from wheat on diet digestibility and performance of feedlot cattle.J Anim Sci 2008,88(4)∶659-65.
12.Dugan MER,Aldai N,Kramer JKG,Gibb DJ,Juárez M,McAllister TA∶Feeding wheat dried distillers grains with solubles improves beef trans and conjugated linoleic acid profiles.J Anim Sci 2010,88(5)∶1842-7.
13.Bauchart D,Roy A,Lorenz S,Chardigny JM,Ferlay A,Gruffat D,Sébédio JL,Chilliard Y,Durand D∶Butters varying in trans 18:1 and cis-9,trans-11 conjugated linoleic acid modify plasma lipoproteins in the hypercholesterolemic rabbit.Lipids 2007,42(2)∶123-33.
14.Vannice G,Rasmussen H∶Position of the academy of nutrition and dietetics:Dietary fatty acids for healthy adults.J Acad Nutr Diet 2014,114(1)∶136-53.
15.CCAC,Olfert ED,Cross BM,McWilliams AA(Eds)∶Guide to the Care and Use of Experimental Animals,Volume 1.2nd edition.Ottawa,Ontario,Canada∶Canadian Council on Animal Care(CCAC);1993.
16.Nielsen JH,Sorensen B,Skibsted LH,Bertelsen G∶Oxidation in pre-cooked minced pork as influenced by chill storage of raw muscle.Meat Sci 1997,46(2)∶191-7.
17.CIE∶Recommendations on uniform color spaces-Color difference equations-Phychometric color terms.In Commission Internationale de l'Eclairage(publication No.15,supplement No.2 8-12.Paris,France∶CIE;1978∶8-12. 18.Shibata K∶Spectrophotometry of opaque biological materials.In Methods of Biochemical Analysis,Volume 9.Edited by Glick D.New York∶Interscience;1966∶217-234.
19.Krzywicki K∶Assessment of relative content of myoglobin,oxymyoglobin and metmyoglobin at the surface of beef.Meat Sci 1979,3(1)∶1-10.
20.Folch J,Lees M,Stanley GHS∶A simple method for the isolation and purification of total lipids from animal tissues.J Biol Chem 1957,226(1)∶497-509.
21.Kramer JKG,Hernandez M,Cruz-Hernandez C,Kraft J,Dugan MER∶Combining results of two GC separations partly achieves determination of all cis and trans 16:1,18:1,18:2 and 18:3 except CLA isomers of milk fat as demonstrated using ag-ion SPE fractionation.Lipids 2008,43(3)∶259-73.
22.Cruz-Hernandez C,Deng Z,Zhou J,Hill AR,Yurawecz MP,Delmonte P,Mossoba MM,Dugan MER,Kramer JKG∶Methods for analysis of conjugated linoleic acids and trans-18:1 isomers in dairy fats by using a combination of gas chromatography,silver-ion thin-layer chromatography/gas chromatography,and silver-ion liquid chromatography.J AOAC Int 2004,87(2)∶545-62.
23.Gómez-Cortés P,Bach A,Luna P,Juárez M,de la Fuente MA∶Effects of extruded linseed supplementation on n-3 fatty acids and conjugated linoleic acid in milk and cheese from ewes.J Dairy Sci 2009,92(9)∶4122-34.
24.AMSA∶Research Guidelines for Cookery,Sensory Evaluation,and Instrumental Tenderness Measurements of Fresh Meat.Champaign,IL.∶American Meat Science Association;1995.
25.SAS∶SAS for Windows,v.9.3.In SAS/STAT on-Line user's Guide:Statistics. Cary,NC,USA∶SAS Institute,Inc;2009.
26.Anderson DB,Kauffman RG,Kastenschmidt LL∶Lipogenic enzyme activities and cellularity of porcine adipose tissue from various anatomical locations.J Lipid Res 1972,13(5)∶593-9.
27.Lee JH,Yamamoto I,Jeong JS,Nade T,Arai T,Kimura N∶Relationship between adipose maturity and fatty acid composition in various adipose tissues of Japanese Black,Holstein and Crossbred(F1)steers.Anim Sci J 2011,82(5)∶689-97.
28.Hood RL,Thornton RF∶Site variation in the deposition of linoleic acid in adipose tissue of cattle given formaldehyde-treated sunflower seed. Aust J Agric Res 1976,27(6)∶895-902.
29.Kramer JKG,Sehat N,Dugan MER,Mossoba MM,Yurawecz MP,Roach JAG,Eulitz K,Aalhus JL,Schaefer AL,Ku Y∶Distributions of conjugated linoleic acid(CLA)isomers in tissue lipid classes of pigs fed a commercial CLA mixture determined by gas chromatography and silver ion-highperformance liquid chromatography.Lipids 1998,33(6)∶549-58.
30.Shingfield KJ,Bonnet M,Scollan ND∶Recent developments in altering the fatty acid composition of ruminant-derived foods.Animal 2013,7(SUPPL.1)∶132-62.
31.Johnson GH,Fritsche K∶Effect of Dietary Linoleic Acid on Markers of Inflammation in Healthy Persons:A Systematic Review of Randomized Controlled Trials.J Acad Nutr Diet 2012,112(7)∶1029-41.e15.
32.Ganesan B,Brothersen C,McMahon DJ∶Fortification of Foods with Omega-3 Polyunsaturated Fatty Acids.Crit Rev Food Sci Nutr 2014,54(1)∶98-114.
33.Wood JD,Enser M,Fisher AV,Nute GR,Sheard PR,Richardson RI,Hughes SI,Whittington FM∶Fat deposition,fatty acid composition and meat quality:A review.Meat Sci 2008,78(4)∶343-58.
34.Vlaeminck B,Fievez V,Cabrita ARJ,Fonseca AJM,Dewhurst RJ∶Factors affecting odd-and branched-chain fatty acids in milk:A review.Animal Feed Sci Tech 2006,131(3-4)∶389-417.
35.Liu SJ,Bu DP,Wang JQ,Sun P,Wei HY,Zhou LY,Yu ZT∶Effect of ruminal pulse dose of polyunsaturated fatty acids on ruminal microbial populations and duodenal flow and milk profiles of fatty acids.J Dairy Sci 2011,94(6)∶2977-85.
36.Pethick DW,Harper GS,Oddy VH∶Growth,development and nutritional manipulation of marbling in cattle:A review.Aust J Exp Agr 2004,44(7)∶705-15.
37.Wongtangtintharn S,Oku H,Iwasaki H,Toda T∶Effect of branched-chain fatty acids on fatty acid biosynthesis of human breast cancer cells.J Nutr Sci Vitaminol 2004,50(2)∶137-43.
38.Cai Q,Huang H,Qian D,Chen K,Luo J,Tian Y,Lin T∶13-Methyltetradecanoic acid exhibits anti-tumor activity on T-cell lymphomas in vitro and in vivo by down-regulating p-AKT and activating caspase-3.PLoS ONE 2013,8(6)∶e65308.
39.Ran-Ressler RR,Khailova L,Arganbright KM,Adkins-Rieck CK,Jouni ZE,Koren O,Ley RE,Brenna JT,Dvorak B∶Branched chain fatty acids reduce the incidence of necrotizing enterocolitis and alter gastrointestinal microbial ecology in a neonatal rat model.PLoS One 2011,6(12)∶e29032.
40.Felix TL,Zerby HN,Moeller SJ,Loerch SC∶Effects of increasing dried distillers grains with solubles on performance,carcass characteristics,and digestibility of feedlot lambs.J Anim Sci 2012,90(4)∶1356-63.
41.Hristov AN,Kennington LR,McGuire MA,Hunt CW∶Effect of diets containing linoleic acid-or oleic acid-rich oils on ruminal fermentation and nutrient digestibility,and performance and fatty acid composition of adipose and muscle tissues of finishing cattle.J Anim Sci 2005,83(6)∶1312-21.
42.Jacome-Sosa M,Lu J,Wang Y,Ruth M,Wright D,Reaney M,Shen J,Field C,Vine D,Proctor S∶Increased hypolipidemic benefits of cis-9,trans-11 conjugated linoleic acid in combination with trans-11 vaccenic acid in a rodent model of the metabolic syndrome,the JCR:LA-cp rat.Nutr Metabol 2010,7(1)∶1-10.
43.Wang Y,Jacome-Sosa MM,Proctor SD∶The role of ruminant trans fat as a potential nutraceutical in the prevention of cardiovascular disease.Food Res Int 2012,46(2)∶460-8.
44.Jaudszus A,Jahreis G,Schlörmann W,Fischer J,Kramer R,Degen C,Rohrer C,Roth A,Gabriel H,Barz D,Gruen M∶Vaccenic acid-mediated reduction in cytokine production is independent of c9,t11-CLA in human peripheral blood mononuclear cells.Biochim Biophys Acta 2012,1821(10)∶1316-22.
45.Sofi F,Buccioni A,Cesari F,Gori AM,Minieri S,Mannini L,Casini A,Gensini GF,Abbate R,Antongiovanni M∶Effects of a dairy product(pecorino cheese)naturally rich in cis-9,trans-11 conjugated linoleic acid on lipid,inflammatory and haemorheological variables:A dietary intervention study.Nutr Metab Cardiovasc Dis 2010,20(2)∶117-24.
46.Roy A,Chardigny JM,Bauchart D,Ferlay D,Lorenz S,Durand D,Gruffat D,Faulconnier Y,Sébédio JL,Chilliard Y∶Butters rich either in trans-10-C18:1 or in trans-11-C18:1 plus cis-9,trans-11 CLA differentially affect plasma lipids and aortic fatty streak in experimental atherosclerosis in rabbits. Animal 2007,1(3)∶467-76.
47.Harfoot CG,Hazlewood GP∶Lipid metabolism in the rumen.In Hobson PN. Edited by Stewart CS.Academic and Professional,London,UK∶The Rumen Microbial Ecosystem;2007∶382-426.
48.Elmore JS,Campo MM,Enser M,Mottram DS∶Effect of lipid composition on meat-like model systems containing cysteine,ribose and polyunsaturated fatty acids.J Agric Food Chem 2002,50(5)∶1126-32.
49.Channon HA,Lyons R,Bruce H∶Sheep Meat Flavour and Odour:A Review.A Final Report Prepared for the Sheep CRC.2003.http∶//www.premier1supplies.com/ sheep-guide/wp-content/uploads/2013/01/Sheepmeat_flavour_review.pdf.(accessed 28 January 2014).
Cite this article as:Mapiye et al.∶Improving beef hamburger quality and fatty acid profiles through dietary manipulation and exploitation of fat depot heterogeneity.Journal of Animal Science and Biotechnology 2014 5∶54.
Submit your next manuscript to BioMed Central and take full advantage of:
• Convenient online submission
• Thorough peer review
• No space constraints or color figure charges
• Immediate publication on acceptance
• Inclusion in PubMed, CAS, Scopus and Google Scholar
• Research which is freely available for redistribution
Submit your manuscript at
www.biomedcentral.com/submit
10.1186/2049-1891-5-54
*Correspondence∶mike.dugan@agr.gc.ca
1Agriculture and Agri-Food Canada,Lacombe Research Centre,6000 C and E Trail,Lacombe,Alberta T4L 1 W1,Canada Full list of author information is available at the end of the article
©2014 Mapiye et al.;licensee BioMed Central Ltd.This is an Open Access article distributed under the terms of the Creative Commons Attribution License(http∶//creativecommons.org/licenses/by/4.0),which permits unrestricted use,distribution,and reproduction in any medium,provided the original work is properly credited.The Creative Commons Public Domain
Dedication waiver(http∶//creativecommons.org/publicdomain/zero/1.0/)applies to the data made available in this article,unless otherwise stated.
Results:Perirenal fat versus SCF hamburgers FA had 14.3%more(P<0.05)18∶0,11.8%less cis(c)9-18∶1(P<0.05),and 1.82%more total trans(t)-18∶1 mainly in the form of t11-18∶1.During sensory evaluation,PRF versus SCF hamburgers had greater(P<0.05)mouth coating,but the difference was less than one panel unit.Examining effects of steer diet within PRF hamburgers,feeding the SS compared to the control diet increased(P<0.05)t-18∶1 by 2.89%mainly in the form of t11-18∶1,feeding DGGS-15 diet led to no further changes(P>0.05),but feeding DDGS-30 diet reduced the proportions of(P<0.05)of t-18∶1 chiefly t11-18∶1.Feeding SS and DDGS diets had small but significant(P<0.05)effects on hamburger sensory attributes and oxidative stability.
Conclusions:Feeding high-forage diets including SS and 15%DDGS,and taking advantage of the FA heterogeneity between fat depots offers an opportunity to differentially enhance beef hamburgers with 18∶2n-6 biohydrogenation products(i.e.,t11-18∶1)with potential human health benefits without compromising their sensory attributes and oxidative stability during retail display.
杂志排行
Journal of Animal Science and Biotechnology的其它文章
- Dietary requirements of synthesizable amino acids by animals:a paradigm shift in protein nutrition
- The liver transcriptome of two full-sibling Songliao black pigs with extreme differences in backfat thickness
- A direct real-time polymerase chain reaction assay for rapid high-throughput detection of highly pathogenic North American porcine reproductive and respiratory syndrome virus in China without RNA purification
- Identification and quantitative mRNA analysis of a novel splice variant of GPIHBP1 in dairy cattle
- Changes in various metabolic parameters in blood and milk during experimental Escherichia coli mastitis for primiparous Holstein dairy cows during early lactation
- Effect of reduced energy density of close-up diets on dry matter intake,lactation performance and energy balance in multiparous Holstein cows
