月季种质资源的倍性变异及核型多样性研究
2015-08-09刘承源何亨辉邱文昌方炎明
刘承源,王 辉,何亨辉,邱文昌,方炎明
(1 南京林业大学 生物与环境学院,南京 210037;2 南京林业大学 南方现代林业创新中心,南京 210037;3深圳市公园管理中心园艺推广部,广东 深圳 518040)
The genus Rosa L.is comprised of about 200 species and 30 000cultivated varieties[1-4],but only 7species have contributed to modern roses:R.chinenesis,R.gigantea,R.multiflora,R.mos-chata,R.wichuraiana,R.gallica and R.foetida[5].Old garden roses play a significant role in the breeding of modern roses[6];however,these germplasm resources are not always sufficiently used.The genetic background of modern roses is relatively narrow compared to the abundant genetic diversity of wild species and old garden roses[7-8].China was considered as the home of Rosa[6,9].Between the late 18th century and early 19th century,wild species and old garden roses native to China were introduced into western countries,such as Great Britain and France.The European breeders used these materials to repeatedly hybridize with indigenous species and finally obtained‘La France’in 1867,the first Hybrid Tea rose.At present,more efficient breeding strategies are needed due to the increasing demand for new modern roses.Many species and old garden roses have desirable traits,such as superior disease resistance,higher fragrance levels,winter-hardiness and the absence of thorns[10-11].Breeders can be innovative in the production of new varieties,provided that the desirable traits from wild species and old garden roses can be used in the breeding of modern roses[12].
Chromosomes,as the carriers of genes,dominate the reproductive process and breeding behavior of plants through their structure and behavior.Therefore,studies of the number,structure and cytological behavior of chromosomes are of great significance for understanding the genetics of plants.Rosa was among the first genera of garden flowers to attract the attention of cytologists and molecular biologists because of the importance of cultivated roses as horticultural plants,the small chromosome sizes and the small nuclear genomes[13-15].Cytological studies in Rosaincluded chromosome numbers,karyotypes and meiotic configuration frequencies using traditional squashing and pressing methods[16-27],C-banding[28],FISH[29-32],chromosome doubling[33]and flow cytometry[34].The genus Rosa is characterized by a typical polyploid chromosome series based on multiples of seven.The chromosome numbers of wild species range from 2n=2x=14to 2n=10x=70[22,35-37],old garden roses are 2n=2x,3xand 4x=14,21and 28,respectively[21,26,34,38],and the modern cultivars are mostly tetraploid[4].
In this study,22taxa from the genus Rosa,in-cluding 5wild species,8old garden roses and 9 modern roses(Table 1),were analyzed using karyological techniques.The main aims of this study were:(1)detecting the role of wild species and old garden roses in modern rose breeding;(2)providing a cytological basis for directional hybridization and germplasm resource innovations in rose.
1 Material and methods
1.1 Plant materials
In total,22taxa,including 5wild species,8old garden roses and 9modern roses,growing in the rose germplasm resources nursery of Shenzhen Park Service(Guangdong,China)were selected for this study(Table 1).Among the nine modern roses,five miniature roses with unknown names were recorded as M1,M2,M3,M4and M5in this paper.‘Mount Shasta×Kosai’and‘Charles de Gaull×Kardinal 85’were the F1of a hybridization,and‘Fragrant Butterfly’was bred by crossing climber‘Crimson Glory’with ‘May Day’.The other plants were observed to confirm their identities using morphological traits and literary descriptions[2,4,11].Tender branch cuttings were used in this study.
1.2 Pretreatment,dissociation and staining
All cytological observations were made from root tips.In the middle of April,appropriate numbers of central branches from each variety(species)were selected and cut into pieces(5-8cm),and then the stem segments were cultivated on rice husk ash.Branches were immersed into 150mg/L indolebutyric acid for 2-3hto improve the rooting rate.Shoot tips were collected when the roots grew to 1-2cm.They were then pretreated in 0.002mol/L 8-hydroxyquinoline for 4hat 20 ℃,then fixed with Carnoy’s fluid(absolute ethyl alcohol:acetic acid=3∶1)at 4℃for 24h.The fixed tips were dissociated with 5mol/L HCl for 30min at room temperature,stained with carbol fuchsin for 2hand squashed on glass slides for observation.Photos were taken under a Nikon E800microscope(NIKON JAPAN).
1.3 Data analysis
Observations were made on nuclei at the somatic mitotic metaphase,and measurements of chromosome arms were taken from at least 30wellspread metaphases of 5root tips.The standards of Levan et al.[39]and Li and Chen[40]were adopted for the analysis of the relative lengths,arm ratios and chromosome patterns.The karyotype symmetry was in accordance with the classification of Stebbins[41].The asymmetry index was calculated according to Arano[42]and the constitution of relative length of the genome was calculated according to Kuo et al.[43].Ikaros software was used to analyze the chromosomes.
2 Results and analysis
The metaphase chromosomes,karyotypes and karyotype pattern for each studied material are shown in Fig.1and 2,respectively.Karyotype information for the studied materials are listed in Table 2.
2.1 Wild species
The five wild species studied were all diploid(2n=2x=14),except R.multifloravar.cathayensis(2n=3x=21),and all of the chromosomes of R.multifloraand R.bracteatacontained median centromeres(2n=2x=14=14m),while the other four species consisted of median and sub-median centromeric chromosomes.The genome of R.bracteata was composed of all four types of chromosomes.Those of R.multiflora and R.odorata were composed of long chromosomes(L),medium long chromosomes(M2)and medium short chromosomes(M1),but not short chromosomes(S).The genome of R.multiflora var.catheyensis was composed of medium long chromosomes(M2),medium short chromosomes(M1)and short chromosomes(S),while that of R.laevigata was composed of only medium long chromosomes(M2)and medium short chromosomes(M1).Asymmetry indices ranged from 57.57%to 62.75%,and the ratio of the longest to the shortest chromosome varied from 1.60 to 1.85.The karyotype of R.multiflora var.cathayensis was 2A,and the rest were 1A.

Table 1 Rosa L.materials used in this study
2.2 Chinese old garden roses

Fig.1 Metaphase chromosome morphology of 22 Rosa L.materialsThe numbers are provided in Table 1next to the material names;The same as below
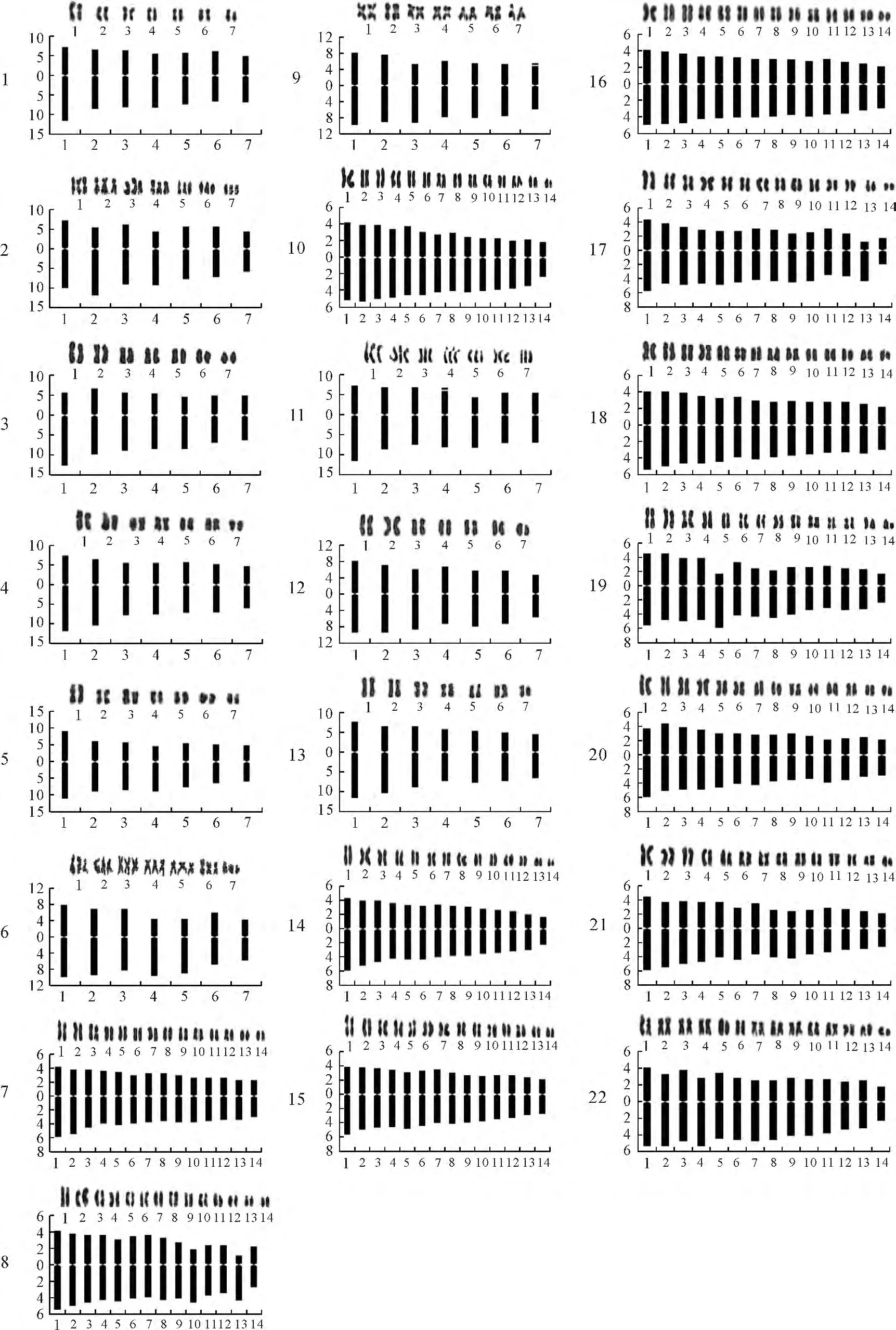
Fig.2 Karyotypes and karyotype models of the 22 Rosa L.materialsThe chromosomes of the tetraploid materials were paired in the same way as the diploid materials because it is unknown whether they are homologous or heterogenous
Marked differences in chromosome numbers were found among the eight Chinese old garden roses.‘Lüe’(R.chinensis var.viridiflora),‘Yuling-long’and‘Zihongxiang’are diploid with 14chromosomes(2n=2x=14),‘Chunshui Lübo’and‘Simianjing’are triploid with 21chromosomes(2n=3x=21),‘Dafugui’,‘Huzhongyue’and‘Qinglian Xueshi’are tetraploid with 28chromosomes(2n=4x=28).The genomes of‘Chunshui Lübo’and‘Yulinglong’were composed of three types of chromosomes,medium long chromosomes(M2),medium short chromosomes(M1)and short chromosomes(S).Those of‘Simianjing’and‘Zihongxiang’were composed of long chromosomes(L),medium long chromosomes(M2)and medium short chromosomes(M1).The genomes of the other cultivars were composed of all four types.Asymmetry indices ranged from 56.04%to 58.96%,and the ratio of the longest to the shortest chromosome varied from 1.66to 2.22.Satellites were found in the seventh chromosome in‘Lüe’and the fourth chromosome in ‘Simianjing’.One pair of sub-terminal centromeric chromosomes were found in‘Huzhongyue’(2n=4x=28=24m+2sm+2st),whereas the other cultivars all consisted of median and sub-median centromeric chromosomes.The karyotype of‘Qinglian Xueshi’was 1B,those of‘Chunshui Lübo’and‘Huzhongyue’were 2A,and the rest were 1A.

Table 2 Karyological characteristics of the materials
2.3 Modern roses
The nine modern roses were all tetraploid with 28chromosomes(2n=4x=28).Genomes of‘M4’and‘Mount Shasta×Kosai’were composed of median,sub-median and sub-terminal centromeric chromosomes,and they shared a karyotype formula(2n=4x=28=22m+4sm+2st).The chromosomes of the other four miniature roses contained median centromeres(2n=4x=28=28m).The other three cultivars were composed of median and sub-median centromeric chromosomes.The genomes of these nine cultivars were comprised of all four types of chromosomes.Asymmetry indices ranged from 55.72%to 60.28%,and the ratio of the longest to the shortest chromosome varied from 1.78to 2.76.The karyotypes of‘M4’and‘Mount Shasta×Kosai’were 2B,‘M1’,‘Crimson Glory’and‘Fragrant Butterfly’were 1B,and the others were 1A.
3 Discussion
Li et al[44]noted that it was difficult to observe chromosomes using root somatic cells from Rosa because the roots regenerated from the cutting are thin and solid.Moreover,colchicine and aqueous solutions of saturated paradichlorobenzene were worth recommending for processing the materials.Shoot tips and aqueous solution of saturated paradichlorobenzene are often chosen for pretreatment when observing the chromosomes of Rosa[16,18-19,21-25,38].However,in our experiment,we found that,it is also a good method to pretreat root tips regenerated from cuttings with 0.002mol/L 8-hydroxyquinoline,coupled with proper sampling times and temperatures.
It is generally acknowledged that the basic chromosome of Rosoideae is x=7-9[45].The first report that Rosa was x=7was made by T ckholm in 1920[46].Later,Hurst[35]performed a statistical analysis of the chromosome numbers of thousands of species,mutations and varieties,and achieved the same conclusion.On the basis of both arm ratio and chromosome length,the authors concluded that karyotypes of Rosa were largely symmetrical among the genus[18-27].Our study confirmed those previous reports.The materials were composed of a basic chromosome number of seven.Also,the karyotypes of most of the studied materials contained median and sub-median centromeric chromosomes.
Many species occurred at only one ploidy level,but a few formed a polyploid series[3,24,36].According to Liu and Li[16],there were diploids and tetraploids in R.multiflora var.cathayensis(2n=2x=14or 2n=4x=28,respectively).In this study,where more than 30cells were observed,however,the chromosome number of the genome was determined to be 2n=3x=21.This intraspecific polyploidy had been reported in R.laxa[27],several species(R.macrophylla,R.sertata and R.webbiana)of Section Cinnamomeae[24],R.odorata var.erubescens[23]and R.platyacantha[2,27].Polyploidy has long been recognized as a prominent force in evolutionary diversification and is an important cytogenetic mechanism in plant evolution and rapid speciation[41].Thus,a comprehensive study would require a greater number of population samples,representing all the variations and the full range of habitats.What is more,the results indicated that different ploidy levels were associated with the different regions and habitats where the germplasm resources grew[27].
The chromosomes of the tetraploid materials were paired in the same way as the diploid materials because it is unknown whether they are homologous or heterogenous.Different ploidy levels were also observed among three old garden roses.According to Jian et al[21]and Zhang et al[38],‘Huzhongyue’and ‘Qinglian Xueshi’were triploid(2n=3x=21),and ‘Zihongxiang’was tetraploid(2n=4x=28).In our study,‘Huzhongyue’and ‘Qinglian Xueshi’were tetraploid,whereas‘Zihongxiang’was diploid(2n=2x=14).Further studies combining materials and cultivation locations are necessary to determine whether these results indicate a polyploidy of cultivars or another situation.Additionally,there were also several slight differences in karyotypes and karyotype formulae among some materials that had already been studied[21,26,38],which may be due to different degrees of chromosome contraction caused by the various conditioning fluids and/or pretreatment times
It is believed that wild species,old garden roses and modern roses exhibit karyological diversity and lack sub-terminal centromeric chromosomes[18-27].Our study confirmed these beliefs.Four karyotypes(1A,2A,1Band 2B)were found among the accessions in our research,and all of the karyotypes contained median and sub-median centromeric chromosomes,except three accessions(sample no.9,18and 20).Levizky[47]first suggested the concepts of karyotype symmetry and asymmetry,with the basic trend of karyotype evolution in plants progressing from symmetry to asymmetry[41].The accessions used in this study were consistent with that view-point.The karyotypes of wild species were 1Aand 2A,old garden roses were 1A,2Aand 1B,and modern roses were 1A,1Band 2B.
The ploidy levels in wild species ranged from 2n=2x=14to 2n=10x=70[22,35-37].However,genomes with more than a tetraploid chromosome number were rare in old garden roses and modern roses.Several authors predicted that high-ploidy hybrids must have emerged during the distant hybridization process and may have been discarded by breeders[16,48].What the appearances of high-ploidy cultivars and whether they are worth selecting require further study in the future.
[1]REHDER A.Manual of Cultivated Trees and Shrubs(2nd ed)[M].New York:MacMillan,1940:996-998.
[2]KU T C,ROBERTSON K R.Rosa(Rosaceae)[M]//WU Z Y,RAVEN P H.Flora of China(vol 9).Beijing:Science Press,St Louis:Missouri Botanical Garden Press,2003:339-381.
[3]WISSEMANN V.Conventional taxonomy of wild roses[M]//ROBERTS A,DEBENER T,GUDIN S.Encyclopedia of Rose Sciences,1st edn.Oxford:Elsevier Science,2003:111-117.
[4]CAIRNS T.Modern roses XII[M].New York:Academic Press,2007.
[5]WYLIE A P.The history of garden roses[J].J.Roy.Hort.Soc.,1954,79:555-571.
[6]WANG G L.A study on the history of Chinese roses from ancient works and images[J].Acta Hort.,2007,751:347-356.
[7]MATSUMOTO S,KOUCHI M,YABUKI J,et al.Phylogenetic analyses of the genus Rosa using the matK sequence:molecular evidence for the narrow genetic background of modern roses[J].Sci.Hort.,1998,77:73-82.
[8]BRUNEAU A,STARR J R,JOLY S.Phylogenetic relationships in the genus Rosa:new evidence from chloroplast DNA sequences and an appraisal of current knowledge[J].Syst.Bot.,2007,32(2):366-378.
[9]陈俊愉.中国花卉品种分类学[M].北京:中国林业出版社,2001:132-135.
[10]SCARIOT V,AKKAK A,BOTTA R.Characterization and genetic relationship of wild species and old garden roses based on microsatellite analysis[J].J.Amer.Soc.Hort.Sci.,2006,131(1):66-73.
[11]张佐双,朱秀珍.中国月季[M].北京:中国林业出版社,2006:170-172.
[12]DEBENER T,MATTIESCH L.Construction of a genetic linkage map for roses using RAPD and AFLP markers[J].Theor.Appl.Genet.,1999,99((5):891-899.
[13]ROWLEY G D.Chromosome studies and evolution in Rosa[J].B.Jard.Bot.Nat.Belg.,1967,37(1):45-52.
[14]DICKSON E E,ARUMUGANATHAN K,KRESOVICH S,et al.Nuclear DNA content variation within the Rosaceae[J].Amer.J.Bot.,1992,79(9):1 081-1 086.
[15]YOKAYA K,ROBERTS A V,MOTTLEY J,et al.Nuclear DNA amounts in roses[J].Ann.Bot.,2000,85(4):557-561.
[16]LIU D H(刘东华),LI M X(李懋学).A study on karyotypes of some flowers of Rosain China[J].J.Wuhan Bot.Res.(武汉植物学研究),1985,3(4):403-408(in Chinese).
[17]MA Y(马 燕),CHEN J Y(陈俊愉).Chromosome studies of seven roses[J].J.Fujian For.Coll.(福建林学院学报),1991,11(2):215-218(in Chinese).
[18]MA Y(马 燕),CHEN J Y(陈俊愉).A study on chromosomes of some modern rose cultivars[J].J.Hebei For.Coll.(河北林学院学报),1992,7(1):12-18(in Chinese).
[19]MA Y,CRANE C F,BYRNE D H.Karyotypic relationships among some Rosaspecies[J].Caryologia,1997a,50(3-4):317-326.
[20]LIU J(刘 佳),DING X L(丁晓六),YU CH(于 超),et al.Karyotype analysis of 7 Rosa hybrid and 5 Rosa rugosa cultivas[J].J.Northwest Agric.&For.Uni.(Nat.Sci.Edi.)(西北农林科技大学学报·自然科学版),2013,41(5):1-8(in Chinese).
[21]JIAN H Y(蹇洪英),ZHANG H(张 颢),WANG Q G(王其刚),et al.Karylogical study of Chinese old garden roses[J].Acta Hort.Sin.(园艺学报),2010,37(1):83-88(in Chinese).
[22]JIAN H Y,ZHANG H,TANG K X,et al.Decaploidy in Rosa praelucens Byhouwer(Rosaceae)endemic to Zhongdian Plateau,Yunnan.China[J].Caryologia,2010,63(2):162-167.
[23]JIAN H Y,ZHANG H,ZHANG T,et al.Karyotype analysis of different varieties of Rosa odorata Sweet[J].J.Plant Genet.Res.,2010,11(4):457-461.
[24]JIAN H Y,ZHANG T,WANG Q G,et al.Karyological diversity of wild Rosain Yunnan,Southwestern China[J].Genet.Resour.Crop Evol.,2013,60(1):115-127.
[25]WANG J Y(王金耀),YU CH(于 超),LUO L(罗 乐),et al.Karyotype analysis of Rosa laxa,modern rose and their interspecific hybrids[J].Acta Bot.Boreal.-Occident.Sin.(西北植物学报),2014,34(3):488-494(in Chinese).
[26]LUO L(罗 乐),ZHANG Q X(张启翔),BAI J R(白锦荣),et al.Karyotype analysis of sixteen Chinese traditional rose cultivars[J].J.Beijing For.Uni.(北京林业大学学报),2009,31(5):90-95(in Chinese).
[27]YU C,LUO L,PAN H T,et al.Karyotype analysis of wild Rosaspecies in Xinjiang,Northwestern China[J].J.Amer.Soc.Hort.Sci.,2014,139(1):39-47.
[28]PRICE L,SHORT K C,ROBERTS A V.Poor resolution of C-bands and the presence of B-chromosomes in Rosa rugosa ‘scabrosa’[J].Caryologia,1981,34(1):69-72.
[29]MA Y,ISLAM-FARIDI M N,CRANE C F,et al.In situ hybridization of ribosomal DNA to rose chromosomes[J].J.Hered.,1997b,88(2):158-161.
[30]JIAN H Y,MIN T,TING Z,et al.Chromosome variation from sect.chinensis(RosaL.)through Chinese old garden roses to modern rose cultivars[J].Acta Hort.,2013b,977:157-166.
[31]AKASAKA M,UEDA Y,KOBA T.Karyotype analysis of wild rose species belonging to septets A by fluorescence in situ hybridization[J].Chromosom.Sci.,2002,6:17-26.
[32]AKASAKA M,UEDA Y,KOBA T.Karyotype analysis of wild rose species belonging to septets B,C,and D by molecular cytogenetic method[J].Breed.Sci.,2003,53(2):177-182.
[33]KHOSRAVI P,KERMANI M J,NEMATZADEH G A,et al.Role of mitotic inhibitors and genotype on chromosome doubling of Rosa[J].Euphytica,2008,160(2):267-275.
[34]LI L H,OGASAWARA R,MA C,et al.Ploidy analysis of Chinese old garden roses and their progenies[J].Agric.Sci.&Tech.,2013,14(4):620-623.
[35]HURST C C.Chromosome numbers and characters in Rosaand their significance in the origin of species[J].Exp.Genet.,1925,37:534-550.
[36]DARLINGTON C D,WYLIE A P.Chromosome Atlas of Flowering Plants[M].London:Geroge Allan & Unwin Ltd,1955:134-138.
[37]CRANE Y M,BYRNE D H.Karyology.[M]//ROBERTS A,DEBENER T,GUDIN S.Encyclopedia of Rose Sciences(1st ed).Oxford:Elsevier Science,2003:267-285.
[38]ZHANG T(张 婷),JIAN H Y(蹇洪英),WANG Q G(王其刚),et al.Study on karyotype of eleven Chinese old garden roses[J].Southwest Chin.J.Agric.Sci.(西南农业学报),2010,23(5):1 656-1 659(in Chinese).
[39]LEVAN A,FREDGA K,SANDBERG A A.Nomenclature for centromeric position on chromosomes[J].Hereditas,1964,52(2):201-220.
[40]LI M X(李懋学),CHEN R Y(陈瑞阳).The standardization about the karyotype analysis[J].J.Wuhan Bot.Res.(武汉植物学研究),1985,3(4):297-302(in Chinese).
[41]STEBBINS G L.Chromosomal Evolution in Higher Plants[M].London:Edward Arnold,1971.
[42]ARANO H.Cytological studies in subfamily Carduoideae(Compositae)of Japan[J].Bot.Mag.Tokyo,1963,76(2):32-39.
[43]KUO S R,WANG T T,HUANG T C.Karyotype analysis of some Formosana gymnosperms[J].Taiwania,1972,17(1):66-80.
[44]李懋学,张赞平.作物染色体及其研究技术[M].北京:中国农业出版社,1996:292-297.
[45]RAVEN P H.The bases of angiosperm phylogeny:cytology[J].Ann.Mo.Bot.Gard.,1975,62:724-764.
[46]T CKHOLM G.On the cytology of the genus Rosa.A preliminary note[J].Svensk.Bot.Tidskr.,1920,14(2-3):300-311.
[47]LEVIZKY G A.The karyotype in systematic[J].Bull.Appl.Bot.Genet.Plant Breed.,1931,27:220-240.
[48]HEINICH O,DARLINGTON C D.Chromosome botany and the origins of cultivated plants[J].Biometrical J.,1966,8(4):283-284.
