Effect of solvent on the crystal morphology of royal demolition explosive
2015-04-22WANGDongxu王东旭CHENShusen陈树森LIYanyue李燕月YANGJiayun杨佳云WEITianyu魏田玉LILijie李丽洁
WANG Dong-xu(王东旭), CHEN Shu-sen(陈树森), LI Yan-yue(李燕月),YANG Jia-yun(杨佳云), WEI Tian-yu(魏田玉), LI Li-jie(李丽洁),
(1.School of Materials Science and Engineering, Beijing Institute of Technology, Beijing 100081, China;2.Gansu Yinguang Chemical Industry Group Co Ltd, Baiyin 730900, China)
Effect of solvent on the crystal morphology of royal demolition explosive
WANG Dong-xu(王东旭)1, CHEN Shu-sen(陈树森)1, LI Yan-yue(李燕月)1,YANG Jia-yun(杨佳云)1, WEI Tian-yu(魏田玉)2, LI Li-jie(李丽洁)
(1.School of Materials Science and Engineering, Beijing Institute of Technology, Beijing 100081, China;2.Gansu Yinguang Chemical Industry Group Co Ltd, Baiyin 730900, China)
Important crystal faces that dominate the crystal morphology of royal demolition explosive (RDX) in vacuum were analyzed with the attachment energy (AE) method. Molecular dynamics (MD) simulations were used to calculate the interaction energies between these crystal faces and different solvent molecules for an attachment energy correction. Growth habits in the presence of different solvents were generated. The results showed that some crystal faces in solutions became morphologically more important than that in vacuum while others became less important. Thus, crystal shape and surface property changed a lot with the variation of crystal faces. The results from calculation were in agreement with those from the re-crystallization experiment, which indicated that cyclohexanone (CH) was a promising solvent to modify the crystal morphology of RDX for obtaining products with regular shape and high purity, while butyrolactone (BL) played a great role in improving the surface electrostatic property of RDX.
royal demolition explosive (RDX); crystal morphology; solvent; molecular dynamics; attachment energy
Crystal growth proceeds in the interface between crystal and solvent (the crystallization surface)[1]. The solvent used for the crystallization has a great influence on the crystal morphology, thus resulting in the variation of crystal surface property.
Generally, explosive crystal with regular shape and smooth surface has a high packing density, one of the most important product performance parameters[2-3], and a low sensitivity[4]. royal demolition explosive (RDX) is an excellent single compound explosive used for various high performance propellants and mixed explosives. Recently, much attention has been paid on improving the crystal morphology of RDX (such as particle size, crystal shape and defects) through re-crystallization experiment to enhance its detonation and safety performance[5-12].
As a tool to predict a certain spatial form of the organic molecules, “molecular dynamics simulation”[13-14]has been widely used in the investigation on the properties of condensed-phase materials. Up to date, there has been a great number of studies by MD simulations focusing on the solvent effect on the crystal morphology[15-18]. Ter Horst et al. studied the effect of solvent on the crystal morphology of RDX through MD simulation and predicted the influence of acetone (AC), CH, BL and DP on the morphology of RDX based on a molecular modeling technique[2-3]. However, no further study was done to give a comprehensive and detailed interpretation of the effects of different solvents on RDX crystal morphology and crystal surface property.
In this study, we investigated the effects of different solvents on the crystal morphology of RDX with the MD method. Crystal habits of RDX were calculated with the AE method after MD simulations were performed, and then the corresponding re-crystallization experiment was carried out for the comparison with calculations.
1 Computational
1.1 Selection of force-field
Condensed-phase optimized molecular potential for atomistic simulation study (COMPASS)[19-21]was chosen as the force-field for simulations. Electrostatic and van der Waals interaction were calculated by Ewald[22]and atom-based method, respectively. The accuracy for Ewald was 0.1 J·mol-1, and the cutoff distance for atom-based method was set to be 1.55 nm.
1.2 Attachment energy method
For the crystal which grows under steady-state, the center-plane spacinghhklis proportional to the relative linear growth rate for crystal faceRhklas well as attachment energyEa, that is
hhkl∝Rhkl∝Ea
(1)
In the theoretical prediction of the crystal morphology,the AE is one of the most reliable methods[23-25].
1.3 Calculation of the crystal morphology of RDX in vacuum
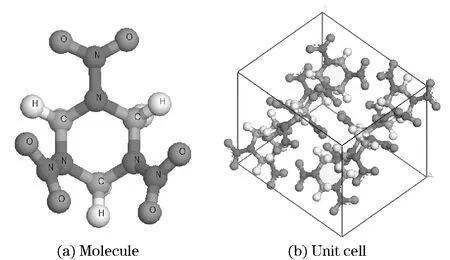
Fig.1 Molecule and unit cell of RDX
According to the cell parameters of RDX[26], the unit cell model was constructed (Fig.1). Molecular mechanics geometry optimization was performed on the unit cell (Tab.1).
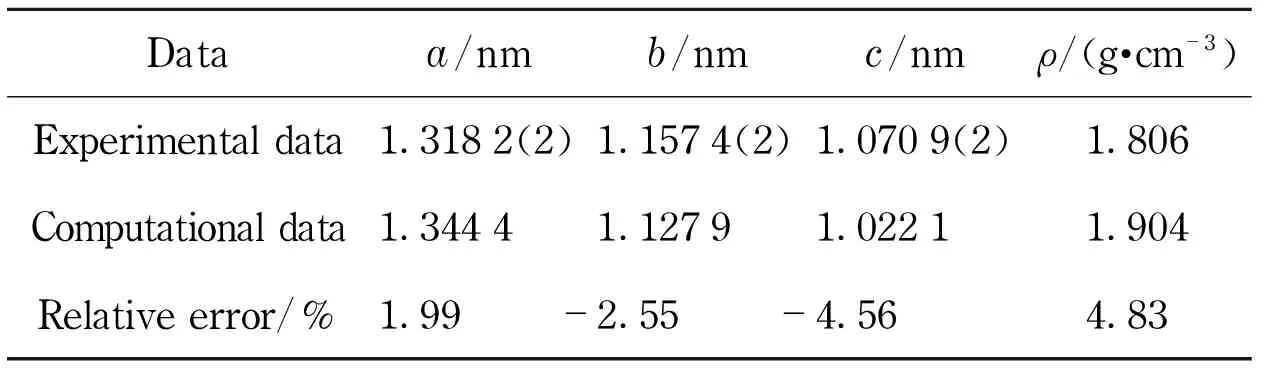
Tab.1 Experimental and computational data of RDX cell parameters
1.4 Attachment energy correction

(2)
ThecorrectiontermEsdescribing the energy of solvent binding on the crystal face (hkl) can be calculated using
Es=EiAac/Asu
(3)
whereEiis the interaction energy between the solvent layer and the crystal surface,Asuis the surface area of the simulated model in the (hkl) direction, andAacis the accessible solvent surface area of the crystal face in unit cell. The interaction energyEiis evaluated by the relationship as
Ei=Et-Esu-Eso
(4)
whereEtis the total energy of the crystal surface and the solvent layer,Esuis the energy of the crystal surface without the solvent layer, andEsois the energy of the solvent layer without the crystal surface.
The accessible solvent surfaceAacis obtained by calculating the Connolly surface. The solvent molecule is modeled by a sphere which is, in effect, rolled over the molecule to generate a smooth outer-surface contour, i.e. the Connolly surface[27]. It can provide a quantitative approach to locate the regions of the crystal surface which would be accessible areas for solvent binding[28]. To predict the crystal morphology in solutions instead of vacuum, Eq.(1) is transformed to
(5)
1.5 Calculation of the crystal morphology of RDX in different solutions
The crystal face structure of RDX, parallel to the (hkl) plane with a depth of three unit cells, was constructed as a periodic super cell of 2×2 unit cells. Subsequently, the structure was optimized by the molecular mechanics (MM).
Six solvents, AC, acetonitrile (AN), CH, cyclopentanone (CP), BL and dimethyl sulfoxide (DMSO), were chosen to modify the RDX crystal morphology. Despite different (hkl) directions, all the faces were constructed as super cells with the same unit size (2×2). Therefore, a solvent layer containing 100 additive molecules was constructed and optimized by MM and MD in turn.
In order to study the effect of solvents on the crystal morphology, double-layer interfacial model (Fig.2) was built, i.e. the solvent layer was adsorbed on the (hkl) crystal faces alongcaxis. The crystal face structure was constrained during the following process. Vacuum slab with 5 nm thickness was built above the solvent layer to eliminate the effect of additional free boundaries on the structure.

Fig.2 Double-layer interfacial model for calculation
After the potential energy minimization, the model described above was considered as ensemble of fixed particle number with constant volume at constant temperature (NVT) and simulated by a MD simulation at the original temperature of 298 K. The temperature control was set following Andersen[13]. The time step of 1fs was used for all the simulations. Equilibration stage with the total simulation time of 100 ps (100 000 fs) was performed until the whole system reached a steady state when the variation of temperature and energy tended to be smooth with a fluctuation of 10% or below, following by production stage of 100 ps during which the data were collected for subsequent analysis.
2 Experimental
2.1 Materials
The raw RDX material (99.6% with HMX about 0.4%, analyzed by high performance liquid chromatography, HPLC) was provided by Gansu Yinguang Chemical Industry Group Co, Ltd. All solvents used were analytical reagent.
2.2 Re-crystallization experiment
50 g RDX was added into 200 mL AC, AN, CH, CP, BL or DMSO under agitation at a stirring rate of 200 r/min and then the solution was slowly heated up to 90 ℃ on a water bath maintained at constant temperature. The heating was stopped and the stirring rate was decreased to 80 r/min after 2 h when RDX was completely dissolved. 0.2 g seed ultrafine RDX (D50-3μm) crystal was added into the solution when a small amount of crystals started to precipitate as the solution was cooled down to 70-75 ℃. The cooling rate was set to be 0.5 ℃/min under well-controlled agitation as the temperature continuously decreased to the ambient temperature (20-25 ℃). After the crystallization finished, RDX crystals were separated by vacuum filtration, washed with water (6×50 mL), and dried.
2.3 Characterization
The morphology of the final RDX crystal was observed using the biological microscope (XSP-8CA). The purity of the final product was analyzed by the HPLC analyzer (Varian5000). The surface electrostatic voltage of the final RDX crystal was determined using the electrostatic voltmeter (EST101).
3 Results and discussions
3.1 Crystal morphology of RDX in vacuum
Crystal morphology in vacuum was calculated based on the AE method, as displayed in Fig.3. The morphologically important (exhibiting in grown crystals) faces with their corresponding parameters were listed in Tab.2.

Fig.3 RDX crystal habit in vacuum calculated using the AE method
Tab.2 Morphologically important faces of RDX crystal in vacuum calculated using the AE method
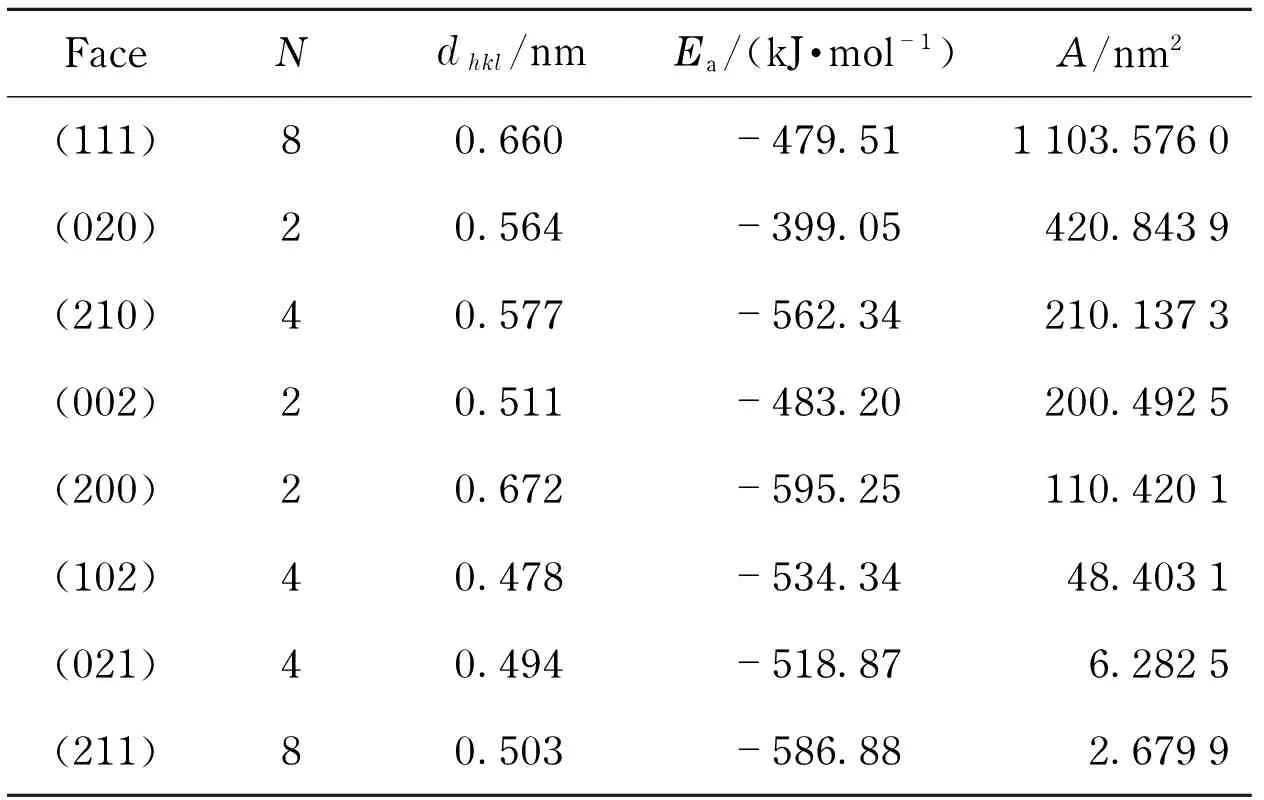
FaceNdhkl/nmEa/(kJ·mol-1)A/nm2(111)80.660-479.511103.5760(020)20.564-399.05420.8439(210)40.577-562.34210.1373(002)20.511-483.20200.4925(200)20.672-595.25110.4201(102)40.478-534.3448.4031(021)40.494-518.876.2825(211)80.503-586.882.6799
N-number of a certain crystal face (hkl) of a grown crystal;dhkl-lattice-plane spacing;A-total facet area
3.2 Properties of the morphologically important crystal faces of RDX in vacuum
Molecular arrangement of the morphologically important crystal faces (Fig.4) illustrated the surface property intuitively. As seen from Fig.4, the (210) face had a roughest surface, while the (020) face was found to be the smoothest on the molecular level. Groups such as nitro, methylene of RDX molecules were distributed on the crystal faces, different only from the orientations, positions and densities. The (211) (102) (210) faces had nitro groups completely stretching out of the surface while nitro groups were observed to be exposed at the (111) (021) faces. The (002) (200) faces had a distribution of both oxygen and hydrogen atoms, while the (020) face allowed only the exposure of hydrogen atoms.

Fig.4 Molecule arrangement of the morphologically important crystal faces of RDX (2×2 unit cells)
For the accessible surface area calculation, the grid interval was set to be 0.04 nm at a probe radius of 0.10 nm. The ratios ofAactoAhklin Tab. 3, which reflected the degree of contact between crystal faces and solvent molecules, were in agreement with the results observed from Fig.4. As a rough surface dotted with gaps available for solvent molecules to step in, the (210) face had the maximum of the possibility, while the smooth face (020) had the minimum.
Tab.3 Surface and accessible surface areas of the morphologically important faces of RDX from calculation
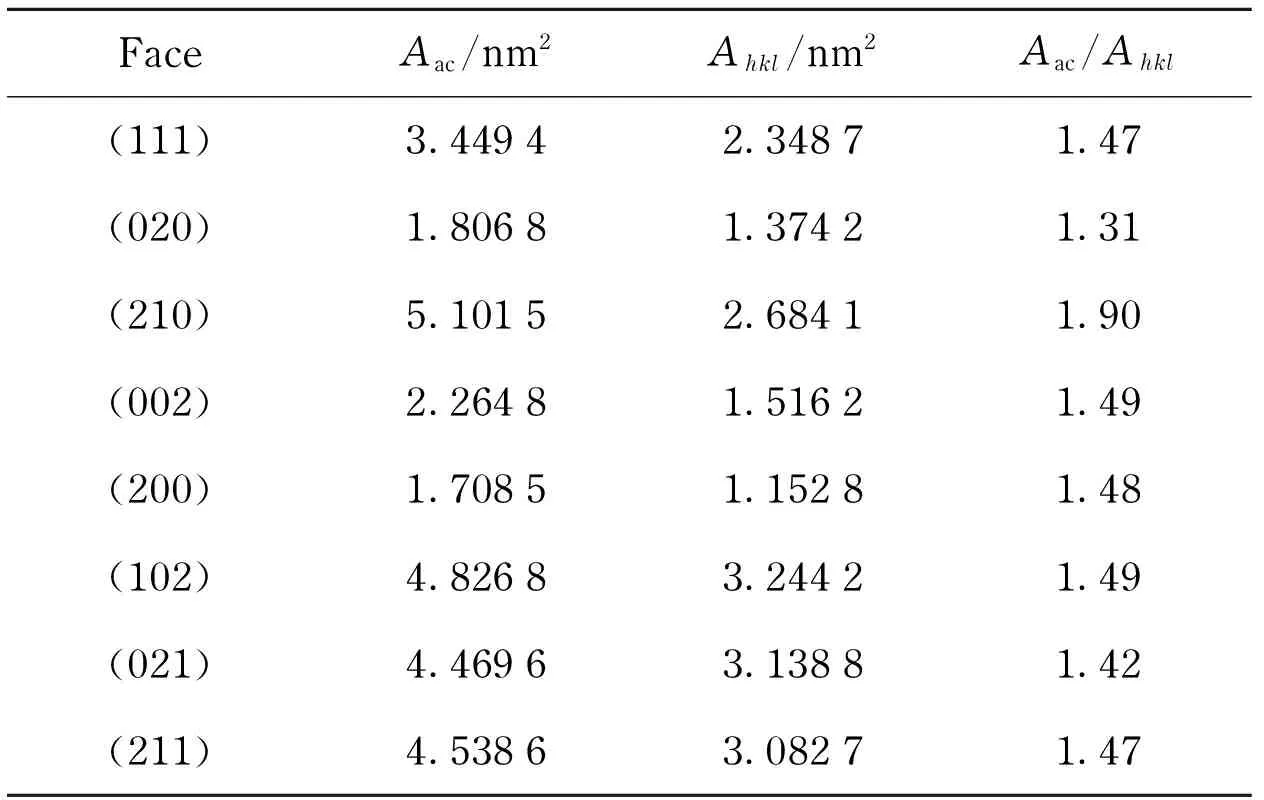
FaceAac/nm2Ahkl/nm2Aac/Ahkl(111)3.44942.34871.47(020)1.80681.37421.31(210)5.10152.68411.90(002)2.26481.51621.49(200)1.70851.15281.48(102)4.82683.24421.49(021)4.46963.13881.42(211)4.53863.08271.47
Ahkl-surface area of the crystal face (hkl) in unit cell
In the morphology calculation, charges were assigned by the COMPASS force-field. As listed in Tab.4, crystal faces had quite different electric charge distributions from each other due to different atomic distributions, resulting in the differ-ence in surface charge property. The (211) face presented high electronegativity owing to the nitro groups stretching out of the surface. By contrast, the (020) was even positive charged mainly because the surface was occupied by hydrogen atoms.
Tab.4 Surface charge of the morphologically important crystal faces of RDX from calculation
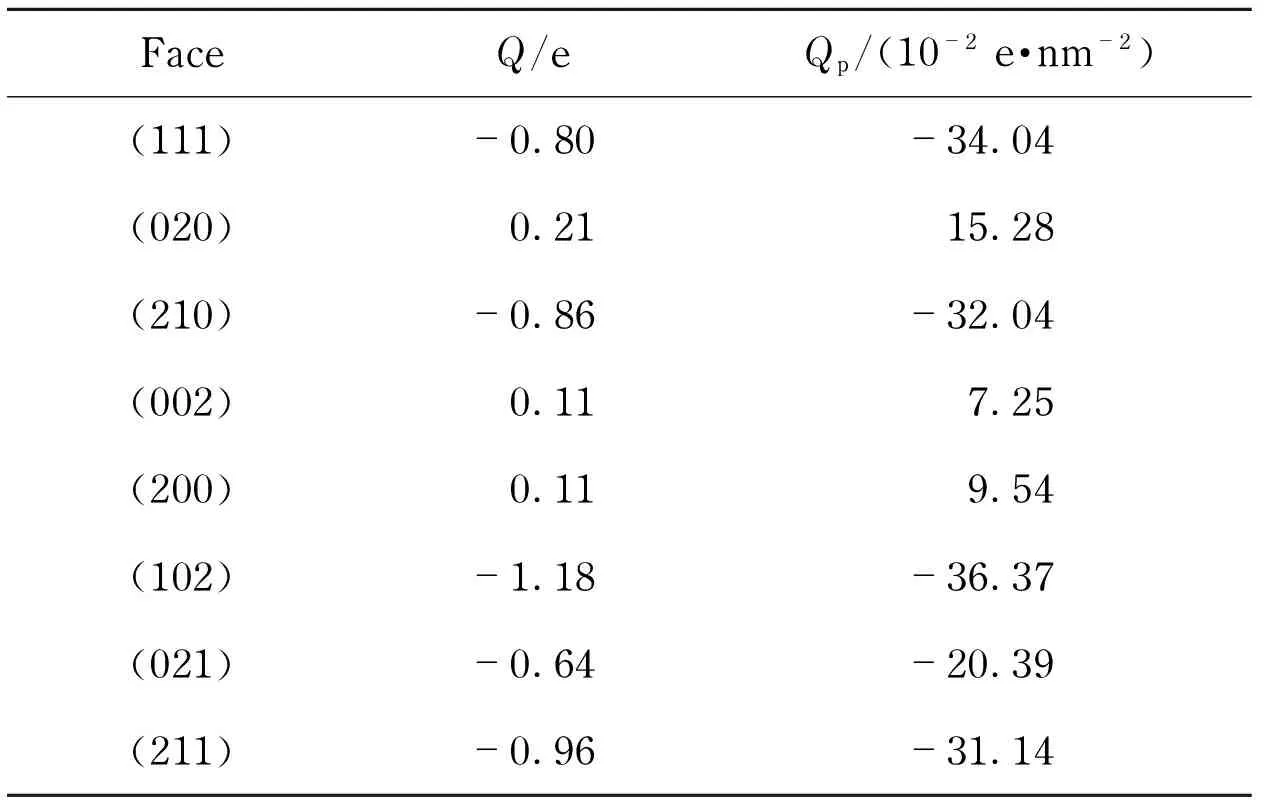
FaceQ/eQp/(10-2e·nm-2)(111)-0.80-34.04(020)0.2115.28(210)-0.86-32.04(002)0.117.25(200)0.119.54(102)-1.18-36.37(021)-0.64-20.39(211)-0.96-31.14
Q-surface charge of each crystal slice in unit cell;e-the electric charge carried by a proton, 1.6×10-19Coulomb;Qp-surface charge of the crystal face per unit area
3.3 Attachment energy
Interaction energies between different solvent molecules and different crystal faces were listed in Tab.5. Six solvents were sorted from left to right approximately in descending order of interaction energies. From Tab. 5, the strongest interaction was found between crystal face and BL or AN, followed by ketone, and the last was observed in DMSO.
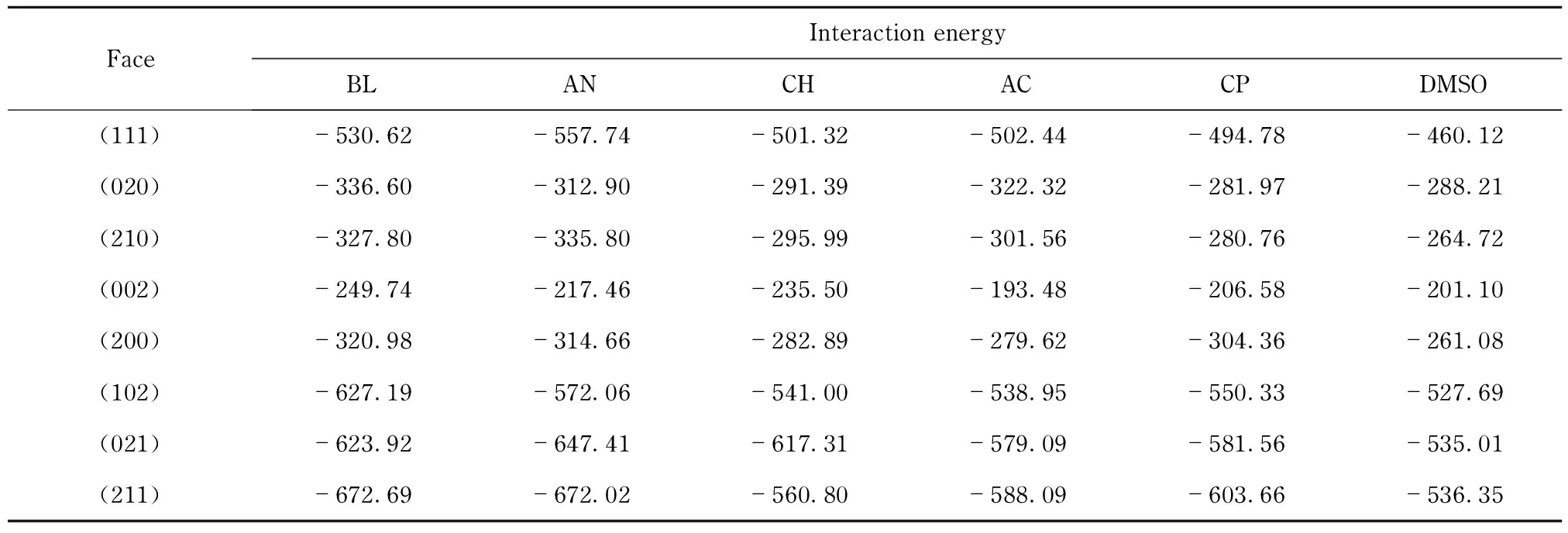
Tab.5 Interaction energies between different solvent molecules and RDX crystal faces from calculation kJ·mol-1
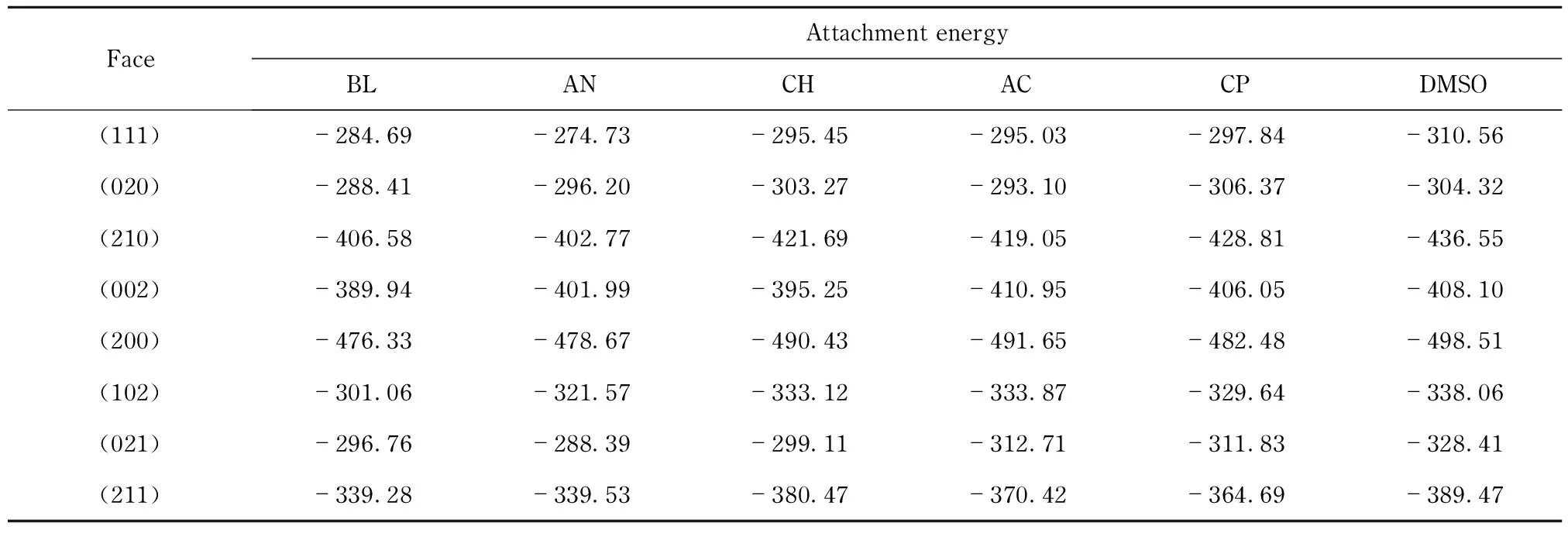
Tab.6 Attachment energies of RDX crystal faces in different solutions from calculation kJ·mol-1
3.4 Crystal morphology of RDX in different solutions
Crystal habits in different solutions were calculated using the AE method based on the results of Tab. 6. Compared with that in vacuum, all the crystal habits were found to have a remarkable change either in crystal shape or exhibiting crystal faces.
The spheroidization degree is an important aspect to characterize the regularity of explosive crystals. Compared with the normal RDX, the spherical RDX had a higher packing density[29]. Here, the relative specific surface area (the ratio of specific surface area to that of a sphere with the same volume) and aspect ratio (the ratio of length to width) were introduced to measure the spheroidization degree of RDX crystals, as listed in Tab. 7. A decline was found in the data of relative specific surface areas in the order from “BL” to “DMSO” with the descending of the interaction energies between solvents and crystal surfaces (Tab. 5), revealing that high interaction energy might be propitious to the spheroidization degree[7]. The aspect ratios, however, showed a minor fluctuation around that of vacuum with the maximum up to 1.681 and the minimum down to 1.580.
Morphologically important faces of RDX crystal habits in vacuum and different solutions with their percentage areas were listed in Tab. 8. Considering the percentage area was too small, the (021) (211) faces were negligible in the calculation. The (002) (200) faces were invisible in almost all solutions owing to high growing rates, and the (102) face became morphologically more important with the percentage area from 2.3% to over 20%. Except the (020), all the other faces had obvious changes in the percentage area. On the whole, the number of exhibiting faces decreased from original eight to five at most, and the percentage areas of exhibiting faces tended to be average.
Tab.7 Spheroidization parameters of RDX crystals in vacuum and different solutions from calculation
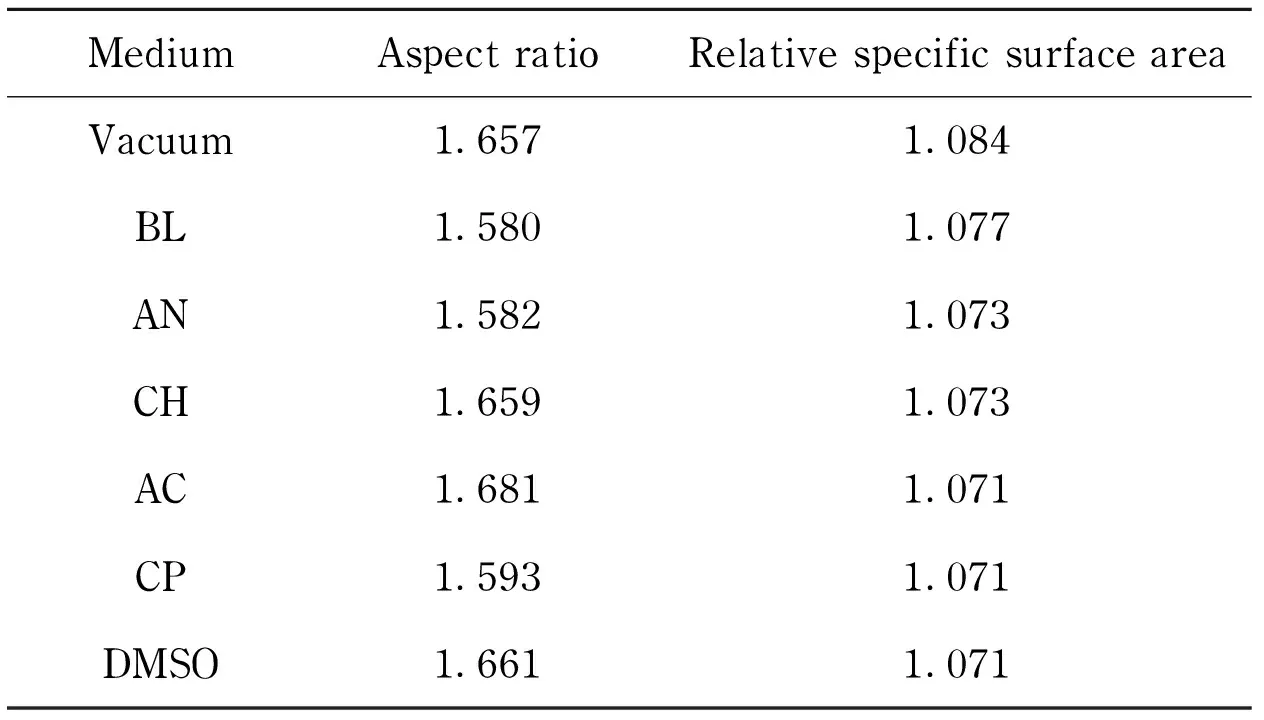
MediumAspectratioRelativespecificsurfaceareaVacuum1.6571.084BL1.5801.077AN1.5821.073CH1.6591.073AC1.6811.071CP1.5931.071DMSO1.6611.071
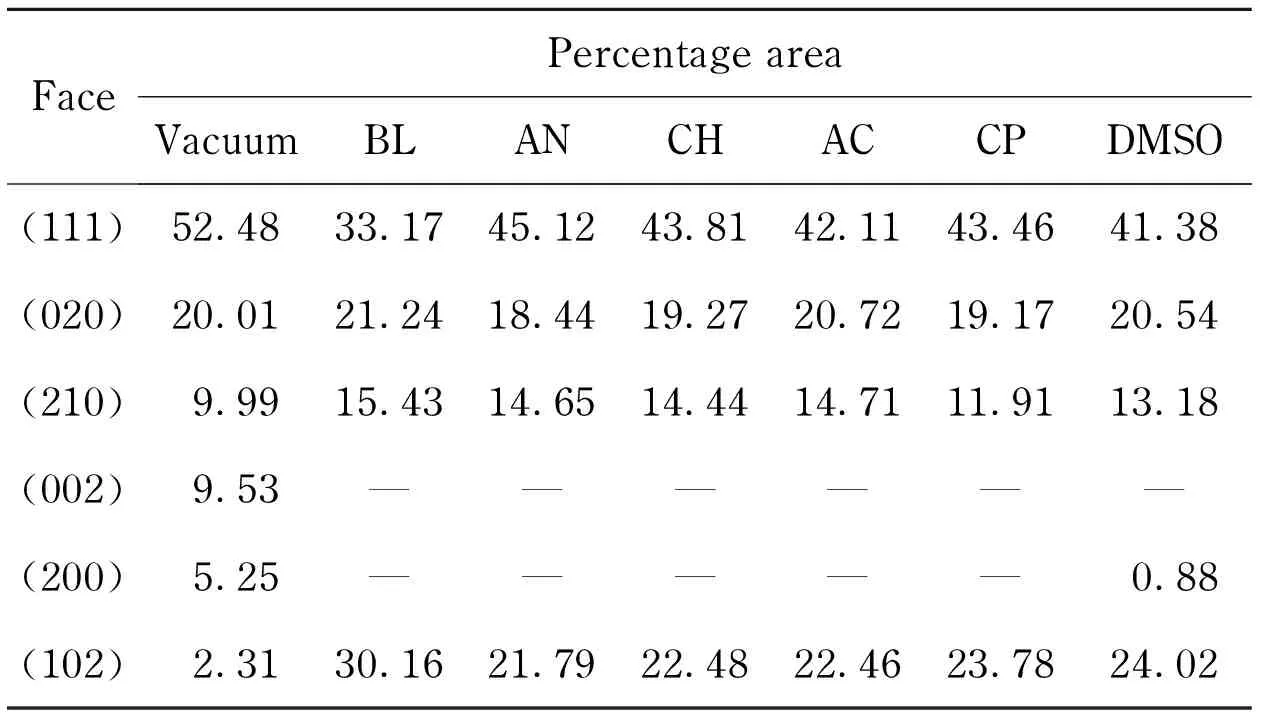
Tab.8 Percentage areas of RDX crystal faces in vacuum and different solutions from calculation %
Crystal habits of RDX re-crystallized from different solutions were observed in experiments (Fig.5). Generally, they were similar to each other in crystal shape and morphology. However, as could be seen, not all solvents achieved desirable results. Crystal habits of RDX grown from DMSO and CP solutions had an irregular shape with excessive edges and corners, while products from BL had too many defects. By comparison, CH could provide products of high quality with regular shapes and minor defects. In addition, HPLC results showed that crystals of RDX re-crystallized from solutions had a higher purity up to 99.9% with HMX<0.1%.
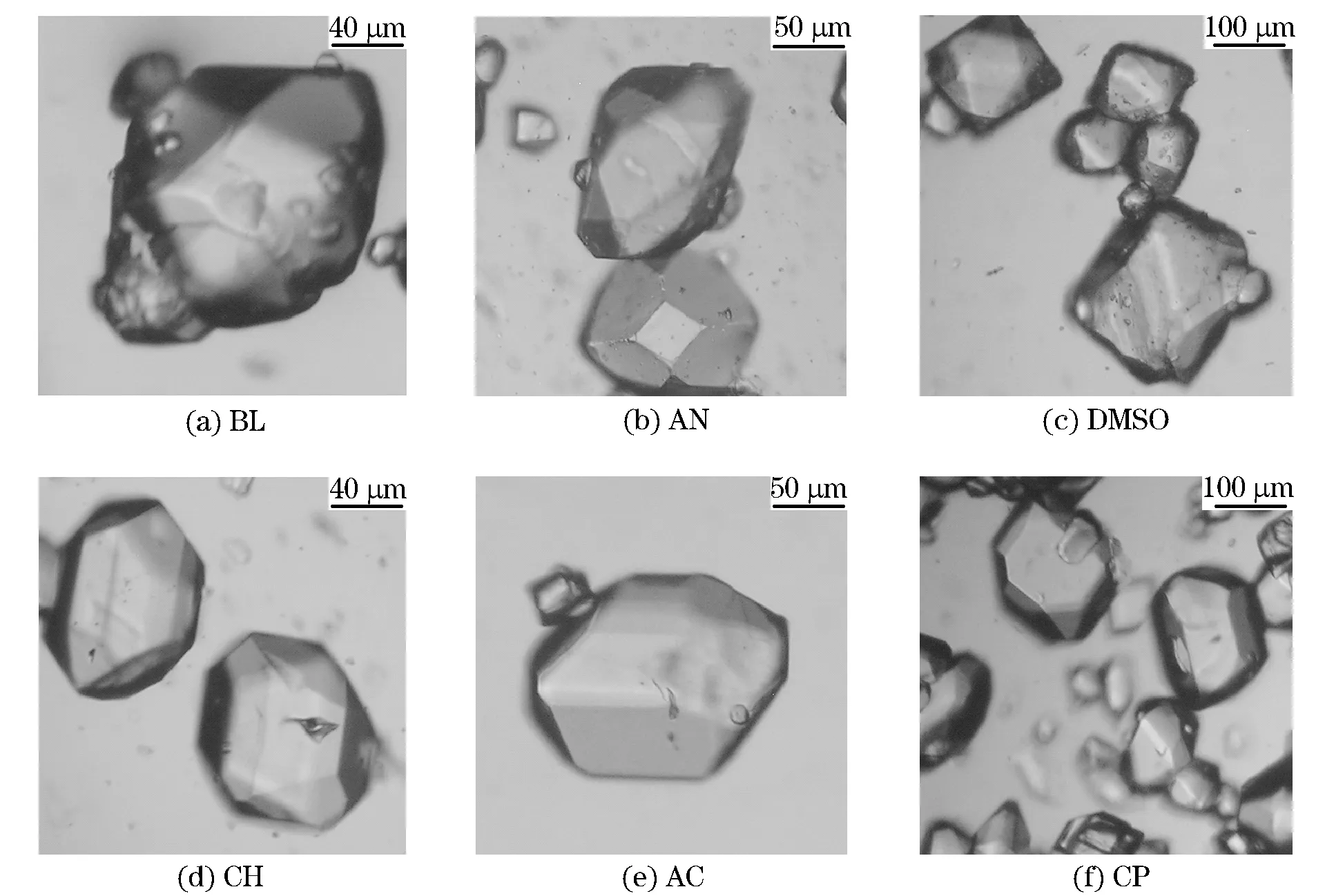
Fig.5 Micrographs for RDX crystal habits re-crystallized from different solvents
3.5 Surface electrostatic property of RDX crystals in different solutions
Total surface charge of RDX crystalsQtcould be calculated based on the data ofQpin Tab.4 andAin Tab.2 (areas of the solvent-effected crystal faces were not listed) by
(6)
whereQpiandAidenote the surface charge and total facet area of each crystal face (hkl), respectively.
For the comparison with the results from calculation, surface electrostatic voltage (Usu) of the final products was analyzed experimentally, as listed in Tab. 9. It was obvious that |Qt| decreased with the addition of solvents, which revealed that the solvent effects consumed a portion of charge by the interaction with the electric groups at the faces, making the crystal surface more stable.
Tab.9 Total surface charge of RDX crystals from calculation and surface electrostatic voltage of RDX products re-crystallized from solutions

MediumQt/(102e)Usu/kVVacuum-3.73—BL-3.30-1.37AN-3.29-1.07CH-3.28-0.83AC-3.24-0.77CP-3.21-0.57DMSO-3.14-0.37
Furthermore, we could find that there was an approximate positive correlation between the surface electrostatic voltage determined and the total surface charge. It meant that the corresponding |Usu| was low when the |Qt| was small. Fig.6 made a further illustration of the correlation of these two parameters. Therefore, the calculation of the total surface charge could provide a relatively reasonable way for the prediction of the surface electrostatic voltage.
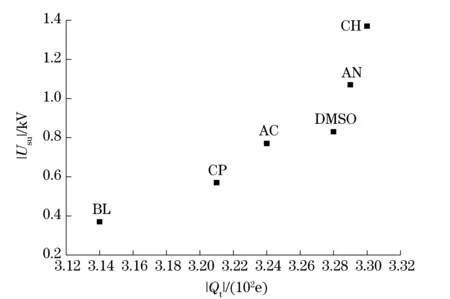
Fig.6 Scatter diagram of |Usu| as function of |Qt| for RDX crystals in different solutions
The electrostatic performance of an explosive is an important aspect to estimate its safety[30]. Since electrostatic charge accumulating on the surface greatly affects safe use of RDX, the solvent is used for the modification of crystal morphology and crystal surface property during the re-crystallization, aiming to control surface static electricity of RDX. As could be found from Tab.9, BL was expected to be the best selection to reduce the surface electrostatic charge of RDX.
4 Conclusions
① The morphologically important crystal faces of RDX in vacuum had different atomic and charge distributions with each other, resulting in different surface properties.
② The number of exhibiting faces of RDX crystal habits calculated using the AE method decreased and the percentage areas of exhibiting faces tended to be average due to the effects of solvents. Crystal shapes of RDX had obvious change with the variation of percentage areas of crystal faces and became more spherical in solutions.
③ Crystal habits of RDX re-crystallized from different solutions were similar to each other in crystal morphology. As solvent in the re-crystallization, CH could provide RDX products of high quality in shape, transparency and purity.
④ The total surface charge of RDX crystals obtained from calculation had an approximate correlation with the surface electrostatic voltage of final products determined experimentally, which provided a way to predict the surface electrostatic property of RDX. Both results indicated that BL was expected to be an effective solvent to reduce the surface electrostatic phenomenon of RDX.
[1] Li Guohua, Wang Dawei, Huang Zhiliang. Study on interface-phase crystal growth[J]. Journal of Synthetic Crystals, 2001, 30(2): 171-177. (in Chinese)
[2] ter Horst J H, Geertman R M, van der Heijden A E, et al. The influence of a solvent on the crystal morphology[J]. Journal of Crystal Growth, 1999, 198-199(1): 773-779.
[3] ter Horst J H, Geertman R M, van Rosmalen G M. The effect of solvent on crystal morphology[J]. Journal of Crystal Growth, 2001, 230(1-2): 277-284.
[4] Li Hongzhen, Kang Bin, Li Jinshan, et al. Effects of RDX crystal characteristics on shock sensitivities[J]. Chinese Journal of Energetic Materials, 2010, 18(5): 487-491. (in Chinese)
[5] Zhao Ruixian, Li Guangming, Gao Tianping, et al. Study on RDX granulate stage process[J]. Chinese Journal of Explosives & Propellants, 2003, 26(4): 67-70. (in Chinese)
[6] Yu Xianhan, Sima Tianlong, Sun kuande. Analysis of mechanical properties of RDX crystals obtained from different solvents[J]. Chinese Journal of Energetic Materials, 2004, 12(2): 78-81. (in Chinese)
[7] Feng Xuesong, Zhao Shengxiang, Li Xiaoping. Study of lowering sensitivity of RDX by recrystallization[J]. Chinese Journal of Explosives & Propellants, 2007, 30(3): 45-47. (in Chinese)
[8] Feng Xuesong, Zhao Shengxiang, Li Xiaoping. The shock sensitivity of a recrystallizing RDX[J]. Chinese Journal of Energetic Materials, 2007, 15(6): 581-582. (in Chinese)
[9] Kim D Y, Kim K J. Correlation between quantity of defect and supersaturation in RDX crystallization usingγ-butyrolactone and water as solvent[J]. Chemical Engineering Research & Design, 2010, 88(11): 1461-1466.
[10] Kim D Y, Kim K J, Kim H S. Semi-quantitative study on the inclusion in cooling crystallization of RDX using various solvents[J]. Propellants, Explosives, Pyrotechnics, 2010, 35(1): 38-45.
[11] Spitzer D, Baras C, Schafer M R, et al. Continuous crystallization of submicrometer energetic compounds[J]. Propellants, Explosives, Pyrotechnics, 2011, 36(1): 65-74.
[12] Zhao Xue, Rui Jiuhou, Feng Shunshan. Recrystallization method for preparation of spherical RDX[J]. Transactions of Beijing Institute of Technology, 2011, 31(1): 5-7. (in Chinese)
[13] Andersen H C. Molecular dynamics simulations at constant pressure and/or temperature[J]. Journal of Chemical Physics, 1980, 72(4): 2374-2383.
[14] Wu X. A simple method for calculating detonation parameters of explosives[J]. Journal of Energetic Materials, 1985, 3(4): 263-277.
[15] Chen J X, Wang J K, Zhang Y, et al. Crystal growth, structure and morphology of hydrocortisone methanol solvate[J]. Journal of Crystal Growth, 2004, 265(1-2): 266-273.
[16] Piana S, Gale J D. Understanding the barriers to crystal growth: dynamical simulation of the dissolution and growth of urea from aqueous solution[J]. Journal of the American Chemical Society, 2005, 127(6): 1975-1982.
[17] Stoica C, Verwer P, Beekes H, et al. Understanding the effect of a solvent on the crystal habit[J]. Crystal Growth & Design, 2004, 4(4): 765-768.
[18] Li C L, Choi P. Molecular dynamics study on the effect of solvent adsorption on the morphology of glycothermally producedα-Al2O3particles[J]. Journal of Physical Chemistry C, 2008, 112(27): 10145-10152.
[19] Sun H, Rigby D. Polysiloxanes: ab initio force field and structural, conformational and thermophysical properties[J]. Spectrochimica Acta Part A, 1997, 53(8): 1301-1323.
[20] Sun H. COMPASS: An ab initio force field optimized for condensed-phase applications overview with details on alkane and benzene compounds[J]. Journal of Physical Chemistry B, 1998, 102(38): 7338-7364.
[21] Rigby D, Sun H, Eichinger B E. Computer simulations of poly (ethylene oxides): force field, PVT diagram and cyclization behavior[J]. Polymer International, 1998, 44(3): 311-330.
[22] Ewald P P. Evaluation of optical and electrostatic lattice potentials[J]. Annals of Physics, 1921, 64(3): 253-287.
[23] Hartman P. The attachment energy as a habit controlling factor I: Theoretical considerations[J]. Journal of Crystal Growth, 1980, 49(1): 145-156.
[24] Hartman P. The attachment energy as a habit controlling factor II: Application to anthracene, tin tetraiodide and ohrtorhombic sulphur[J]. Journal of Crystal Growth, 1980, 49(1): 157-165.
[25] Hartman P, Bennema P. The attachment energy as a habit controlling factor III: Application to corundum[J]. Journal of Crystal Growth, 1980, 49(1): 166-176.
[26] Choi C S, Prince E. The crystal structure of cyclotrimethylene-trinitramine[J]. Acta Crystallographica B, 1972, 28(9): 2857-2862.
[27] Connolly M L. Analytical molecular surface calculation[J]. Journal of Applied Crystallography, 1983, 16(5): 548-558.
[28] Jorgensen W L, Duffy E M. Prediction of drug solubility from structure[J]. Advanced Drug Delivery Reviews, 2004, 54(3): 355-366.
[29] Zhao Xue, Rui Jiuhou, Feng Shunshan. Effects of RDX physical morphology on properties of RDX base melt-cast explosive[J]. Journal of Nanjing University of Science and Technology, 2011, 35(5): 714-716. (in Chinese)
[30] Li Zhimin, Zhang Tonglai, Yang Li, et al. Progress on electrostatic performances of explosive[J]. Science & Technology Review, 2011, 29(26): 74-79. (in Chinese)
(Edited by Cai Jianying)
10.15918/j.jbit1004-0579.201524.0219
TF 55; O 7 Document code: A Article ID: 1004- 0579(2015)02- 0260- 09
Received 2013- 10- 09
E-mail: ndy12306@163.com
猜你喜欢
杂志排行
Journal of Beijing Institute of Technology的其它文章
- Nonlinear symbolic LFT model for UAV
- Novel scheme of high precision inertial measurement for high-speed rotating carriers
- Study on influencing factors of adapters separating with the underwater missile
- Fast-solving method for air-to-surface guided bombs’ allowable attack area
- Design and analysis of mechanical self-destruction and self-neutralization mechanism for submunition fuze
- Resilience approach for heterogeneous distributed networked unmanned weapon systems
