Microbially induced deposition of barium phosphates and its ingredient, morphology and size under different pH values
2015-03-01YuXiaoniuQianChunxiangWangXin
Yu Xiaoniu Qian Chunxiang Wang Xin
(School of Materials Science and Engineering, Southeast University, Nanjing 211189, China)(Research Institute of Green Construction Materials, Southeast University, Nanjing 211189, China)
Microbially induced deposition of barium phosphates and its ingredient, morphology and size under different pH values
Yu Xiaoniu Qian Chunxiang Wang Xin
(School of Materials Science and Engineering, Southeast University, Nanjing 211189, China)(Research Institute of Green Construction Materials, Southeast University, Nanjing 211189, China)
Abstract:A phosphate-mineralization microbe was used to induce barium phosphates precipitation, and the precipitates with different types were obtained under different pH values. The average crystallite size of the barium phosphates was calculated by particle size distribution curves, and the size of the products was 33.40, 29.37, 24.13, 47.76 and 96.53 μm when the pH values of the mixed solution are 7, 8, 9, 10 and 11, respectively. The results of X-ray diffraction (XRD) show that the structures of the particles controlled by the mixed solution are mainly BaHPO4when pH<10; the barium phosphates are synthesized by biological deposition which is the mixture of BaHPO4and Ba5(PO4)3OH when pH=10; when pH=11, the barium phosphates are also the mixtures, which are Ba5(PO4)3OH and BaNaPO4. The above results indicate that the phosphate-mineralization microbe can produce a certain enzyme which constantly hydrolyzes phosphate monoester in the mixed solution, and then ions are obtained.
Key words:phosphate-mineralization microbe; barium phosphates; morphology; X-ray diffraction; phosphate monoester
Received 2015-05-09.
Biographies:Yu Xiaoniu(1988—),male,graduate; Qian Chunxiang(corresponding author), female, doctor, professor, cxqian@seu.edu.cn.
Foundation items:The National Natural Science Foundation of China (No.51372038,No.51178104), the Scientific Research Foundation of Graduate School of Southeast University (No.YBJJ1453), the 333 Project of Jiangsu Province.
Citation:Yu Xiaoniu, Qian Chunxiang, Wang Xin.Microbially induced deposition of barium phosphates and its ingredient, morphology and size under different pH values[J].Journal of Southeast University (English Edition),2015,31(4):506-510.[doi:10.3969/j.issn.1003-7985.2015.04.013]
Microbially induced precipitation of inorganic compounds are natural inorganic/organic hybrid materials formed through a cooperative interaction of inorganic materials with organic macromolecules, where the macromolecules control the nucleation, growth, morphology, structure, and crystal orientation of the inorganic components[1-2]. The morphology and size of inorganic crystals are usually controlled by adding organic matrices, such as BaHPO4[3],CaCO3[4-6],LiFePO4/C[7],CdS[8],Ag2S[9-10], etc. Therefore, inorganic materials via bio-inspired precipitation have achieved much attention from biologists, chemists, material scientists, and so on[11-15].
Barium phosphates have been extensively studied in the past for their applications in different fields, such as catalysts, bioceramics, ionic conductivity, ferroelectrics, luminescence, metal-doped, corrosion resistance, etc. These applied domains show that barium phosphates are important inorganic phosphate materials. Barium phosphates are usually prepared by the sol-gel method, hydrothermal synthesis, chemical precipitation, and so on. However, few investigations focused on the synthesis, ingredients, size and morphology of barium phosphates through microbiological technique. In this work, the influence of pH on the ingredient, morphology and size of barium phosphates precipitated in the mixed solution of bacteria and substrate is studied. Bacterial body and biomacromolecules can tailor the size and shape of synthesized particles, which have important influences on the size and morphology of the products. Therefore, barium phosphates precipitated in the mixture solution is taken as a more complicated process than other methods because the microbial cells of negative charge and functional groups of organic matrix can chelate metal ionsand regulate nucleation, growth, size, and orientation of the crystals[3, 16-18].
1Materials and Methods
All reagents and solvents from commercial sources were used without further purification. Deionized water was self-made by our laboratory. The phosphate-mineralization microbe was selected to investigate microbially induced barium phosphates precipitation. Cultivation of the phosphate-mineralization microbe was conducted in a medium containing 3 g/L of beef extract, 5 g/L of peptone and 1 g/L of sodium chloride. The microbe with the OD600value of 1.69 was used in this study. In general, the harvested microorganisms were kept in a refrigerator at (4±0.2)℃ for stock prior to use.
Microbially induced deposition of barium phosphates was prepared as follows: 20 mmol of phosphate monoester was completely dissolved in deionized water (40 mL), and the solution was poured into 200 mL of bacterial solution. The mixed solution of bacteria and substrate was allowed to stand under static conditions for 24 h at (30±2)℃. The pH values of the mixed solution were adjusted to 7, 8, 9, 10 and 11 by using 18%HCl and 5%NaOH solution. Next, 20 mmol of BaCl2·2H2O were added to the above solution. After stirring, the precipitated solution was allowed to stand under static conditions for 24 h at ambient temperature. Finally, all precipitates were collected and characterized.
The phase purity of products was examined by the powder X-ray diffraction (XRD) with Bruker D8-Discover diffractometer using graphite-monochromatized high-intensity Cu Kα radiation (λ=0.154 06 nm). The scanning electron microscope (SEM, FEI Company, Netherlands, operating voltage 20 kV) with a Genesis 60S energy dispersive X-ray spectroscopy (EDS) spectroscopy system was used to conduct morphological studies and to measure the elemental compositions of the samples. The particle size was determined using a Microtrac S3500 particle size analyzer (Advanced Research Tools Corporation, Downers Grove, Illinois, USA) for dispersions of barium phosphates particles in pure water.
2Results and Discussion
The XRD of materials analysis confirms that the barium phosphates at pH<10, patterns can be readily indexed to the reported structures of BaHPO4(JCPDS No.17-0929, 17-0929 and 72-1370, respectively), and no peaks attributable to impurities are observed (see Fig.1). The precipitates are mainly mixtures when the pH values of the mixed solution are 10 and 11. The standard XRD patterns of the reported structures of the mixture of BaHPO4and Ba5(PO4)3OH used are JCPDS No.72-1370 and 78-1141 when pH=10 (see Fig.1). When pH=11, the standard XRD patterns of the reported structures by the mixture of Ba5(PO4)3OH and NaBaPO4used are JCPDS No.06-0272 and 33-1210 (see Fig.1). The barium phosphates are precipitated in the mixed solution under different pH values, which can be explained by the following steps:
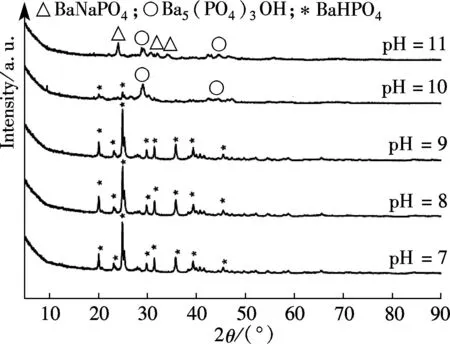
Fig.1 XRD patterns of barium phosphates at different pH values
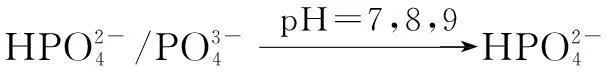
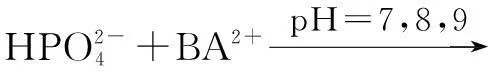
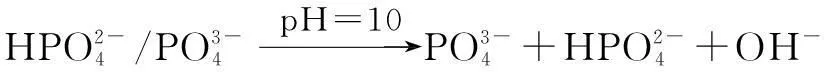

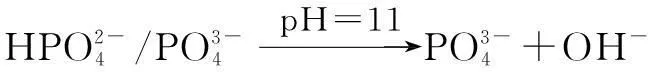
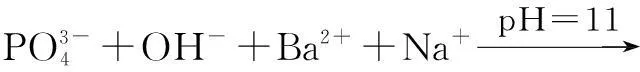
The distribution coefficients of ionsδcan be used here to explain the above results of XRD and the forms of phosphate ions with different values. The distribution coefficient of phosphate ions in the solution depends on the concentration of the hydrogen ion, and the distribution coefficient can be calculated by the distribution coefficient equations as
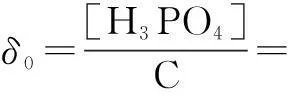
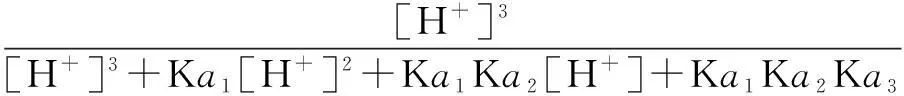
(1)
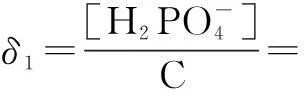
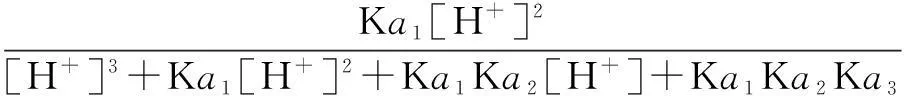
(2)
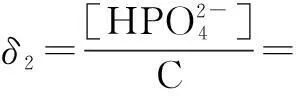
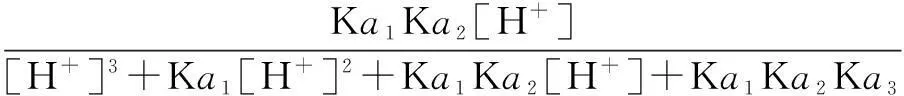
(3)
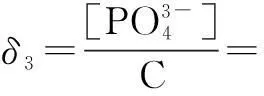
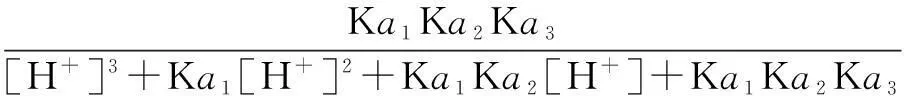
(4)
where Ka1=7.25×10-3, Ka2=6.31×10-8, Ka3=4.80×10-13.
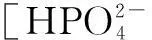
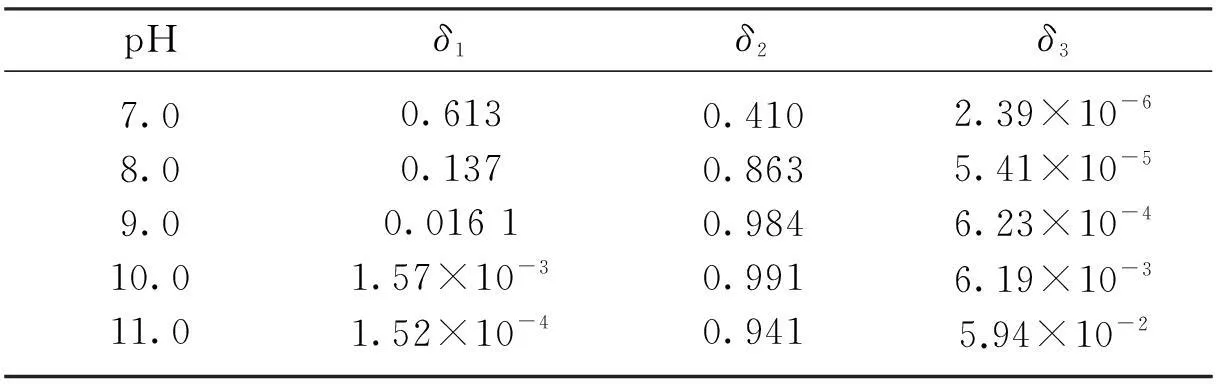
Tab.1 Distribution coefficients of ions under different pH
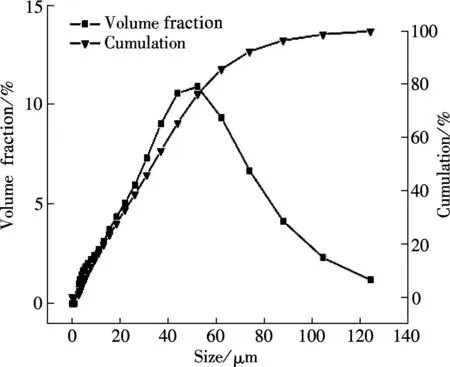
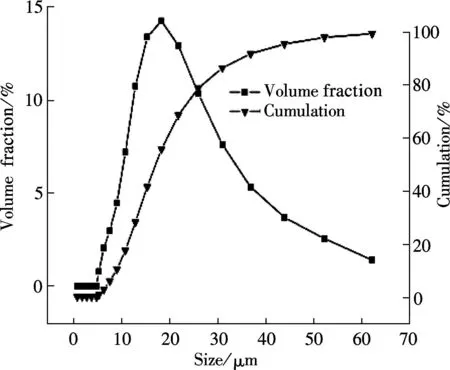
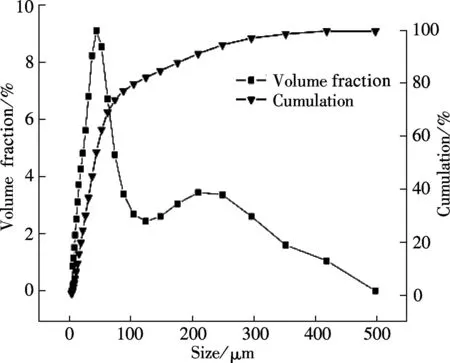
The SEM images and particle size distribution curves of barium phosphates obtained in the mixed solution of bacteria and substrate under different pH values are shown in Figs.2 and 3, respectively. When the pH of the mixed solution is 7, the morphology of precipitates is an irregular particle, and the length and width of the particle is less than 124.40 μm (see Fig.2(a) and Fig.3(a)). When pH=8, the spheres are accompanied by some irregular sphere appearing, as shown in Fig.2(b). The size of particles is nonuniform and the diameter ranges from 4.62 to 87.99 μm (see Fig.3(b)). Fig.2(c) and Fig.3(c) show that the shape of the products is dumbbell-shaped and the size of particles is 5.50 to 62.22 μm when the pH of the mixed solution is 9. When the pH of the mixed solution is increased up to 10, the shape of the mixture of BaHPO4and Ba5(PO4)3OH displays irregularly with the nonuniform size, as shown in Fig.2(d). The irregular particles’ diameters ranges from 3.89 to 176.00 μm (see Fig.3(d)). When the pH of the mixed solution is increased to 11, the shape of the mixture of Ba5(PO4)3OH and NaBaPO4is also irregular with the nonuniform size, as shown in Fig.2(e). The irregular particles size is 4.62 to 418.60 μm (see Fig.3(e)). The average crystallite size of barium phosphates is 33.40, 29.37, 24.13, 47.76, and 96.53 μm when the pH values of the mixed solution are 7, 8, 9, 10 and 11, respectively (see Fig.3).

(a) (b) (c)

(d) (e)
3Conclusion
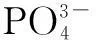
(a) (b)
(c) (d)
(e)
References
[1]Xu X R, Han J T, Cho K. Formation of amorphous calcium carbonate thin films and their role in biomineralization [J].ChemistryofMaterials, 2004, 16(9):1740-1746.
[2]Mann S. Molecular recognition in biomineralization [J].Nature, 1988, 332(6160):119-124.
[3]Wang F, Xu G Y, Zhang Z Q. The effect of pH on morphological control of barium hydrogen phosphate crystal by a double-hydrophilic copolymer [J].MaterialsLetters, 2005, 59(7):808-812.
[4]Ma Y F, Qiao L, Feng Q L. In-vitro study on calcium carbonate crystal growth mediated by organic matrix extracted from fresh water pearls [J].MaterialsScience&EngineeringC, 2012, 32(7): 1963-1970.
[5]Mann S, Heywood B R, Rajam S, et al. Controlled crystallization of CaCO3under stearic acid monolayers [J].Nature, 1988, 334(6184): 692-695.
[6]Fu G, Valiyaveettil S, Wopenka B, et al. CaCO3biomineralization: acidic 8-kDa proteins isolated from aragonitic abalone shell nacre can specifically modify calcite crystal morphology [J].Biomacromolecules, 2005, 6(3):1289-1298.
[7]Zhang X D, He W, Yue Y Z, et al. Bio-synthesis participated mechanism of mesoporous LiFePO4/C nanocomposite microspheres for lithium ion battery [J].JournalofMaterialsChemistry, 2012, 22(37):19948-19956.
[8]Lin J, Cates E, Bianconi P A. A synthetic analogue of the biomineralization process: Controlled crystallization of an inorganic phase by a polymer matrix [J].JournaloftheAmericanChemicalSociety, 1994, 116(11):4738-4745.
[9]Chen J, Kong Y F, Ji J J, et al. Protein-induced structural evolution of silver sulfide at the nanoscale: from hollow particles to solid spheres [J].Nanoscale, 2012, 4(15):4455-4458.
[10]Qin D Z, Zhang L, He G X, et al. Synthesis of Ag2S nanorods by biomimetic method in the lysozyme matrix [J].MaterialsResearchBulletin, 2013, 48(9):3644-3647.
[11]Zhu W K, Luo X G, Zhang C, et al. Microbiological precipitation of barium carbonate [J].ChineseJournalofInorganicChemistry, 2011, 27(10):2053-2060.
[12]Cheng L, Cord-Ruwisch R, Shahin M A. Cementation of sand soil by microbially induced calcite precipitation at various degrees of saturation [J].CanadianGeotechnicalJournal, 2013, 50(1):81-90.
[13]DeJong J T, Fritzges M B, Nusslein K. Microbially induced cementation to control sand response to undrained shear [J].JournalofGeotechnicalandGeoenvironmentalEngineering, 2006, 132(11): 1381-1392.
[14]Schüler D, Baeuerlein E. Dynamics of iron uptake and Fe3O4biomineralization during aerobic and microaerobic growth of magnetospirillum gryphiswaldense [J].JournalofBacteriology, 1998, 180(1): 159-162.
[15]Rong H, Qian C, Li L. Influence of molding process on mechanical properties of sandstone cemented by microbe cement [J].ConstructionandBuildingMaterials, 2012, 28(1): 238-243.
[16]Höppe H A, Daub M, Oeckler O. Synthesis, crystal structure, infrared spectrum and thermal behavior of α-BaHPO4[J].SolidStateSciences, 2009, 11(8):1484-1488.
[17]Chaabane T B, Smiri L, Bulou A. Vibrational study and crystal structure refinement of BaHPO4[J].SolidStateSciences, 2004, 6(2):197-204.
[18]Nallamuthu D, Selvarajan P, Freeda T H. Studies on various properties of pure and Li-doped barium hydrogen phosphate (BHP) single crystals [J].PhysicaB:CondensedMatter, 2010, 405(24): 4908-4913.
doi:10.3969/j.issn.1003-7985.2015.04.013
杂志排行
Journal of Southeast University(English Edition)的其它文章
- Mitigation of inter-cell interference in visible light communication
- Modified particle swarm optimization-based antenna tiltangle adjusting scheme for LTE coverage optimization
- Distribution algorithm of entangled particles for wireless quantum communication mesh networks
- Kernel principal component analysis networkfor image classification
- CFD simulation of ammonia-based CO2 absorption in a spray column
- Simulation and performance analysis of organic Rankine cycle combined heat and power system
