Endogenous neurotrophin-3 promotes neuronal sprouting from dorsal root ganglia
2015-02-07XuyangWangPeiyuanGuShiwenChenWenweiGaoHengliTianXiangheLuWeimingZhengQichuanZhugeWeixingHu
Xu-yang Wang, Pei-yuan Gu Shi-wen Chen Wen-wei Gao Heng-li Tian, Xiang-he Lu, Wei-ming Zheng, Qi-chuan Zhuge, Wei-xing Hu
1 Department of Neurosurgery, First Af liated Hospital of Nanjing Medical University, Nanjing, Jiangsu Province, China
2 Department of Neurosurgery, Shanghai Jiao Tong University Af liated 6thPeople’s Hospital, Shanghai, China
3 Department of Neurosurgery, First Af liated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang Province, China
Endogenous neurotrophin-3 promotes neuronal sprouting from dorsal root ganglia
Xu-yang Wang1,2,3, Pei-yuan Gu1, Shi-wen Chen2, Wen-wei Gao2, Heng-li Tian2,*, Xiang-he Lu3, Wei-ming Zheng3, Qi-chuan Zhuge3, Wei-xing Hu1,*
1 Department of Neurosurgery, First Af liated Hospital of Nanjing Medical University, Nanjing, Jiangsu Province, China
2 Department of Neurosurgery, Shanghai Jiao Tong University Af liated 6thPeople’s Hospital, Shanghai, China
3 Department of Neurosurgery, First Af liated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang Province, China
In the present study, we investigated the role of endogenous neurotrophin-3 in nerve terminal sprouting 2 months after spinal cord dorsal root rhizotomy. The left L1–5and L7–S2dorsal root ganglia in adult cats were exposed and removed, preserving the L6dorsal root ganglia. Neurotrophin-3 was mainly expressed in large neurons in the dorsal root ganglia and in some neurons in spinal lamina II. Two months after rhizotomy, the number of neurotrophin-3-positive neurons in the spared dorsal root ganglia and the density of neurite sprouts emerging from these ganglia were increased. Intraperitoneal injection of an antibody against neurotrophin-3 decreased the density of neurite sprouts. These f ndings suggest that endogenous neurotrophin-3 is involved in spinal cord plasticity and regeneration, and that it promotes axonal sprouting from the dorsal root ganglia after spinal cord dorsal root rhizotomy.
nerve regeneration; neurotrophin-3; sensory neurons; dorsal root ganglion; cats; nerve terminal; neural regeneration
Funding: This study was supported by grants from the Shanghai Municipal Commission of Health and Family Planning, No. 20114351, the Traditional Chinese Medicine Science Funding of Zhejiang Province of China, No. 2010ZB091 and the Natural Science Foundation of Zhejiang Province of China, No. Y2090864.
Wang XY, Gu PY, Chen SW, Gao WW, Tian HL, Lu XH, Zheng WM, Zhuge QC, Hu WX (2015) Endogenous neurotrophin-3 promotes neuronal sprouting from dorsal root ganglia. Neural Regen Res 10(11):1865-1868.
Introduction
Neurotrophic factors are endogenous signaling proteins that promote the survival, dif erentiation and function of neurons. Neurotrophic factors are produced by various cell types, including target neurons and muscle cells, as well as microglia and Schwann cells (Ekestern, 2004). Among the various neurotrophic factors that are involved in spinal cord regeneration (Li et al., 2007, 2008), neurotrophin-3 (NT-3) has been particularly well studied (Blits et al., 2003; Liu et al., 2012; Tuinstra et al., 2012; Wang et al., 2013). NT-3 plays important roles in regulating the growth of muscle sensory neurons and in maintaining proprioceptive sensory organs (Chen et al., 2002; Gorokhova et al., 2009). NT-3 contributes to the survival of muscle spindle sensory af erent f bers. NT-3 also promotes the elaboration of terminal projections to motor neurons during the late stages of development, and in addition, potentiates group Ia synaptic projections to motor neurons. Delivering NT-3 to peripherally axotomized af erent f bers promotes the growth of axons. The monosynaptic projections from spindle af erent f bers to motor neurons also exhibit acute potentiation when exposed to NT-3 in the isolated spinal cord. NT-3 also enhances the mechanical sensitivity of the neuroma of spindle af erents that have been axotomized.
Previous studies have shown that rhizotomy can signif -cantly increase the expression of NT-3 in the spared dorsal root ganglion (DRG) (Wang et al., 2002) and in neurons and glial cells in lamina II of the af erent segments (L5and L7) in cats (Zhou et al., 2002). In addition, DRG cells from cats with spinal cord injury produce signif cantly more neuronal spheres and longer axonal projections than those from normal control cats (Zhang et al., 2004). However, whether endogenous NT-3 secreted by the DRG is involved in neuroplasticity after rhizotomy remains unclear. In this study, we examined the function of NT-3 at an extended period (2 months) after rhizotomy in cats.
Materials and Methods
Establishment of spinal cord dorsal root rhizotomy model A total of 25 adult male outbred cats (1 year old, clean grade, weighing 3–3.5 kg) were provided by the Laboratory Animal Center of Nanjing Medical University (Nanjing, Jiangsu Province, China). The cats were individually housed in a vivarium with a 12-hour light/dark cycle for at least 3 days before surgery, with free access to food and water. The experimental procedures used in this study were approved by the Ethics Committee of Nanjing Medical University Af liated First Hospital (Nanjing, Jiangsu Province, China). The cats were randomly assigned to the following threepentobarbital solution (3.5%, 1.3 mL/kg), the lumbar laminae and part of the sacral vertebrae were removed. The dura was incised to expose the L1–5and L7–S2DRGs. The DRGs along with 2–3-mm segments of the associated dorsal roots were then removed at the intervertebral foramina on the left side, leaving the L6DRG and its associated dorsal root intact (Figure 1).
NT-3 blocking
NT-3 blocking in the cats was performed as previously described (Liu et al., 2009). Brief y, after the cats were anesthetized by intraperitoneal injection of sodium pentobarbital solution (3.5%, 1.3 mL/kg), the L5–6processes and vertebral arches were resected, exposing the lumbar subarachnoid space. A catheter was inserted into the subarachnoid space and fixed by suturing the muscles and skin. NT-3-specific antibody (1:1,500; 50 μL; rabbit anti-cat; Santa Cruz Biotechnology, Dallas, TX, USA) was injected through the catheter once every week starting on postoperative day 1 during the f rst month, then every 2 weeks during the second month.
Immunohistochemical staining
All cats were sacrificed 2 months post-surgery and transcardially perfused. Five animals from each of the normal control and rhizotomy groups were used for immunohistochemical staining. The L6DRG and spinal cord segment were harvested and f xed in 4% paraformaldehyde. The tissues were dehydrated in 20% sucrose solution overnight and cryosectioned into 30-μm sections for the spinal cord and 15-μm sections for the DRG. For unbiased sampling of data, a systematic sampling method was used, and the 10th, 20th, 30th, 40thand 50thsections were selected for analysis. Immunohistochemical staining was performed using rabbit anti-cat NT-3 monoclonal antibody (1:1,500; Santa Cruz Biotechnology), with a 48-hour incubation at 4°C. Horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:200; Santa Cruz Biotechnology) was added at 37°C for 1.5 hours. Sections were then incubated with 3,3′-diaminobenzidine for staining. PBS instead of primary antibody was used for the negative control. NT-3-positive neurons in the L6DRG and spinal cord lamina II were counted in a randomly selected square of 1.5 × 1.5 μm2under a microscope (X51, Olympus, Shanghai, China). The results of all sections were averaged.
Retrograde tracing
Retrograde tracing was performed on axons of DRG neurons using a previously described protocol (Liu et al., 2009). Brief y, 5 days before the animals were sacrif ced, the cats were given general anesthesia and the bilateral lumbosacral trunks were isolated. Cholera toxin B subunit conjugated to horseradish peroxidase (CB-HRP) 15 μL (30%; Sigma-Aldrich, St. Louis, MO, USA) was injected into the bilateral lumbosacral trunks for retrograde labeling of DRG neurons. Staining was performed using tetramethylbenzidine (TMB). Brief y, the L6DRG and spinal cord segment were f xed in 4% paraformaldehyde and stained with TMB (1%; Sigma-Aldrich). PBS instead of CB-HRP was used for the negative control. The area of the spinal dorsal horn was measured. CB-HRP-positive nerve f bers were counted under a microscope (X51, Olympus) to calculate their density in the dorsal horn. The f nal measurements were the average of the f ve selected sections from each cat.
Statistical analysis
All data are expressed as the mean ± SD. Inter-group comparisons were performed with unpaired t-test or one-way analysis of variance using SPSS 10.0 software (SPSS, Chicago, IL, USA). A P-value < 0.05 was considered statistically signif cant.
Results
Quantitation of NT-3-positive neurons in the L6DRG and lamina II after rhizotomy
Immunohistochemical staining showed that 2 months after rhizotomy, the number of NT-3-positive neurons in the L6 DRG was signif cantly increased compared with the normal control group (P < 0.05). However, no signif cant dif erence in the number of NT-3-positive neurons in the L6 spinal cord lamina II was found between the rhizotomy and normal control groups (P > 0.05; Figure 2).
Density of CB-HRP-labeled af erent f bers in the spinal dorsal horn after rhizotomy
Two months after rhizotomy, TMB staining revealed labeled neurons and nerve f bers in the L6DRG in both the normal control and rhizotomy groups (Figure 3A, B), suggesting that the CB-HRP retrograde tracing was successful. In the normal control group, the stained L6DRG neurons projected into laminae III, IV and V, but not lamina II (Figure 3C). In the rhizotomy and rhizotomy plus NT-3 blocking groups, the projections of CB-HRP-labeled nerve f bers appeared similar to that in the normal control group (Figure 3D, E). However, the density of CB-HRP-labeled nerve f bers was signif cantly increased in the rhizotomy group compared with the normal control group (P < 0.05; Figure 3F). This suggests that rhizotomy stimulates neurite sprouting from the spared L6DRG neurons into the dorsal horn. The neurites mainly projected into laminae III, IV and V. In the rhizotomy plus NT-3 blocking group, there was a signif cant decrease in the density of CB-HRP-labeled nerve fibers compared with the rhizotomy group (P < 0.05; Figure 3F). Our f nding suggests that the NT-3 antibody inhibits neurite growth from DRG neurons.
Discussion
In this study, we found that rhizotomy signif cantly increased the number of NT-3-positive neurons in the spared L6DRG in cats 2 months after rhizotomy. This ef ect was inhibited by an NT-3 antibody, suggesting that NT-3 plays an important role in neural regeneration after rhizotomy.

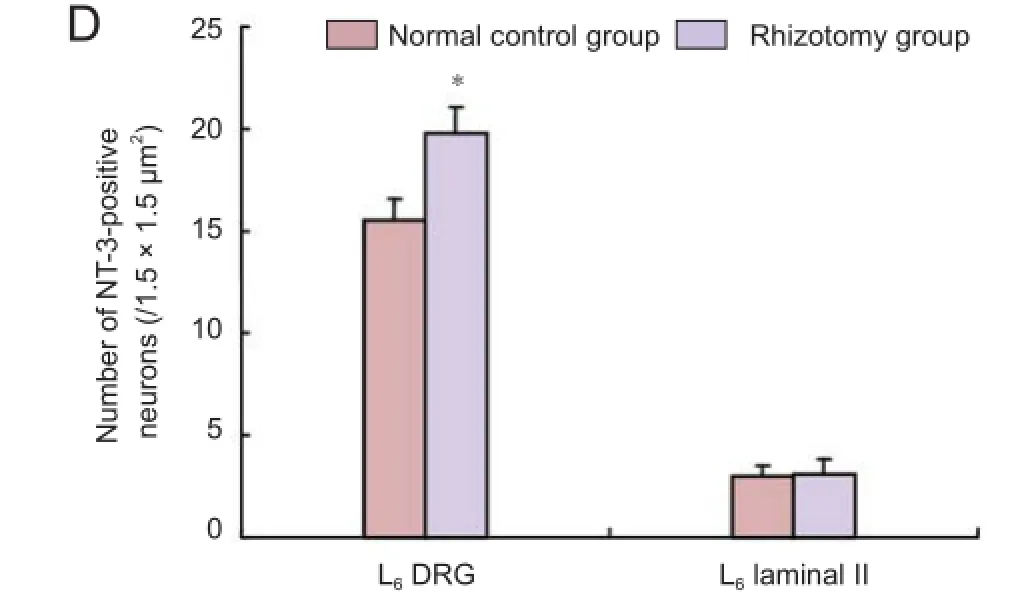
Figure 2 Neurotrophin-3 (NT-3)-positive neurons in the L6dorsal
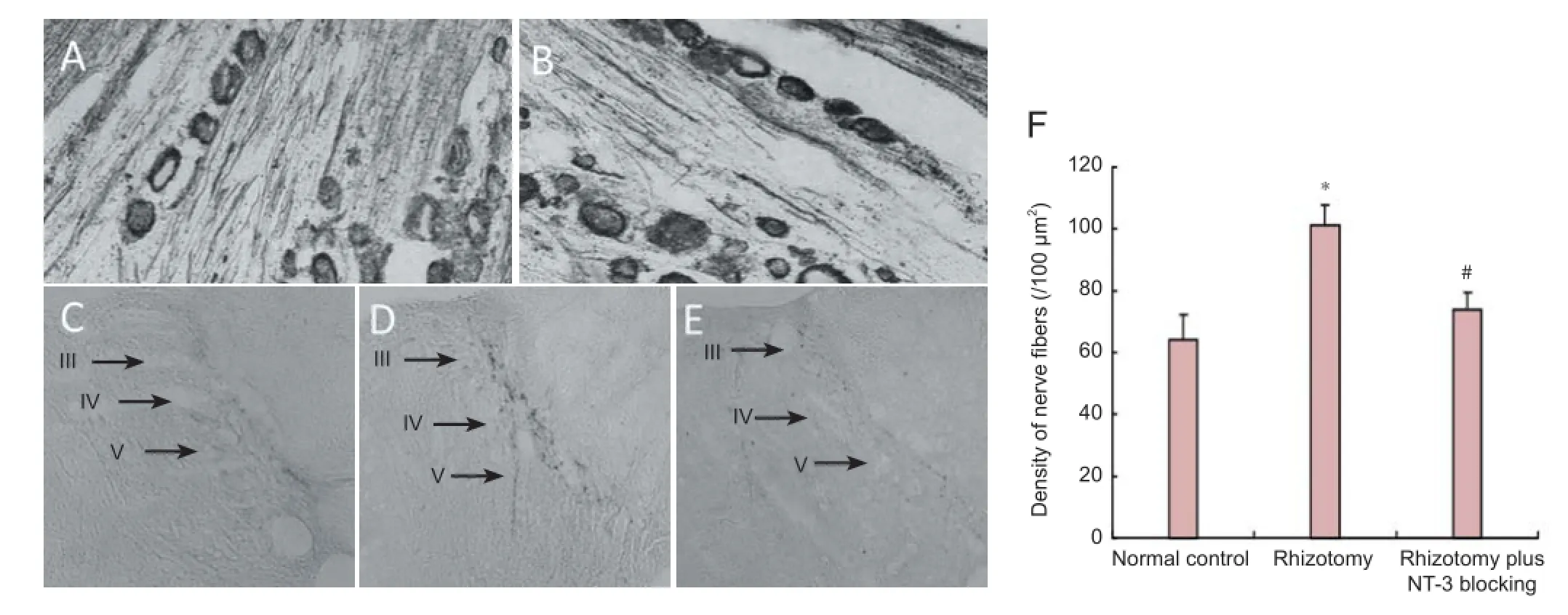
Figure 3 Labeling of af erent f bers in the spinal dorsal horn with cholera toxin B subunit conjugated to horseradish peroxidase (CB-HRP) 2 months after rhizotomy
NT-3 is an important neurotrophic factor that plays important roles in nervous system development and synaptic plasticity (Maisonpierre et al., 1990; Schnell et al., 1994; Liu et al., 2009). NT-3 is expressed in motor neurons of the spinal ventral horn, in axons, in the spinal dorsal horn, and in glial cells (Ernfors et al., 1990; Li et al., 2007). In addition, groups: normal control group (n = 10), rhizotomy group (n =10; given unilateral spinal cord dorsal root rhizotomy), and rhizotomy plus NT-3 blocking group (n = 5; given unilateral spinal cord dorsal root rhizotomy and NT-3 blocking). Five animals from each of the normal control and rhizotomy groups were used for immunohistochemical staining and retrograde labeling. The rhizotomy plus NT-3 blocking group was only used for retrograde staining.
Spinal cord dorsal root rhizotomy was performed as previously described (Liu et al., 2009). Brief y, after the cats were anesthetized by intraperitoneal injection of sodiumNT-3 is expressed in DRG neurons and their axons (Ni et al., 2001; Wang et al., 2002, 2007, 2009; Zhou et al., 2002). However, the role of NT-3 in regeneration following rhizotomy remained unclear.
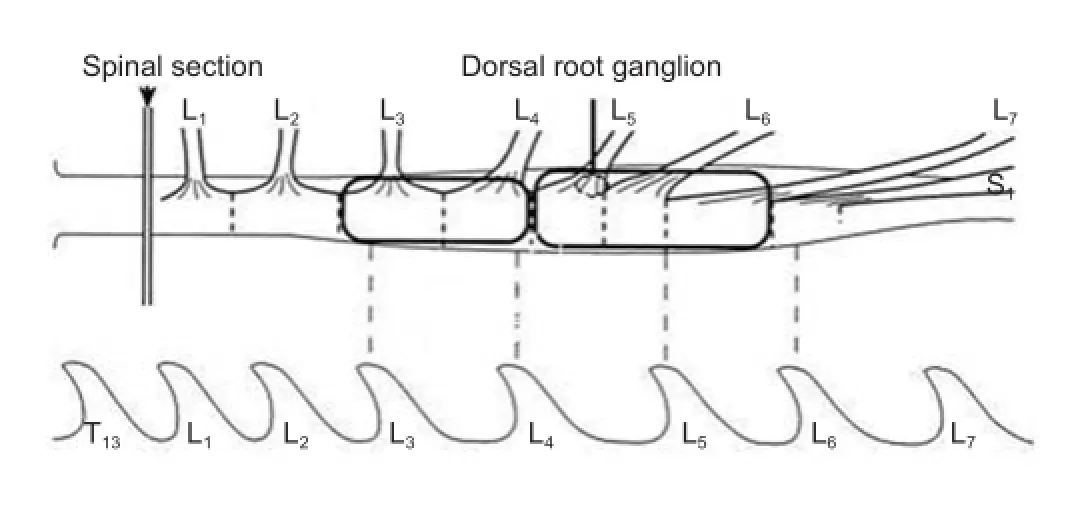
Figure 1 Establishment of spinal cord dorsal root rhizotomy model.
In the present study, we found that rhizotomy in cats signif cantly increased the number of NT-3-positive neurons in the DRG, suggesting that NT-3 in the spared DRG might be involved in regeneration following spinal cord injury. This is consistent with a previous f nding that neurons in the spared DRG extend longer axonal projections than neurons in the normal DRG (Zhang et al., 2004). These results suggest that the increased number of NT-3-positive neurons in the spared DRG is associated with axonal regrowth from sensory neurons. Therefore, NT-3 may promote the growth of sensory nerve f bers after rhizotomy.
We also found that there was a signif cant increase in the number of CB-HRP-labeled f bers 2 months after rhizotomy in cats. This might be attributed to increased NT-3 expression in DRG neurons. We speculate that NT-3 secreted by DRG neurons may help in the formation of a microenvironment conducive to neurite sprouting from sensory neurons in the spinal cord. This may ultimately enhance repair after rhizotomy and promote neuronal plasticity. Previous studies have shown that during the early stage of repair after rhizotomy, NT-3-expressing neurons in the spared DRG were mostly medium and small-sized (Liu et al., 2009). However, in this study, the NT-3-expressing neurons in the spared DRG 2 months after rhizotomy were mostly large-sized. We speculate that NT-3 is expressed by different groups of neurons in the DRG after rhizotomy. Changes in NT-3 expression in the DRG might also alter NT-3 levels in the spinal cord, which may further enhance repair following rhizotomy. To evaluate the function of NT-3 in the spared DRG after rhizotomy in cats, we used an NT-3-specif c antibody to block its function. We found that the number of NT-3-positive f bers was signif cantly decreased by this antibody in rhizotomized cats. This is consistent with our previous f nding that blocking NT-3 inhibits axonal growth from DRG neurons in vitro (Zhang et al., 2004).
In conclusion, we show that the number of NT-3-positive large neurons in the spared DRG is increased after rhizotomy in cats, which may promote neurite sprouting and repair after rhizotomy.
Author contributions: WXH and HLT designed the study. XYW, PYG, SWC, XHL and WWG established the animal models and performed the experiments. WMZ and QCZG performed the statistical analysis. XYW drafted the paper. All the authors approved the f nal version of the paper.
Conf icts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded, stringently reviewed by international expert reviewers.
Chen HH, Tourtellotte WG, Frank E (2002) Muscle spindle-derived neurotrophin 3 regulates synaptic connectivity between muscle sensory and motor neurons. J Neurosci 22:3512-3519.
存取控制也是最早采用的安全技术之一,它一般与身份验证技术一起使用,赋予不同身份的用户以不同的操作权限,以实现不同安全级别的信息分级管理。
Ekestern E (2004) Neurotrophic factors and amyotrophic lateral sclerosis. Neurodegener Dis 1:88-100.
Ernfors P, Ibáñez CF, Ebendal T, Olson L, Persson H (1990) Molecular cloning and neurotrophic activities of a protein with structural similarities to nerve growth factor: developmental and topographical expression in the brain. Proc Natl Acad Sci U S A 87:5454-5458.
Gorokhova S, Gaillard S, Gascon E (2009) Spindle-derived NT3 in sensorimotor connections: principal role at later stages. J Neurosci 29:10181-10183.
Li XL, Zhang W, Zhou X, Wang XY, Zhang HT, Qin DX, Zhang H, Li Q, Li M, Wang TH (2007) Temporal changes in the expression of some neurotrophins in spinal cord transected adult rats. Neuropeptides 41:135-143.
Li XL, Liu J, Wang XY, Li LY, Ni W, Zheng RY, Yang HJ, Lu YC, Qi JG, Wang TH (2008) Temporal changes in the expression of TGF-beta 1 and EGF in the ventral horn of the spinal cord and associated precentral gyrus in adult Rhesus monkeys subjected to cord hemisection. J Neurol Sci 268:163-171.
Liu F, Zhang JM, Zhang C, Wang XY, Liao M, Ren YH, Tu WZ, Lei YN (2009) Ef ect of endogenous NT-3 on spinal cord injury treated with electro-acupuncture. Jiefangjun Yixue Zazhi 34:1000-1008.
Liu T, Xu J, Chan BP, Chew SY (2012) Sustained release of neurotrophin-3 and chondroitinase ABC from electrospun collagen nanof ber scaf old for spinal cord injury repair. J Biomed Mater Res A 100:236-242.
Maisonpierre PC, Belluscio L, Squinto S, Ip NY, Furth ME, Lindsay RM, Yancopoulos GD (1990) Neurotrophin-3: a neurotrophic factor related to NGF and BDNF. Science 247:1446-1451.
Ni S, Wang T, Chen Y (2001) The expression of BDNF and NT-3 in dorsal root ganglion following peripheral and central axotomy--an immunohistochemical study. Hua Xi Yi Ke Da Xue Xue Bao 32:350-352.
Schnell L, Schneider R, Kolbeck R, Barde YA, Schwab ME (1994) Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature 367:170-173.
Tuinstra HM, Aviles MO, Shin S, Holland SJ, Zelivyanskaya ML, Fast AG, Ko SY, Margul DJ, Bartels AK, Boehler RM, Cummings BJ, Anderson AJ, Shea LD (2012) Multifunctional, multichannel bridges that deliver neurotrophin encoding lentivirus for regeneration following spinal cord injury. Biomaterials 33:1618-1626.
Wang TH, Wang XY, Li XL, Chen HM, Wu LF (2007) Effect of electroacupuncture on neurotrophin expression in cat spinal cord after partial dorsal rhizotomy. Neurochem Res 32:1415-1422.
Wang TH, Wu LF, Liao DY, Zhou X, Chen YH, Liu S, A T (2002) The change of NT-3 and its mRNA in spared dorsal root ganglion and spinal dorsal horn lamina following partial dorsal rhizotomy. Shenjing Jiepou Xue Zazhi 18:59-62.
Wang X, Li Y, Gao Y, Chen X, Yao J, Lin W, Chen Y, Liu J, Yang Y, Wang X (2013) Combined use of spinal cord-mimicking partition type scaffold architecture and neurotrophin-3 for surgical repair of completely transected spinal cord in rats. J Biomater Sci Polym Ed 24:927-939.
Wang XY, Li XL, Hong SQ, Xi-Yang YB, Wang TH (2009) Electroacupuncture induced spinal plasticity is linked to multiple gene expressions in dorsal root deaf erented rats. J Mol Neurosci 37:97-110.
Zhang W, Zhou X, Wang TH, Wang TW, Liu S, Chen SX, Ou KQ (2004) The neurotrophic ef ect of endogenous NT-3 from adult cat spared dorsal root ganglion on ganglionic neurons. Sichuan Da Xue Xue Bao Yi Xue Ban 35:25-28.
Zhou X, Wu L, Chen H, Wang T, Chen L, Bao T, Liao D (2001) The spatiotemporal change of neurotrophin family and their mRNA expression in spinal cord of cats after partial rhizotomy. Hua Xi Yi Ke Da Xue Xue Bao 33:165-168, 191.
Blits B, Oudega M, Boer GJ, Bartlett Bunge M, Verhaagen J (2003) Adeno-associated viral vector-mediated neurotrophin gene transfer in the injured adult rat spinal cord improves hind-limb function. Neuroscience 118:271-281.
Copyedited by Patel B, Robens J, Yu J, Yang Y, Li CH, Song LP, Zhao M
*Correspondence to: Wei-xing Hu, M.D., Ph.D. or Heng-li Tian, M.D., weixinghu@njmu.edu.cn or ltianhengli@163.com.
orcid: 0000-0001-7463-4493 (Wei-xing Hu) 0000-0002-1219-4952 (Heng-li Tian)
10.4103/1673-5374.170318 http://www.nrronline.org/
Accepted: 2015-07-21
猜你喜欢
杂志排行
中国神经再生研究(英文版)的其它文章
- Intracellular sorting pathways of the amyloid precursor protein provide novel neuroprotective strategies
- The role of the Rho/ROCK signaling pathway in inhibiting axonal regeneration in the central nervous system
- VEGF in the nervous system: an important target for research in neurodevelopmental and regenerative medicine
- Studying neurological disorders using induced pluripotent stem cells and optogenetics
- Ef cacy of glucagon-like peptide-1 mimetics for neural regeneration
- Compliant semiconductor scaf olds: building blocks for advanced neural interfaces
