Preoperative serum liver enzyme markers for predicting early recurrence after curative resection of hepatocellular carcinoma
2015-02-06
Nanjing, China
Preoperative serum liver enzyme markers for predicting early recurrence after curative resection of hepatocellular carcinoma
Zhong-Xia Wang, Chun-Ping Jiang, Yin Cao, Guang Zhang, Wei-Bo Chen and Yi-Tao Ding
Nanjing, China
BACKGROUND: Early recurrence of hepatocellular carcinoma (HCC) is associated with worse prognosis after liver resection. This study aimed to investigate the prognostic value of common liver enzyme markers in HCC early recurrence after curative hepatectomy and to establish a simple predictive model for HCC early recurrence.
METHODS: A total of 200 patients who had undergone curative resection for HCC were retrospectively analyzed. The patients were divided into early recurrence (within 2 years) and non-early recurrence groups. Demographical characteristics, preoperative liver function parameters, surgical factors and tumor related factors of the patients were assessed by univariate analysis to identify potential signifcant predictors for early recurrence after resection of HCC. Parameters with statistical signifcance were entered into a Cox proportional hazard model to fnd independent risk factors. Receiver operating characteristic analysis was done to determine optimal cut-off values and the number of combined factors in multi-factor predictive model.
RESULTS: Of 13 potential risk factors for early recurrence identifed by univariate analysis, high lactate dehydrogenase (LDH>206 U/L, HR=1.711,P=0.006), high aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio (AST/ ALT>0.96, HR=1.769,P=0.006), elevated alpha-fetoprotein(AFP>8.6 ng/mL, HR=2.079,P=0.007), small resection margin (≤1 cm, HR=2.354,P<0.001) and advanced TNM stage (TNM III-IV, HR=2.164,P<0.001) were independent risk factors for early recurrence of HCC shown by multivariate analysis. Patients with three or more concurrent independent risk factors had signifcantly higher risk for early recurrence than those with low risk factors. The sensitivity and specifcity of this predictive model are 53.6% and 80.7%, respectively (area under curve=0.741, 95% CI 0.674-0.800,P<0.0001).
CONCLUSIONS: Preoperative common liver enzyme markers, LDH and AST/ALT ratio, were independently associated with early recurrence of HCC. The combination of serum liver enzyme markers with AFP, resection margin and TNM stage better predicted early recurrence of HCC after curative resection in a simple multi-factor model.
(Hepatobiliary Pancreat Dis Int 2015;14:178-185)
hepatocellular carcinoma;liver enzyme; recurrence; resection; hepatectomy
Introduction
Hepatocellular carcinoma (HCC) is one of the most prevalent cancers with an increasing incidence worldwide.[1]In recent decades, hepatectomy has become a safe and effective procedure for HCC with a low perioperative mortality.[2]Although hepatectomy remains a most frequently used curative therapy for HCC, longterm prognosis after liver resection remains unsatisfactory because of a high incidence of recurrence.[3,4]Tumor recurrence after hepatectomy may originate from either intrahepatic metastasis of primary HCC orde novocarcinogenesis from the remnant liver, which could be discriminated by different time of recurrence and different risk factors. The prognosis of patients who suffer from early recurrence is extremely dismal.[4]Therefore, early recurrence is considered as an important adverse prog-nostic factor after curative therapy for HCC.[5,6]
Since early recurrence is associated with worse clinical outcome, identifying patients at high risk for early recurrence may help improving the prognosis of this population by active surveillance, prevention and treatment of recurrent disease. Although studies[4,7]suggested tumor pathologic factors and types of surgery[8,9]as risk factors for early recurrence of HCC, the use of these parameters is of technical diffculties and could not dynamically predict recurrence risk in daily medical practice. In this study, we investigated whether widely-available liver enzyme tests in combination with other readily-available clinical parameters could predict early recurrence of HCC.
Methods
Patients
The patients who had undergone curative resection for HCC at the Department of Hepatobiliary Surgery, the Affliated Drum Tower Hospital of Nanjing University Medical School between May 2007 and May 2011 were retrieved from a prospectively maintained database and were analyzed retrospectively. Only patients with complete follow-up data and required clinical information were included. The data of the patients were collected and cross-checked by two authors (WZX and JCP) and reviewed by a senior author (DYT) for determination of inclusion. Reasons for exclusion included previous surgical interventions for primary HCC at other institutions, loss of required data, unavailable follow-up data, perioperative mortality and non-curative resections, etc. Finally, 200 patients were enrolled in this analysis. Curative resection was defned as complete excision of HCC without identifable gross or microscopic tumor and no residual tumor demonstrated by ultrasonography (US) or contrast-enhanced computed tomography (CT) at one month after surgery. Diagnosis of HCC was confrmed by at least two pathologists. This study was approved by the Institutional Ethics Committee of the Affliated Drum Tower Hospital of Nanjing University Medical School. The principles outlined in theDeclaration of Helsinkiwere followed.
Follow-up
The patients were followed up regularly in our outpatient clinic. Monthly serum alpha-fetoprotein (AFP) tests were conducted in the frst three months postoperatively. Regular serum AFP, chest X-ray and abdominal US or contrast-enhanced CT was done at least every three months in the frst two years. The patients were followed up every six months during the 3rd to 5th years. After fve years, the patients were asked to visit outpatient clinic annually. Recurrence was diagnosed when recurrent tumor was confrmed by imaging. Recurrence within two years after surgery was determined as early recurrence.[5,10]
Analyzed parameters
The parameters included in the analysis were categorized into patient characteristics, preoperative liver function markers, surgical factors and tumor-related factors. Patient characteristics included gender, age, body mass index (BMI), hypertension, diabetes, smoking, and alcohol consumption. Status and type of hepatitis were recorded. HBV DNA was quantitatively determined and analyzed in HBV-infected patients.
Preoperative serum liver enzyme tests were regularly conducted. Parameters used in this analysis included alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), γ-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), total bilirubin (TBIL), direct bilirubin (DBIL), cholinesterase (CHE), albumin (ALB), prothrombin time (PT), international normalized ratio (INR) and AST/ALT ratio. The status of cirrhosis, portal hypertension and detectable ascites were examined. The diagnosis of portal hypertension was established according to the criteria of Barcelona Clinic Liver Cancer (BCLC) group defned as the presence of either esophageal varices detected by endoscopy or splenomegaly (major diameter >12 cm) with platelet count less than 100 000/mm3.[11]Child-Turcotte-Pugh (CTP) classifcation and Model for End-stage Liver Disease (MELD) score were also evaluated.
Surgical factors subjected to analysis included the use of vascular occlusion, total blood loss, blood transfusion, and the distance from resection margin to tumor. Hepatectomies involving three or more segments were defned as major resections. Liver resections of less than three segments, wedge resections or tumor enucleations were defned as minor resections.
Tumor related clinicopathologic factors that potentially associated with HCC recurrence were analyzed. The parameters included preoperative serum AFP level (upper limit in our hospital: 5 ng/mL), size of tumor, number of tumor, tumor capsule, microvascular invasion, macrovascular invasion, adjacent organ invasion and differentiation of HCC (categorized by poor/moderate/well or Edmonson-Steiner grade I-II/III-IV). TNM staging was performed according to theAmerican Joint Committee on Cancer Staging Manual(7th edition). BCLC stage was determined as previously described.[12]
Statistical analysis
Univariate analysis was used to identify potential riskfactors. Categorical variables were analyzed by the Chisquare test or Fisher's exact test. Student'sttest was used to evaluate continuous variables with normal distribution. Non-normal distribution variables were analyzed by the Mann-WhitneyUtest.P<0.05 (two-tailed) indicates statistical signifcance. Cox proportional hazard ratio model was used for multivariate analysis to identify independent risk factors. Signifcant parameters in univariate analysis were subjected to a forward stepwise regression model. Step selections were based on maximal likelihood ratio test. Only signifcant variables were kept in the model. Cumulative recurrence-free survival (RFS) was evaluated by the Kaplan-Meier method and the difference between groups was compared by the log-rank test. Receiver operating characteristic (ROC) curve was used to evaluate predictive value and to determine optimal cut-offs of continuous parameters. Cut-off values were determined by seeking the maximal sum of sensitivity and specifcity. All other statistical evaluations were conducted by PASW statistics 18 (Chicago, IL, USA) and MedCalc 13 software (Ostend, Belgium).
Results
Patient characteristics
Demographic data of the patients are listed in Table 1. The study population consists of 170 males (85.0%) and 30 females (15.0%). The median follow-up period was 39.4 months (range 3.0-97.6). The average age of the patients was 54.0 years and the average BMI was 23.0. Forty-one patients had hypertension and sixteen of them had diabetes as comorbidities. A minority of patients had habits of smoking or alcohol consumption. Most of the patients were infected with hepatitis B virus (HBV, 158/200, 79.0%) while only 1.0% (2/200) of patients were hepatitis C virus (HCV)-positive. Moreover, 89.5% (179/200) of the patients had cirrhosis. None of the patients belonged to CTP C grade. Only 8 patients had CTP B grade liver function. The mean MELD score was 7.8, indicating most of the patients had well-compensated liver function. Most of the patients (136/200, 68%) belonged to stage I-II in TNM staging system and the rest had advanced HCC (stage III-IV). BCLC staging showed that 4 patients had stage 0 disease and 50% (100/200) of the patients were in stage A. However, a considerable proportion of patients had stage B or C HCC.
Cumulative RFS
During follow-up, 136 patients developed recurrent tumor. The median RFS time of the patients was 15.6 months. In this series, 112 patients suffered from early recurrence after surgery. The 1-, 3- and 5-year RFS rates were 53.0%, 35.1% and 25.0%, respectively. The Kaplan-Meier survival curve is shown in Fig. 1.
Univariate analysis of risk factors correlated with early recurrence
The study population was stratifed by whether early recurrence occurred. Multiple univariate analyses were made by comparing patient characteristics, preoperative liver function, surgical factors and tumor related factors between early recurrence group (ER) and non-early recurrence group (non-ER). The results of univariate analysis are shown in Table 2.
None of the tested patient demographic characteristics was associated with early recurrence. However, forpreoperative liver function markers, univariate comparison indicated signifcantly higher AST, LDH, GGT, ALP and AST/ALT ratio in the ER group. The existence of cirrhosis, portal hypertension and ascites was comparable between the two groups. Similarly, no signifcant difference between CTP classifcation and MELD score was found.

Table 1.Demographic data of the patients in this study

Fig. 1.Cumulative recurrence-free survival curve of the whole study population (n=200).

Table 2.Univariate analysis of risk factors for early recurrence of HCC
Statistical analysis of surgical factors also revealed signifcant difference between the non-ER and ER groups. The ER group had more blood loss than the non-ER group. The number of patients who received hepatectomy with resection margin less than 1 cm to tumor was significantly more in the ER group than in the non-ER group. Other factors including extent of resection, application of vascular occlusion and the need of blood transfusion were similar between the two groups.
Finally, tumor-related factors were also analyzed. The ER group had a higher preoperative AFP level, a larger tumor size and advanced TNM and BCLC stages. Multicentric tumors and macrovascular invasion were more prevalent in the ER group than in the non-ER group.
Multivariate analysis of risk factors for early recurrence
ROC analysis was done to determine optimal cutoff values of continuous values for the prediction of early recurrence. By seeking the maximum sum of sensitivity and specifcity, we determined the cut-off values (Table 3). Thirteen parameters with statistical signifcance in univariate analysis were subjected to Cox proportional hazard model to identify independent risk factors. Five independent adverse prognostic factors for early recurrence were identifed (Table 4): LDH [hazard ratio (HR) =1.711, 95% confdence interval (95% CI) 1.170-2.502,P=0.006], AST/ALT ratio (HR=1.769, 95% CI 1.180-2.540,P=0.006), AFP (HR=2.079, 95% CI 1.221-3.542,P=0.007), resection margin (HR=2.354, 95% CI 1.490-3.719,P<0.001) and TNM stage (HR=2.164, 95% CI 1.463-3.201,P<0.001).
A simple multi-factor model for the prediction of early recurrence
Based on multivariate analysis, we investigated whether the combination of risk factors better predicts early recurrence. At frst, the cut-off value for the number of combined risk factors was determined by ROC analysis. When the cut-off was set as >2, the sensitivity and specifcity for prediction of early recurrence was 53.6% and 80.7% (area under curve (AUC) =0.741, 95% CI 0.674-0.800,P<0.0001) (Fig. 2). The patients were thus stratifed into a low risk group and a high risk group by this criterion. Kaplan-Meier survival curve (Fig. 3) showed that patients with more than two risk factors had a lower RFS rate in the frst two years after liver resection than those with two or less risk factors (log-rank test,P<0.001).

Table 3.Cut-off values for continuous parameters in multivariate analysis

Table 4.Multivariate analysis of risk factors for early recurrence of HCC
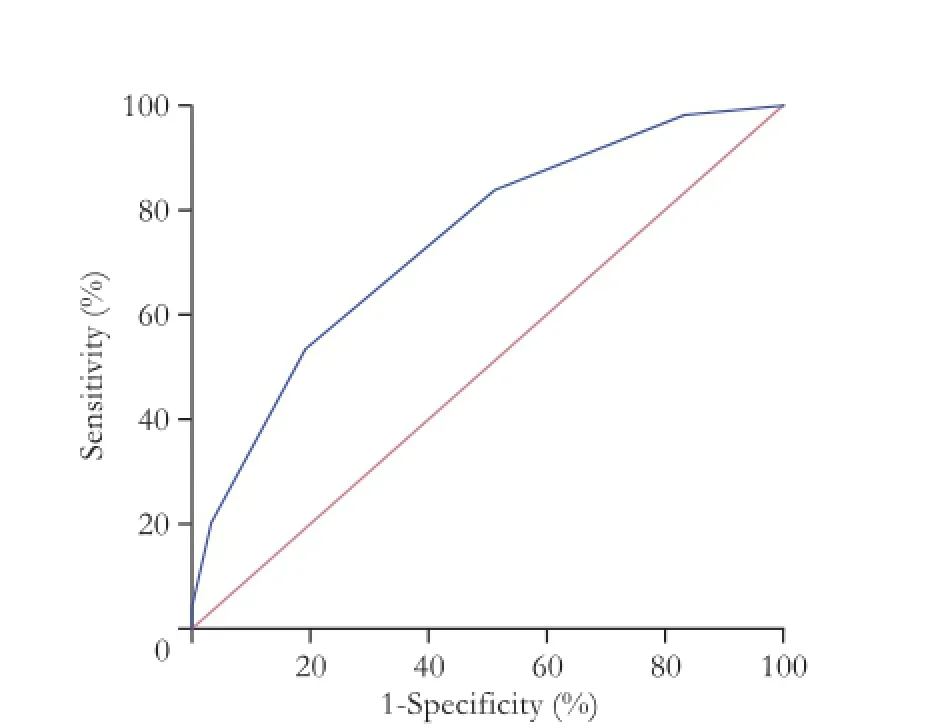
Fig. 2.Receiver operating characteristics curve: predictive value of combining independent risk factors for predicting early recurrence.

Fig. 3.Cumulative recurrence-free survival of low risk patients (blue line, number of risk factors ≤2) and high risk patients (green line, number of risk factors >2) (P<0.001).
Discussion
Early recurrence remains a major cause of treatment failure after curative resection of HCC. In our study, early recurrence developed in 56.0% (112/200) of the patients who received curative liver resection for HCC. The RFS curve steeply descended in the frst two years after surgery, indicating that a majority of patients developed recurrent disease in early phase (Fig. 1). Unfortunately, patients with early recurrence generally have worse prognosis than those with late phase recurrence.[13,14]Therefore, early identifcation of high-risk population and active interventions are crucial.
Various studies have reported risk factors for early recurrence. Poon et al[4]discriminated recurrent HCC into early- and late-phase diseases and identifed different risk factors. They concluded that early recurrence mainly arises from intrahepatic metastasis which is associated with aggressive tumor pathological characteristics. In contrast, late recurrence is more likely to be newly formed multicentric lesion on the background of chronic liver injury. Other reports[10,15,16]suggested that clinicopathologic parameters such as vascular invasion, number of tumors, non-anatomic resection and high AFP level correlate with early recurrence of HCC. Although chronic liver injury contributes to late recurrence throughde novotumor formation, elevated liver injury markers also refect infammatory liver microenvironment,[17,18]which is crucial for intrahepatic metastasis.[19,20]Therefore, we investigated prognostic value of readily-available liver enzyme markers to identify easy-to-use predictors of HCC early recurrence.
We analyzed four groups of parameters that may contribute to early recurrence: patient characteristics, liver injury markers, surgical factors, and tumor-related factors. Univariate analysis found 13 risk factors were statistically signifcant. As expected, cirrhosis, portal hypertension and ascites which indicate advanced liver disease were not correlated with early recurrence. CTP classifcation and MELD score that measure the advancement of liver dysfunction also had no signifcant relationship with early recurrence. In contrast, liver enzymes indicating liver injury and infammatory environment including AST, LDH, GGT, ALP and AST/ALT ratio were closely related to early recurrence. Surgical factors such as excessive blood loss and resection margin adjacent to tumor were also correlated with early recurrence. Factors refecting aggressive tumor behavior such as higher AFP secretion, larger tumor size, multiple tumors, and macrovascular invasion were correlated with early recurrence as expected. Moreover, advanced TNM and BCLC stages were also related to early recurrence.
Multivariate analysis, however, showed that only 5 of the 13 parameters remained as independent risk factors in univariate analysis. Patients with a high AFP level (>8.6 ng/mL) had a higher risk of early recurrence (HR=2.079,P=0.007). This may refect expected correlation between invasive tumor behavior and intrahepatic metastasis.[21]It is easy to understand that TNM stage is also associated with early recurrence (HR=2.164,P<0.001). Among surgical factors, only adjacent surgical margin was independent adverse prognostic factor (HR=2.354,P<0.001) although prolonged vascular occlusion, excessive blood loss and major liver resection were reported to be risk factors for recurrence (not specifc to early recurrence).[22-24]Interestingly, both LDH (HR=1.711,P=0.006) and AST/ALT ratio (HR=1.769,P=0.006) were independent risk factors for early recurrence.
Our results suggest that simple laboratory test of liver enzymes could serve as indicators of early recurrence after curative hepatectomy. LDH has been used as a marker for liver injury for a long history, but the role of this enzyme in HCC is less studied. The high level of LDH (especially LDH 5) has been reported in patients with aggressive HCC. Elevated serum level of LDH may indicate a rapid growth and a highly malignant behavior of HCC.[25]In patients receiving transarterial chemoembolization, high pre-therapeutic LDH level is associated with poor prognosis.[26]Dynamic observation of LDH in patients before and after transarterial chemoembolization revealed that LDH is an indicator of therapeutic effcacy.[27]LDH level also predicts the outcomes of HCC patients treated with sorafenib.[28]Our results showed that the high level of preoperative serum LDH was associated with early recurrence after curative resection of HCC. A possible explanation for the role of LDH in early recurrence observed in our study is that LDH is an indirect marker of tumor hypoxia and angiogenesis. Under hypoxic environment, cancer cells produce a high level of lactate and pyruvate via glycolysis.[29]As a key enzyme,LDH converts pyruvate to lactate under anaerobic condition. Upregulation of LDH ensures glycolytic metabolism under hypoxic condition in HCC. Relationship between hypoxia, LDH and angiogenesis through hypoxia inducible factor-1 is also well-established.[27]These evidences demonstrate that LDH may not only refect liver injury as an intracellular enzyme but also participate in aggressive tumor behavior via energy metabolism adaption and angiogenesis, thus correlates with early recurrence of HCC.
Another liver enzyme-derived marker we investigated is AST/ALT ratio. Both AST and ALT are hepatocyte predominant enzymes.[30]When hepatocytes are damaged, AST and ALT are released into blood, and their activities have been widely used in evaluating liver injury. The ratio of AST to ALT has been used as an indicator for fbrosis and cirrhosis.[17,18]Interestingly, the high AST/ALT ratio is also a risk factor of fbrosis in patients with non-alcoholic fatty liver disease, which is related to infammatory liver microenvironment.[31]Therefore, we hypothesize that the AST/ALT ratio may indicate fbrosis in addition to liver infammatory environment that facilitates the invasion and recurrence of HCC. Moreover, the AST/ALT ratio is an independent predictor of portal vein invasion, which is a well-known risk factor for HCC recurrence.[32]The underlying mechanism of early recurrence in patients with an increased AST/ALT ratio should be investigated in future studies.
In our study, ROC analysis was done to determine the optimal cut-off values of enzymatic markers. Since most patients who had undergone hepatectomy had well-compensated liver function, the cut-off values for predicting early recurrence of HCC were not as high as those traditionally used in the determination of signifcant liver injury. In a study on HCV-related HCC patients without postoperative interferon therapy, the cut-off value of AST or ALT for discriminating the high and low risk of HCC recurrence was 80 U/L.[33]This difference could be explained by several reasons. In the study of Yamashita et al,[33]postoperative AST and ALT were analyzed in HCV-related HCC patients without interferon therapy. Active hepatitis C-induced liver injury and elevated serum liver enzymes could be anticipated. In contrast, we analyzed preoperative serum liver enzymes in patients with well controlled HBV-related HCC (as indicated by low level HBV DNA) and HCC without hepatitis. In patients with elevated liver enzymes, antiviral therapy and liver protection therapy were routinely applied in our center to ensure surgery safety. Therefore, the baseline level of preoperative liver enzymes was relatively lower in the present study. Moreover, Yamashita et al[33]used AST and ALT to refect liver injury caused by HCV activity, and set the cut-off value empirically to 80 U/L. We did not hypothesize the role of liver enzymes in our study, and ROC analysis revealed a lower cut-off value as the best predictive value for early recurrence. The result also suggested that although the analyzed markers are liver enzymes, they may contribute to early recurrence of HCC through mechanisms other than liver injury.
We established a simple model using the fve identifed independent risk factors to predict early recurrence of HCC. ROC analysis revealed that when patients had three or more risk factors, they were more susceptible to early recurrence of HCC. In contrast, patients with two or less risk factors tended to have longer RFS and lower risk of early recurrence (Fig. 3). This model showed satisfactory sensitivity and specifcity.
However, the results should be interpreted cautiously due to some limitations of the study. This is a preliminary study with limited sample size. Population bias could not be ruled out and may distort the true effect of some risk factors of early recurrence. Although we analyzed most of the potentially signifcant parameters, early recurrence of HCC could be affected by other multiple factors. Because of the retrospective nature of this study, future prospective studies are warranted to confrm our results.
In this study, we investigated the prognostic role of common liver enzymes in early recurrence of HCC. Despite of limitations of the study, we found that high LDH, high AST/ALT ratio, elevated AFP level, small resection margin and advanced TNM stage were independent risk factors for early recurrence of HCC. In a simple multifactor predictive model, these risk factors were of value in the identifcation of patients who were at high risk of early recurrence. This readily-available simple model may help improve the management of early recurrence of HCC through early identifcation of high-risk population.
Contributors:JCP and DYT conceived this study. WZX and JCP collected and analyzed the data. CY, ZG and CWB assisted in data analysis. WZX and JCP wrote the manuscript. DYT revised the manuscript and gave fnal approval for publication. WZX and JCP contributed equally to this study. DYT is the guarantor.
Funding:This study was supported by grants from the Key Project of Medical Science and Technology Development Foundation, Nanjing Municipality Health Bureau (ZKX12011), Jiangsu Provincial Innovation Program for PhD Candidates (KYLX_0058) and Scientifc Research Foundation of Graduate School of Nanjing University (2013CL14).
Ethical approval:This study was approved by Institutional Ethics Committee of the Affliated Drum Tower Hospital of Nanjing University Medical School.
Competing interest:No benefts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69-90.
2 Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg 1999;229:322-330.
3 Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg 2002;235:373-382.
4 Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer 2000;89: 500-507.
5 Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg 2006;243:229-235.
6 Chun JM, Kwon HJ, Sohn J, Kim SG, Park JY, Bae HI, et al. Prognostic factors after early recurrence in patients who underwent curative resection for hepatocellular carcinoma. J Surg Oncol 2011;103:148-151.
7 Cha C, Fong Y, Jarnagin WR, Blumgart LH, DeMatteo RP. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg 2003;197:753-758.
8 Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, et al. Recurrence of hepatocellular carcinoma after surgery. Br J Surg 1996;83:1219-1222.
9 Nagasue N, Uchida M, Makino Y, Takemoto Y, Yamanoi A, Hayashi T, et al. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology 1993;105:488-494.
10 Du ZG, Wei YG, Chen KF, Li B. Risk factors associated with early and late recurrence after curative resection of hepatocellular carcinoma: a single institution's experience with 398 consecutive patients. Hepatobiliary Pancreat Dis Int 2014;13:153-161.
11 Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classifcation. Semin Liver Dis 1999;19:329-338. 12 Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis 2010;30:61-74.
13 Shimada M, Takenaka K, Gion T, Fujiwara Y, Kajiyama K, Maeda T, et al. Prognosis of recurrent hepatocellular carcinoma: a 10-year surgical experience in Japan. Gastroenterology 1996;111:720-726.
14 Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg 1999;229:216-222.
15 Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol 2003;38:200-207.
16 Li T, Qin LX, Gong X, Zhou J, Sun HC, Wang L, et al. Clinical characteristics, outcome, and risk factors for early and late intrahepatic recurrence of female patients after curative resection of hepatocellular carcinoma. Surgery 2014;156:651-660.
17 Hann HW, Wan S, Myers RE, Hann RS, Xing J, Chen B, et al. Comprehensive analysis of common serum liver enzymes as prospective predictors of hepatocellular carcinoma in HBV patients. PLoS One 2012;7:e47687.
18 Lin YJ, Lee MH, Yang HI, Jen CL, You SL, Wang LY, et al. Predictability of liver-related seromarkers for the risk of hepatocellular carcinoma in chronic hepatitis B patients. PLoS One 2013;8:e61448.
19 Capece D, Fischietti M, Verzella D, Gaggiano A, Cicciarelli G, Tessitore A, et al. The infammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. Biomed Res Int 2013;2013:187204.
20 Han YF, Zhao J, Ma LY, Yin JH, Chang WJ, Zhang HW, et al. Factors predicting occurrence and prognosis of hepatitis-B-virus-related hepatocellular carcinoma. World J Gastroenterol 2011;17:4258-4270.
21 Giannini EG, Sammito G, Farinati F, Ciccarese F, Pecorelli A, Rapaccini GL, et al. Determinants of alpha-fetoprotein levels in patients with hepatocellular carcinoma: implications for its clinical use. Cancer 2014;120:2150-2157.
22 Makino I, Chijiiwa K, Kondo K, Ohuchida J, Kai M. Prognostic beneft of selective portal vein occlusion during hepatic resection for hepatocellular carcinoma. Surgery 2005;137:626-631.
23 Katz SC, Shia J, Liau KH, Gonen M, Ruo L, Jarnagin WR, et al. Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg 2009;249:617-623.
24 Hasegawa K, Kokudo N. Surgical treatment of hepatocellular carcinoma. Surg Today 2009;39:833-843.
25 Fujiwara Y, Takenaka K, Kajiyama K, Maeda T, Gion T, Shirabe K, et al. The characteristics of hepatocellular carcinoma with a high level of serum lactic dehydrogenase: a case report. Hepatogastroenterology 1997;44:820-823.
26 Kohles N, Nagel D, Jüngst D, Durner J, Stieber P, Holdenrieder S. Prognostic relevance of oncological serum biomarkers in liver cancer patients undergoing transarterial chemoembolization therapy. Tumour Biol 2012;33:33-40.
27 Scartozzi M, Faloppi L, Bianconi M, Giampieri R, Maccaroni E, Bittoni A, et al. The role of LDH serum levels in predicting global outcome in HCC patients undergoing TACE: implications for clinical management. PLoS One 2012;7:e32653.
28 Faloppi L, Scartozzi M, Bianconi M, Svegliati Baroni G, Toniutto P, Giampieri R, et al. The role of LDH serum levels in predicting global outcome in HCC patients treated with sorafenib: implications for clinical management. BMC Cancer 2014;14:110.
29 Warburg O. On the origin of cancer cells. Science 1956;123:309-314.
30 Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ 2005;172:367-379.
31 Cichoż-Lach H, Celiński K, Prozorow-Król B, Swatek J, Słomka M, Lach T. The BARD score and the NAFLD fbrosis score in the assessment of advanced liver fbrosis in nonalcoholic fatty liver disease. Med Sci Monit 2012;18:CR735-740.
32 Shijo H, Okazaki M. Prediction of portal vein invasion by hepatocellular carcinoma: a correlations between portal vein tumor thrombus and biochemical tests. Jpn J Clin Oncol 1991;21:94-99.
33 Yamashita Y, Shirabe K, Toshima T, Tsuijita E, Takeishi K, Harimoto N, et al. Risk factors for recurrence after curative resection of hepatitis C-related hepatocellular carcinoma in patients without postoperative interferon therapy. Hepatol Res 2013;43:1313-1320.
Received November 2, 2014
Accepted after revision February 1, 2015
AuthorAffliations:Department of Hepatobiliary Surgery, the Affliated Drum Tower Hospital of Nanjing University Medical School (Wang ZX, Jiang CP, Cao Y, Chen WB and Ding YT); Department of Hepatobiliary Surgery, Nanjing Drum Tower Hospital Clinical Medical College of Nanjing Medical University (Jiang CP, Zhang G and Ding YT), Nanjing 210008, China
Yi-Tao Ding, MD, Department of Hepatobiliary Surgery, the Affliated Drum Tower Hospital of Nanjing University Medical School, 321 Zhongshan Road, Nanjing 210008, China (Tel: +86-25-83304616ext66866; Fax: +86-25-83307115; Email: yitaoding1950@ gmail.com)
© 2015, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(15)60353-8
Published online March 18, 2015.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Progression-free survival as surrogate endpoint in advanced pancreatic cancer: meta-analysis of 30 randomized frst-line trials
- Preoperative diabetes as a protective factor for pancreatic fstula after pancreaticoduodenectomy: a meta-analysis
- Benefcial mechanisms of aerobic exercise on hepatic lipid metabolism in non-alcoholic fatty liver disease
- Major complications of adult right lobe living liver donors
- Prognostic value of glypican-3 in patients with HBV-associated hepatocellular carcinoma after liver transplantation
- sFRP-4, a potential novel serum marker for chronic hepatitis B-related hepatocellular carcinoma