Screening,Identification and Fermentation Property of a Yeast Strain R6 Accumulating Alpha-ketoglutaric Acid
2015-01-18JingCHENChunyangZHANGChaoLI
Jing CHEN,Chunyang ZHANG,Chao LI
1.School of Life Science,Shandong University of Technology,Zibo 255049,China;
2.Zibo Center for ADR Monitoring,Zibo 255008,China
Responsible editor:Qingqing YIN Responsible proofreader:Xiaoyan WU
Alpha-ketoglutaric acid (alpha-KG)is an important intermediate of tricarboxylic acid (TCA)cycle,which plays a significant role in the metabolism of matter and energy.Alpha-KG is also the precursor of many essential amino acids and proteins,which are widely used in medicine,organic synthesis,functional food,and other fields.But the importance of alpha-KG in TCA cycle and biosynthesis makes it difficult to accumulate and secrete to the outside of cells.It means that there are certain difficulties to achieve alpha-KG accumulation extensively.Though chemical synthesis can produce a lot of alpha-KG,it has several disadvantages such as high cost and complex process.Moreover,it is hard to use in food and cosmetics directly.Meanwhile,microbial fermentation can produce alpha-KG with the advantages of low cost and simple process,which shows a broad application prospect in food and health care products industry[1-2].
Since the first study on alpha-KG fermentation was reported in 1946,natural strain that possesses ability of accumulating alpha-KG has become a research hot spot.In the early decades,the research mainly focused on the category of microorganisms accumulating alpha-KG.Doucette et al.found that Pseudomonas,Enterobacter aerogenes and Serratia marcescens could accumulate alpha-KG extensively.He put forward that the concentration of carbon source and nitrogen source was very important for the excessive accumulation of alpha-KG[3-6].Recently,the research on producing of alpha-KG in thiamine auxotrophic yeast through fermentation have aroused many people’s interests.Liu et al.[7-8]have made great achievements in using Yarrowia lipolytica and Torulopsis glabrata to produce alpha-KG by fermentation.
In the study,thiamine auxotrophy was used as a negative selective indicator,and a strain R6 which had the ability to accumulate alpha-KG was isolated and screened from the edible oilpolluted soil.The fermenting properties,physiological and biochemical characters as well as the phylogeny properties of strain R6 were investigated.It was identified as a Rhodotorula mucilaginosa according to API 20 C AUX yeast identification system and the phylogenetic property of its 18S rDNA gene sequence.The study provides a valuable strain resource and technique support for producing alpha-KG by fermentation.
Materials and Methods
Sample and molecular biological reagents
The edible oil-polluted soil was sampled from Zhangdian District,Zibo,China.Biochemical and molecular biological reagents,including PCR Master Mix (2) (product number:BS9295,Sangon),primers,lysozyme,proteinase K,Taq DNA polymerase,EZ-10 DNA Gel Extraction Kit,DNA Cloning Vector Kit,and an API 20 C AUX yeast identification kit were purchased from Shanghai Sangon Biological Engineering Technology &Services Co.,Ltd.(Shanghai,China).
Isolation and screening
10 g of soil sample was diluted into 90 ml of sterilized water,and 200 μl of 10-fold serial dilutions diluents(10-2-10-7) was spread onto isolation medium plate (1 L of distilled water containing 30 g of glucose,10 g of peptone,0.5 g of KH2PO4,0.2 g of Mg-SO4·7H2O,15 g of agar,pH 5.5) to grow colonies.Then,single colonies were streaked onto solid fermentation medium plate [1 L of distilled water containing 20 g of glucose,10 g of(NH4)SO4,1 g of KH2PO4,0.5 g of Mg-SO4·7H2O,0.020 mg of thiamine (filter-sterilized stock),15 g of agar and 10 ml of trace element solution,pH 7.5].The trace element solution contained(per liter of distilled water)2 g of CaCl2·2H2O,2 g of FeSO4·7H2O,0.5 g of ZnCl2,1.2 g of MnCl2·4H2O and 0.05 g of CuSO4·5H2O.As the negative indicator,negative selection medium plate without thiamine was prepared the same as solid fermentation medium.All cultures were incubated at 30 ℃unless described.The colonies which grew on solid fermentation medium plate but did not grow on negative selection medium plate were selected to be thiamine auxotrophic strains.The selected strains were used for further identification and alpha-KG fermentation analysis.
Physiological and biochemical experiments
Morphological,physiological and biochemical characteristics of strain R6 were completed by referring to Barnett’s Manual of Yeast characteristics and identification,and API 20 C AUX yeast identification system(BioMerieux S.A.,Marcy l ‘Etoile,France,version 4.0) was also applied to the physiological and biochemical properties of the pure culture according to the manufacturer’s directions.
Cloning,sequencing,and phylogenetic analysis of 18S rDNA gene
Genomic DNA of the thiamine auxotrophic strain grown on liquid medium was extracted as previously described[9].Prepared DNA was used as the template for polymerase chain reaction (PCR) for amplifying 18S rDNA gene.Universal primers in fungus:forward primer P1 (5’-ATCTGGTTGATCCTGCCAGT-3’) and reverse primer P2 (5’-GATCTTCCGCAGGTTCACC-3’) were synthesized and used.The following PCR program was used:pre-denaturation at 95 ℃for 10 min,35 cycles consisting of 1 min at 94 ℃,1 min at 55 ℃,and 2 min at 72℃.The last step was continued for 10 min to get enough time for extending.The PCR products were separated by electrophoresis in 1% (w/v) agarose gel with TBE buffer,and the desired PCR products were purified using EZ-10 DNA Gel Extraction Kit as recommended by the manufacturer.The purified 18S rDNA gene fragment was sequenced by Shanghai Biosune Biotechnology Co.,Ltd.(Shanghai,China).
The final 18S rDNA gene sequence was sent to National Center for Biotechnology Information (NCBI)web site to search for the related 18S rDNAs using the BLAST program.Sequences obtained in the study and those retrieved from GenBank database were aligned by ClustalW method,then a phylogenetic tree was constructed using MEGA program version 4.0(Lynnon Biosoft)[10-12].
Addition of impact factor in the fermentation substrate
Fermentation medium[1 L of distilled water contained100 g of glucose,7 g of NH4Cl,6 g of CH3COONa,5 g of KH2PO4,0.8 g of MgSO4·7H2O,PH 5.5]with different concentration of thiamine (10,20,30 and 40 mg/L) and CaCO3(0,10,20,30,40,50 and 60 g/L) were tested respectively to determine the optimal conditions for accumulating alpha-KG[13-15].
Analytical methods
After fermenting for 48 h,the supernatant of fermentation culture was examined through high performance liquid chromatography (HPLC:AGILENT1100) to determine the amount of the alpha-KG.Chromatographic conditions:StableBond C18 reversedphase column,column temperature:28 ℃,detector:UV 210 nm,mobile phase:0.1%(v/v)H3PO4,flow rate:1.0 ml/min and injection volume:10 μl16].
Results and Analysis
Microbe isolation
Athiamine auxotrophic strain R6 which has the ability to accumulate alpha-KG was isolated from the activated sludge through the method of negative selection[17-18].As shown in Fig.1,the strain formed smooth and translucent colonies on solid selective medium.Microscopic observation revealed that strain R6 was a red fungus.
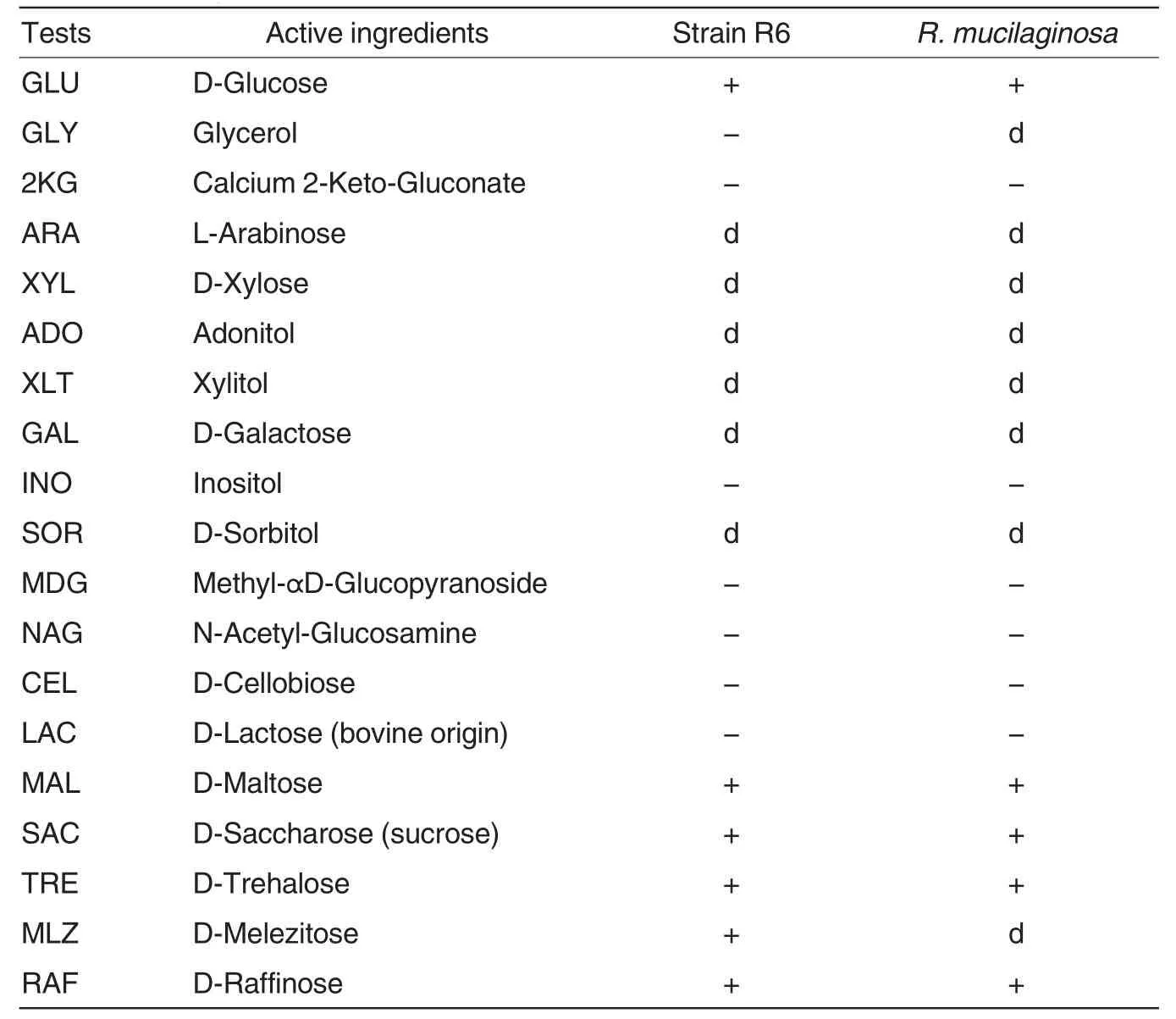
Table1 Main physiological and biochemical characters of strain R6 revealed by API 20 C AUX system
Physiological and biochemical characters
Strain R6 was further identified through physiological and biochemical experiments.Based on the microscopic morphological characteristics of R6,API 20 C AUX yeast identification system was applied to identify the strain.The main physiological and biochemical characters of R6 are shown in Table1.From these results,it could be seen that R6 shared the common feature with R.mucilaginosa both in colors and most of physiological and biochemical characters.So,we suggest that R6 belongs to R.mucilaginosa.
Cloning,sequencing,and phylogenetic analysis of 18S rDNA gene
APCR product about 1 700 bp was amplified with the forward primer P1 and reverse primer P2,from the genomic DNA of strain R6(Fig.2).The PCR product purified by kit was used to sequence and sequence alignment,and the result proved that the sequence was highly homologous with 18S rDNA genes of other species.The 18S rDNA gene sequence of strain R6 was performed to search for the related 18S rDNAs from GenBank database at NCBI with the BLAST program,and the results showed that the identity between strain R6 18S rDNA gene and R.mucilaginosa reach up to 99%,which indicated R6 was a member of genus R.mucilaginosa.Finally,a phylogenetic tree consisting of 18 members was constructed (Fig.3)with MEGA program based on the 18S rDNA gene sequences of strain R6 and the related strains in the GenBank database[19].As described in the phylogenetic tree that strain R6 was located in the lineage of R.mucilaginosa.The 18S rDNA gene of strain R6 and R.mucilaginosa (MAFF237983),R.mucilaginosa (YS6R.mucilaginosa2),R.mucilaginosa (L10-2)gathered into a cluster,which formed a special clade,R.mucilaginosa clade.The results indicated that strain R6 was a member of the species R.mucilaginosa.
Influence of VB1 on alpha-KG accumulation
Strain R6 is a thiamine auxotroph,and thiamine is a coenzyme of alphaR.mucilaginosaketoglutarate dehydrogenase system in tricarboxylic acid cycle simultaneously[20-21].Therefore,the amount of thiamine in substrate had great influence on the volume of production of alpha-KG fermentation.Experimental results (Fig.4) showed that when the concentration of thiamine was 0.03 mg/L,the production of alpha-KG was increased to 3.688 g/L.Nevertheless,with the increase of thiamine,alpha-KG would enter the tricarboxylic acid cycle and transform into succinic acid,leading to that alpha-KG yield gradually decreased.
Influence of CaCO3 on alpha-KG accumulation
CaCO3could regulate the pH value of fermenting broth,and Ca2+could promote the accumulation of alpha-KG by improving the activity of pyruvate carboxylase and alpha-ketoglutarate dehydrogenase system.Thus,changing the amount of CaCO3will affect the production of ketoglutaric acid in the fermenting broth although the CaCO3might be not fully decomposed.Experimental results(Fig.5)showed that the concentration of alpha-KG was increased with the increasing of CaCO3concentration no more than 60 g/L,and reached the maximum value of 6.035 g/L at CaCO3of 60 g/L.Meanwhile,the concentration of CaCO3was increased continually that could not improve the production of alpha-KG effectively[22-23].
Conclusions
Here,a thiamine auxotrophic strain R6 which had the ability of accumulating alpha-KG was isolated from the contaminated soil.Physiological and biochemical characters as well as the phylogenetic analysis based on 18S rDNA gene sequences revealed that strain R6 was a member of R.mucilaginosa.Strain R6 could accumulate alpha-KG adequately when the partial metabolic and growth restriction caused by thiamine auxotrophy was happened[3].This property made it possible to accumulate alpha-KG excessively by controlling the addition of thiamine and CaCO3in the medium and to maintain the activity of alpha-KG dehydrogenase and pyruvate carboxylase in appropriate levels[2].In the study,the accumulation of alpha-KG was increased by optimizing the amount of thiamine and CaCO3in the fermentation medium of strain R6.When the additions of thiamine and CaCO3were 0.03 mg/L and 60 g/L respectively,the alpha-KG accumulation reached a value of 6.035 g/L.However,the nutritional and environmental conditions,such as thiamine,nitrogen source,CaCO3,dissolved oxygen and pH value,still have much space for optimization.
Although there were still many problems to be resolved and the optimal fermentation conditions remained to be further study,the discovery of the natural thiamine auxotrophic strain R6 not only provided a valuable microbial resource for alpha-KG production,but also laid a foundation for further fermentation regulation to achieve excessive alpha-KG accumulation.
[1]LI Y,CHEN J,LUN SY,RUI XS.Efficient pyruvate production by a multi-vitamin auxotroph of Torulopsis glabrata:key role and optimization of vitamin levels[J].Applied Microbiol and Biotechnology,2001,55(6):680-685.
[2]ZHOU JW,YIN XX,MADZAK C,et al.Enhanced α-ketoglutarate production in Yarrowia lipolytica WSH-Z06 by alteration of the acetyl-CoA metabolism [J].Journal of Biotechnology,2012,161(3):257-264.
[3]DOUCETTE CD,SCHWAB DJ,WINGREEN NS,et al.α-Ketoglutarate coordinates carbon and nitrogen utilization via enzyme I inhibition[J].Nature Chemical Biology,2011,7(12):894-901.
[4]MUJIKA JI,UGALDE JM,LOPEZ X.Aluminum interaction with glutamate and α-ketoglutarate:a computational study [J].The Journal of Physical Chemistry B,2014,118(24):6680-6686.
[5]BATES JM,MITTGE E,KUHLMAN J,et al.Distinct signals from the microbiota promote different aspects of zebrafish gut differentiation [J].Developmental Biology,2006,297(2):374-86.
[6]LIN H,VADALI RV,BENNETT GN,et al.Increasing the acetyl-CoA pool in the presence of overexpressed phosphoenolpyruvate carboxylase or pyruvate carboxylase enhances succinate production in Escherichia coli.[J].Biotechnology Progress,2004,20 (5):1599-1604.
[7]LIU LM,LI Y,ZHU Y,et al.Redistribution of carbon flux in Torulopsis glabrata by altering vitamin and calcium level[J].Metabolic Engineering,2007,9 (1):21-29.
[8]MICELI MH,DÍAZ JA,LEE SA.Emerging opportunistic yeast infections [J].The Lancet Infectious Diseases,2011,11(2):142-151.
[9]MARMUR J,ANDERSON WF,MATTHEWS L,et al.The effects of ultraviolet light on the biological and physical chemical properties of deoxyribonucleic acids[J].Journal of Cellular&Comparative Physiology,1961,58(3):33-55.
[10]LI RP,ZHANG HY,LIU WM,et al.Biocontrol of postharvest gray and blue mold decay of apples with Rhodotorula mucilaginosa and possible mechanisms of action [J].International Journal of Food.Microbiology,2011,146(2):151-156.
[11]BERSHTEIN S,TAWFIK DS.Advances in laboratory evolution of enzymes [J].Current Opinion Chemical Biology,2008,12(2):151-158.
[12]BAEK JO,SEO JW,KWON O,et al.Expression and characterization of a second L-amino acid deaminase isolated from proteus mirabilis in Escherichia coli[J].Journal of Basic Microbiology,2011,51(2):129-135.
[13]KUNZ DA,CHEN JL,PAN GL.Accumulation of α-keto acids as essential components in cyanide assimilation by pseudomonas fluoresces NCIMB 11764 [J].Applied and Environmental Microbiology,1998,64 (11):4452-4459.
[14]RAGGI P,LOPEZ P,DIAZ A,et al.Debaryomyces hansenii and Rhodotorula mucilaginosa comprised the yeast core gut microbiota of wild and reared carnivorous salmonids,croaker and yellowtail[J].Environmental Microbiology,2014,16(9):2791-2803.
[15]SCHREIER HJ,SMITH TM,BERNLOHR RW.Regulation of nitrogen catabolic enzymes in Bacillus spp.[J].Journal of Bacteriology,1982,151(2):971-975.
[16]WELBORN JR,SHPUN S,DANTZLER WH,et al.Effect of alpha-ketoglutarate on organic anion transport in single rabbit renal proximal tubules[J].The American Journal of Physiology,1998,274(1 Pt 2):F165-174.
[17]NESTL BM,NEBEL BA,HAUER B.Recent progress in industrial biocatalysis[J].Current Opinion Chemical Biology,2011,15(2):187-193.
[18]REETZ MT,BOCOLA M,CARBALLEIRA JD,et al.Expanding the range of substrate acceptance of enzymes:combinatorial active-site saturation test[J].Angewandte Chemie(International Ed.In English),2005,44(27):4192-4196.
[19]MASEREEUW R,RUSSEL FG,MILLER DS.Multiple pathways of organic anion secretion in renal proximal tubule revealed by confocal microscopy [J].The American Journal of Physiology,1996,271 (6 pt 2):1173-1182.
[20]LO RE V,FISHMAN NO,NACHAMKIN I.Recurrent catheter-related Rhodotorula rubra infection[J].Clinical Microbiology and Infection,2003,9(8):897-900.
[21]YU T,LI HY,ZHENG XD.Synergistic effect of chitosan and Cryptococcus laurentii on inhibition of Penicillium expansum infections[J].International Journal of Food Microbiology,2007,114(3):261-266.
[22]OUE S,OKAMOTO A,YANO T,et al.Redesigning the substrate specificity of an enzyme by cumulative effects of the mutations of non-active site residues[J].The Journal of Biological Chemistry,1999,274(4):2344-2349.
[23]JAEGER KE,EGGERT T,EIPPER A,et al.Directed evolution and the creation of enantioselective biocatalysts[J].Applied Microbiology and Biotechnology,2001,55(5):519-530.
杂志排行
Agricultural Science & Technology的其它文章
- Effects of Exogenous Glycine Betaine on Oxidation Metabolism in Cucumbers during Low-temperature Storage
- A Preliminary Study on Genetic Variation of gE Gene of an Epidemic Pseudorabies Virus Strain and Its Pathogenicity to Piglets
- Development and Application of a Quantitative Competitive PCR Assay for Detecting Mycoplasma hyopneumoniae
- A New Rapid and Batch-oriented Crushing Method for DNA Extraction from Maize Leaves
- Effects of Reducing Application Amount of Base Fertilizer and Increasing Application Time of Leaf Fertilizer on Corn Yield
- Effects of Green Manure Rotation on Rice Growth Dynamics and Nitrogen Uptake and Utilization
