奈诺沙星体外抗菌作用研究
2015-01-05朱德妹吴培澄胡付品叶信予张婴元
朱德妹, 吴培澄, 胡付品, 吴 湜, 叶信予, 张婴元
·论著·
奈诺沙星体外抗菌作用研究
朱德妹, 吴培澄, 胡付品, 吴 湜, 叶信予, 张婴元
目的评价奈诺沙星对临床分离菌的体外抗菌作用。方法琼脂对倍稀释法测定奈诺沙星对1 263株临床分离菌和肉汤微量稀释法测定51株支原体属的体外抗菌活性、杀菌浓度、杀菌曲线以及培养条件对其抗菌活性的影响。结果奈诺沙星对甲氧西林耐药葡萄球菌、青霉素不敏感肺炎链球菌均具高度抗菌活性,对粪肠球菌的抗菌活性略低。其对革兰阳性球菌的抗菌活性明显优于左氧氟沙星、环丙沙星和莫西沙星。奈诺沙星对多数肠杆菌科细菌、洛菲不动杆菌和嗜麦芽窄食单胞菌等不发酵糖革兰阴性杆菌具良好抗菌作用,对嗜血杆菌属、卡他莫拉菌具高度抗菌活性,但对淋病奈瑟菌的抗菌作用差。该药对肠杆菌科细菌和多数不发酵糖革兰阴性杆菌(除了鲍曼不动杆菌和铜绿假单胞菌)的抗菌作用与其他受试喹诺酮类大致相仿,或略差。奈诺沙星对消化链球菌和痤疮丙酸杆菌等厌氧菌以及肺炎支原体具有良好的抗菌活性。奈诺沙星对受试的革兰阳性球菌和革兰阴性菌均具杀菌作用。培养条件中p H和细菌接种菌量的改变对奈诺沙星体外抗菌活性无明显影响,但血清蛋白含量增高时可降低奈诺沙星的抗菌活性。结论奈诺沙星对需氧革兰阳性菌、革兰阴性菌、部分厌氧菌和肺炎支原体等具有广谱抗微生物活性,尤其对甲氧西林耐药金黄色葡萄球菌、青霉素不敏感肺炎链球菌具有高度抑菌和杀菌活性,优于其他受试氟喹诺酮类抗菌药物。
奈诺沙星; 药物敏感性试验; 抗菌药物; 最低抑菌浓度
奈诺沙星(nemonoxacin TG-873870)是近年来TaiGen生物技术公司研制的一种新型不含氟的C-8-甲氧基结构的喹诺酮类抗菌药物[1]。该药通过抑制DNA旋转酶(拓扑异构酶Ⅱ)和拓扑异构酶Ⅳ达到对细菌的广谱抗菌作用。奈诺沙星具有显著的体外抗菌活性[2-4],对来自呼吸道和皮肤感染的许多相关病原菌,包括多重耐药肺炎链球菌和甲氧西林耐药金黄色葡萄球菌(金葡菌)(MRSA)均具有抗菌活性。此外,该药的口服制剂具有良好的药动学和耐受性[5-7]。目前已经完成在中国大陆和台湾地区口服制剂Ⅱ期和Ⅲ期社区获得性肺炎的临床试验。现将我们在临床前研究中奈诺沙星的体外药效学,包括该药对国内临床分离菌的抗菌活性、杀菌作用以及培养条件对其抗菌活性的影响等结果报道如下。
1 材料与方法
1.1 材料
1.1.1 受试菌株 2004—2005年上海、北京等地16所医院临床分离菌共1 263株,均经本研究所重新鉴定确认,无重复株。质控菌为金葡菌ATCC29213(甲氧西林敏感株,MSSA)、N315(MRSA),肺炎链球菌ATCC49619(青霉素中介株,PISP),流感嗜血杆菌ATCC49247,粪肠球菌ATCC29212,脆弱拟杆菌ATCC25282,解脲脲原体ATCC27618和肺炎支原体ATCC25523;均为本研究所保存菌株。
1.1.2 抗菌药物标准品和对照品 苹果酸奈诺沙星(nemonoxacin malate,TG875649)为TaiGen生物技术公司提供,该药品与奈诺沙星(nemonoxacin TG-873870)的分子量换算为1.385∶1;莫西沙星和利奈唑胺为American Custom Chemicals Corp产品;头孢吡肟为中美上海施贵宝制药有限公司产品;亚胺培南为美国默沙东公司产品;甲硝唑为Sigma公司产品。左氧氟沙星等其他21种抗菌药物均为中国药品生物制品鉴定所标准品。
1.1.3 药敏试验用培养基 药敏试验采用的Meuller-Hinton(MH)琼脂、Haemophilus Test Medium Base(HTMB)及SR158添加剂、GC培养基以及L53补充专用培养基、GAM培养基均为英国OXOID公司产品。阳离子调节MH肉汤(Cation Adjusted Mueller Hinton Broth CAMHB)为BBL公司产品。脲原体属10-B肉汤培养基和肺炎支原体属SP-4肉汤培养基为美国Remel公司产品。
1.2 方法
1.2.1 药敏试验 按美国临床和实验室标准化协会(CLSI)[8]推荐,采用琼脂对倍稀释法测定抗菌药物对临床分离菌的最低抑菌浓度(MIC)。支原体属的药物敏感性试验按Lorian等[9]2005年描述的微量稀释法进行。
1.2.2 最低杀菌浓度(MBC)和杀菌曲线 按Lorian等[10]2005年描述的MBC的方法测定奈诺沙星对194株临床分离株的MBC以及对临床分离的MSSA、MRSA、粪肠球菌、肺炎链球菌、大肠埃希菌和铜绿假单胞菌各2株,和质控菌株共18株细菌进行奈诺沙星的杀菌动力学监测。对金葡菌、肺炎链球菌和粪肠球菌以万古霉素作为对照药;大肠埃希菌和铜绿假单胞菌以环丙沙星作对照药。以不加药的105CFU/mL菌液同步培养作为对照。
1.2.3 培养条件对奈诺沙星体外抗菌活性的影响
采用液体微量稀释法[8]在不同培养条件下测定奈诺沙星对60株临床分离株的体外抗菌活性及其对不同p H值(p H 5、p H 7、p H 9)的培养基、不同接种菌量(103CFU/mL、105CFU/mL、107CFU/mL)和不同人血清浓度(25%、50%、75%)的影响。以105CFU/mL的接种菌量、p H 7和不含人血清的CAMHB肉汤为质控对照。
1.2.4 药敏试验结果的判断标准和资料统计 采用SPSS统计软件进行药敏资料的统计,并按CLSI 2006年M100-16的标准[8]判断对需氧菌的药敏结果;对卡他莫拉菌采用CLSI 2006 M45-A[11];对厌氧菌采用NCCLS2001 M11-A5[12]的判断标准。
2 结果
2.1 奈诺沙星的体外抗菌作用
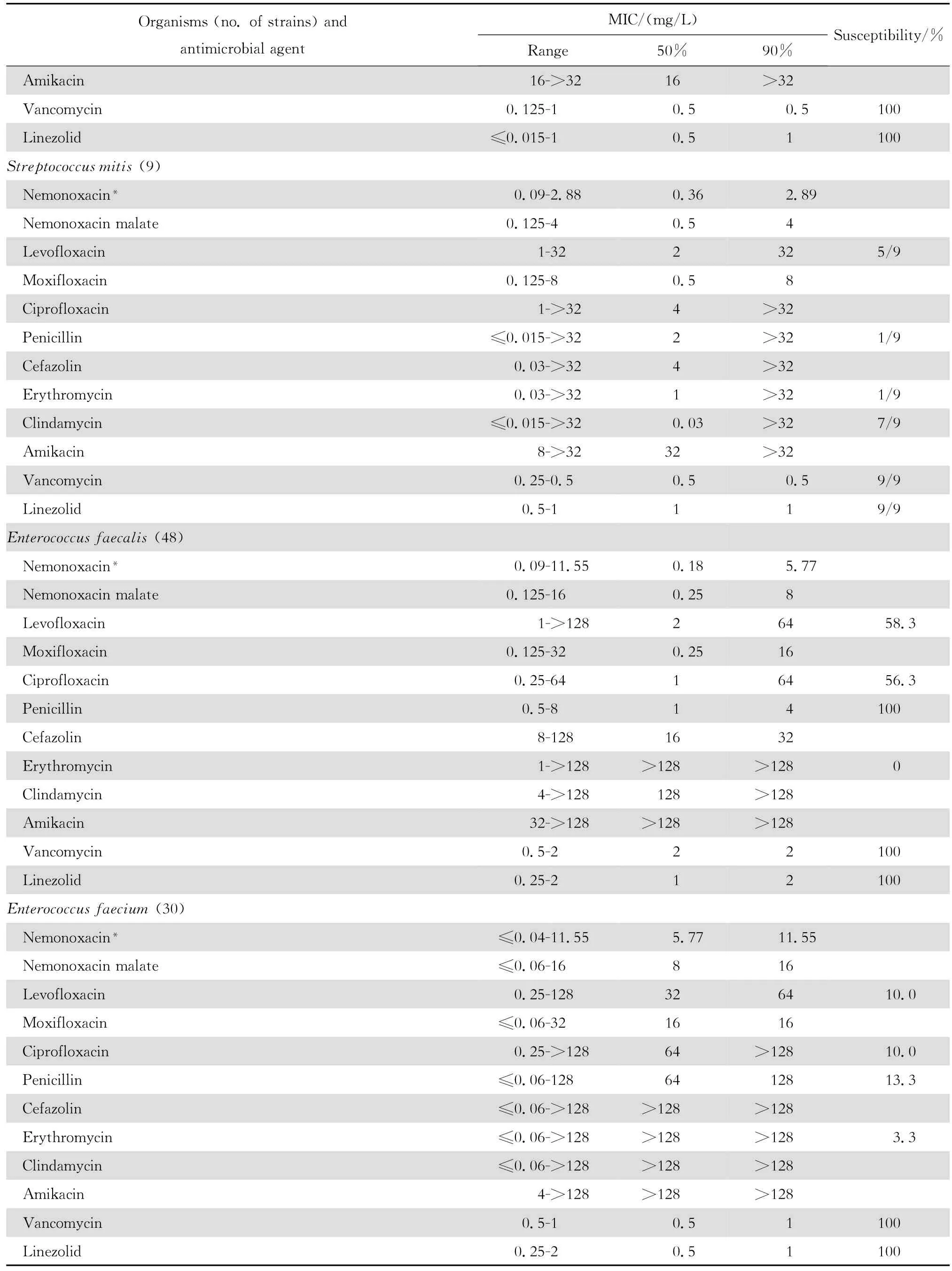
2.1.1 革兰阳性菌 奈诺沙星对所有受试的需氧革兰阳性菌具强大的抗菌活性(表1)。0.72 mg/L浓度的奈诺沙星可抑制90%~100%受试的甲氧西林敏感葡萄球菌(MSSA、MSCNS)和甲氧西林耐药葡萄球菌(MRSA和MRCNS)。0.04~0.72 mg/L浓度的奈诺沙星可抑制90%~100%受试的青霉素敏感和不敏感肺炎链球菌、A组链球菌和B组链球菌。奈诺沙星2.89 mg/L浓度仍可抑制87.5%的粪肠球菌;本品对屎肠球菌的作用较差。奈诺沙星对甲氧西林耐药葡萄球菌和青霉素耐药肺炎链球菌(PRSP)的抗菌活性远高于其他喹诺酮类,对粪肠球菌的抗菌活性亦高于受试的其他喹诺酮类。
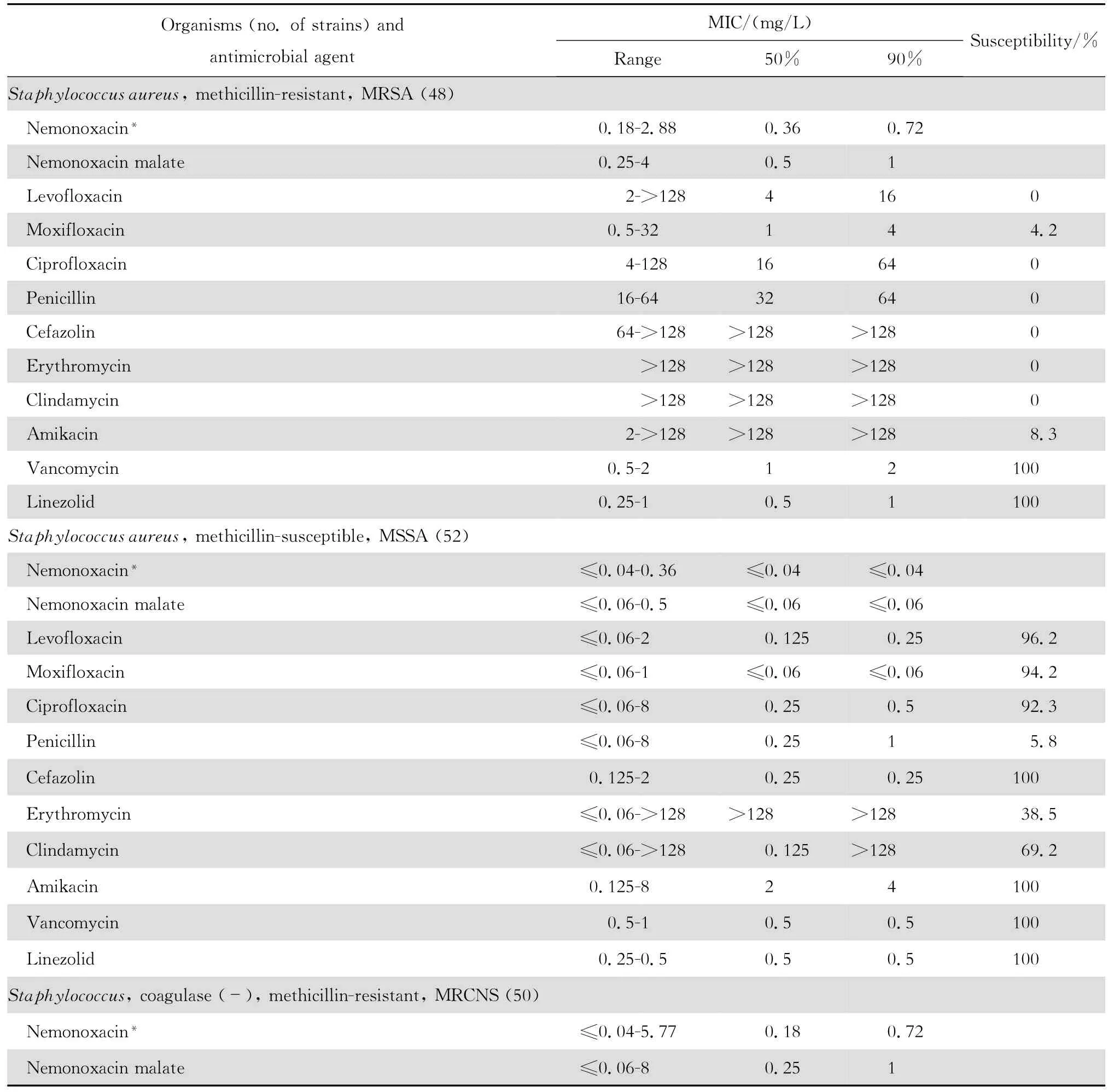
表1 奈诺沙星等抗菌药物对革兰阳性菌470株的体外药敏试验结果Table1 In vitro activities of nemonoxacin and comparative antimicrobial agents against aerobic gram-positive microorganisms(n=470)
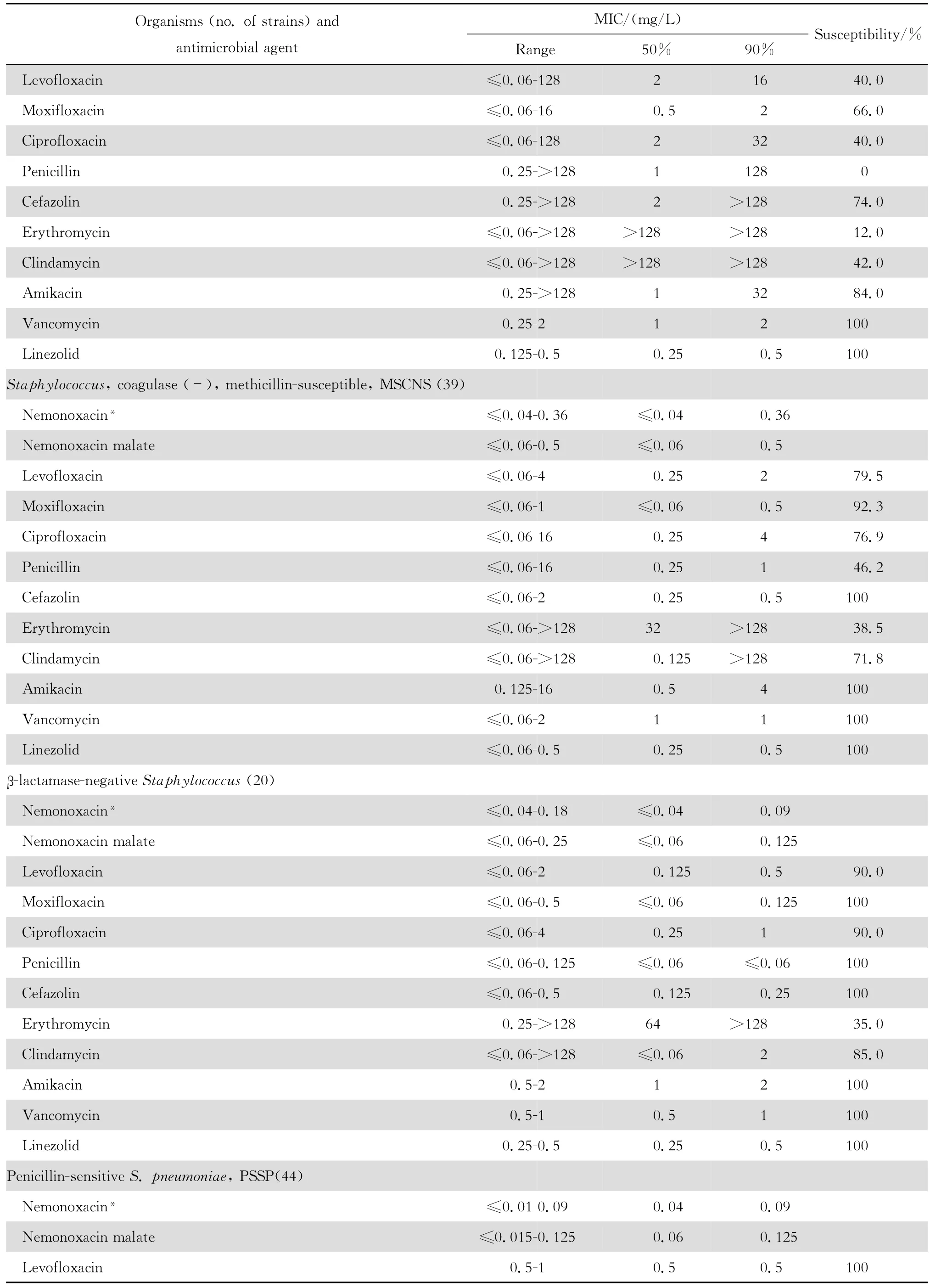
continued table1
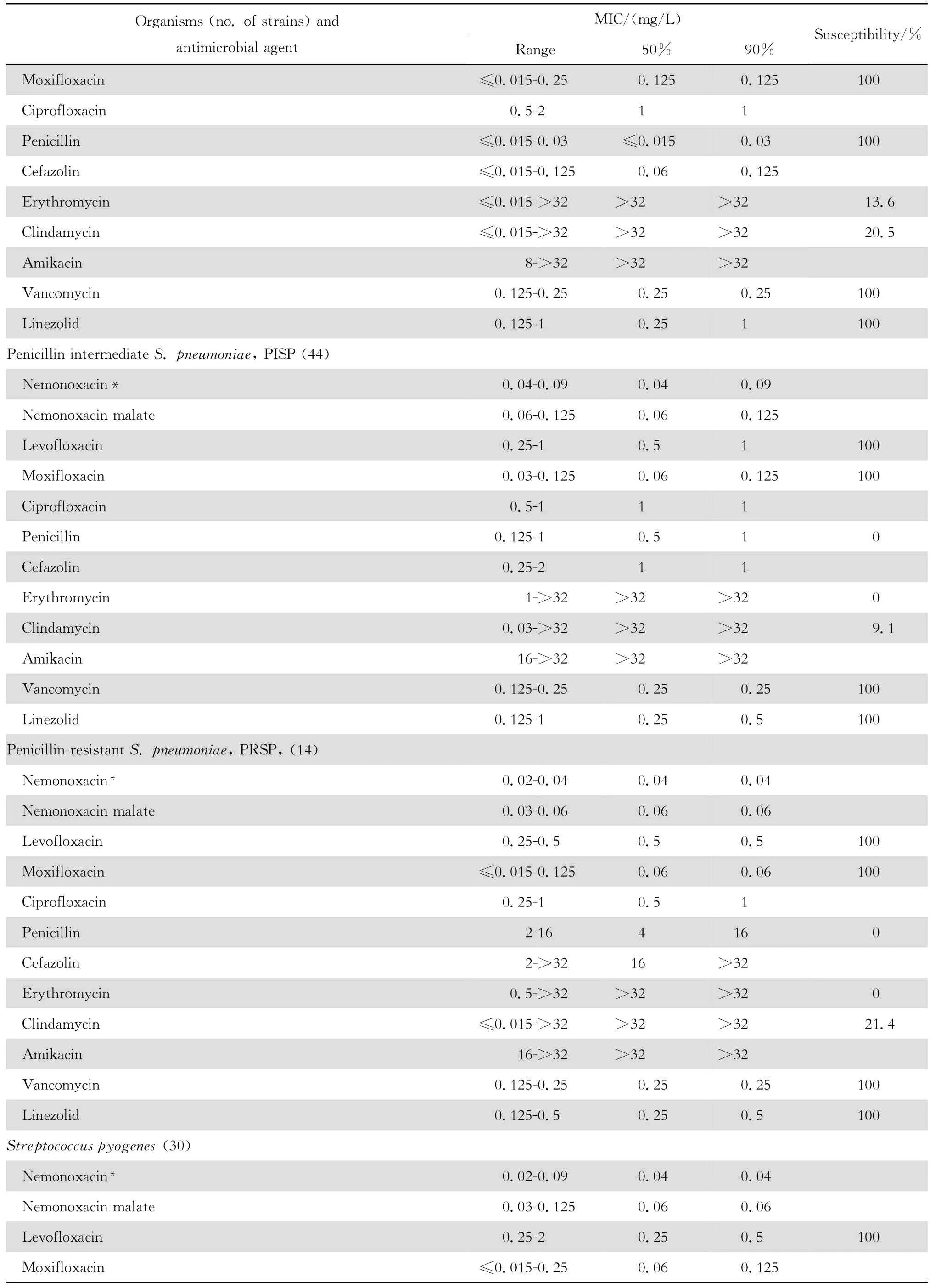
continued table1
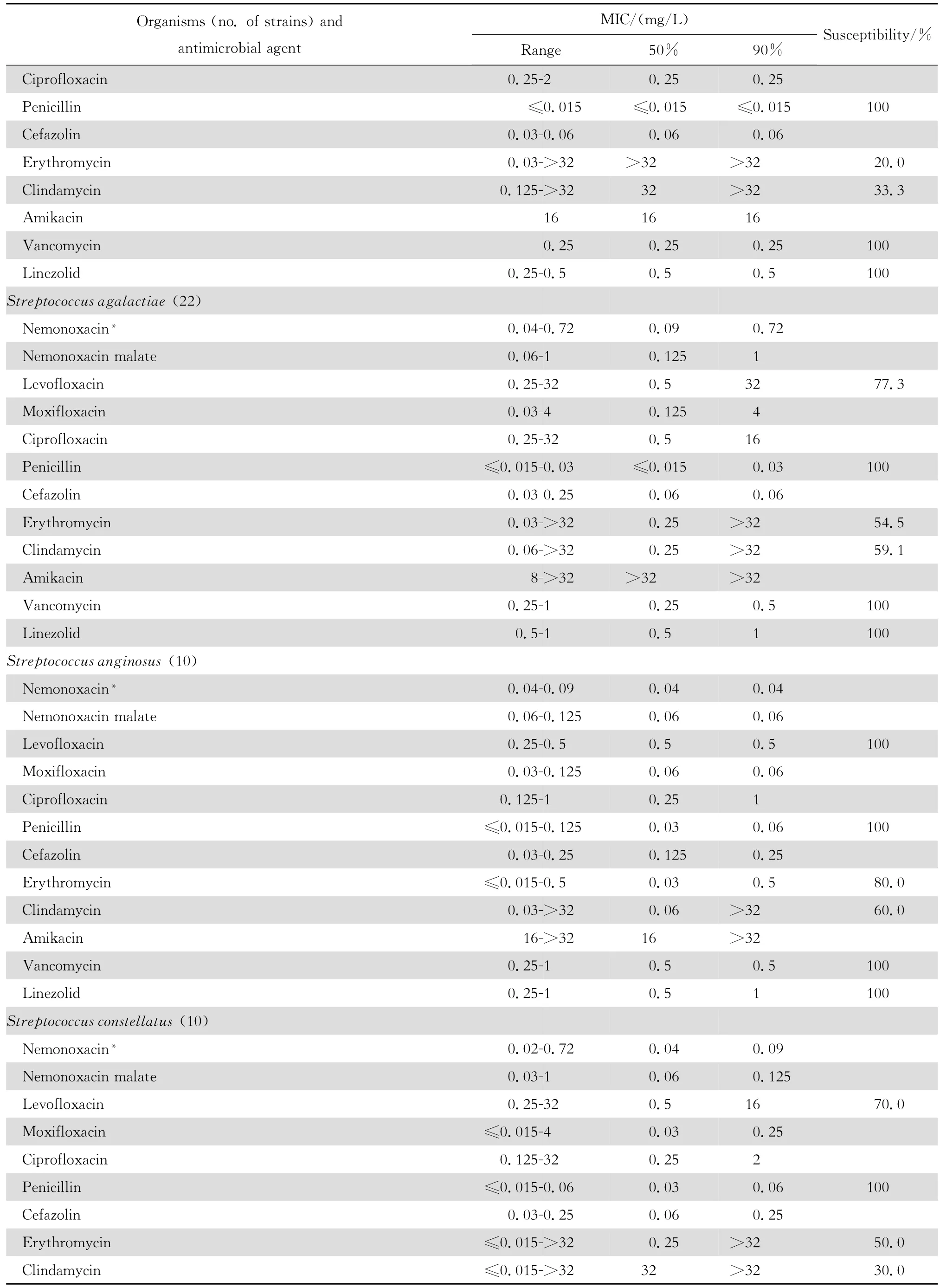
continued table1
continued table1
2.1.2 革兰阴性菌 奈诺沙星对多数肠杆菌科细菌具有良好抗菌作用(表2)。0.09~1.44 mg/L浓度的本品可抑制60%~100%多数肠杆菌科细菌生长,但本品对环丙沙星耐药大肠埃希菌的作用差。奈诺沙星对多数不发酵糖革兰阴性杆菌包括铜绿假单胞菌和鲍曼不动杆菌等的抗菌活性差(表3),其抗菌活性略低于环丙沙星和左氧氟沙星;0.72~ 1.44 mg/L浓度的本品可抑制60%~90%嗜麦芽窄食单胞菌和洛菲不动杆菌的生长。此外,奈诺沙星对流感嗜血杆菌和卡他莫拉菌具有高度抗菌活性,0.18 mg/L浓度的本品可抑制90%~98%细菌生长;但对淋病奈瑟菌的抗菌作用差(表4),与受试的其他喹诺酮类相仿。
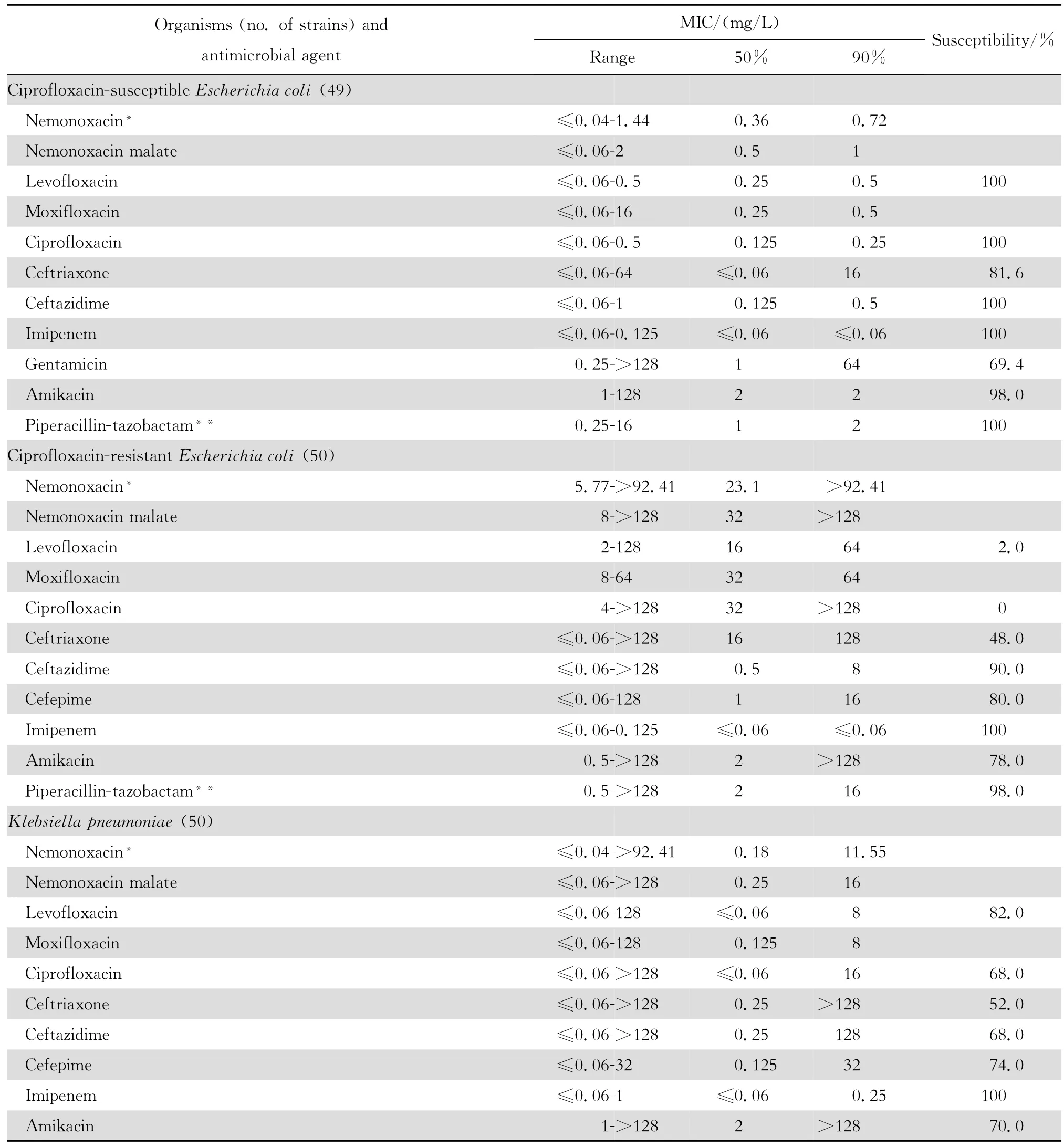
表2 奈诺沙星等抗菌药物对肠杆菌科细菌414株的体外药敏试验结果Table2 In vitro activities of nemonoxacin and comparative antimicrobial agents against Enterobacteriaceae strains(n=414)
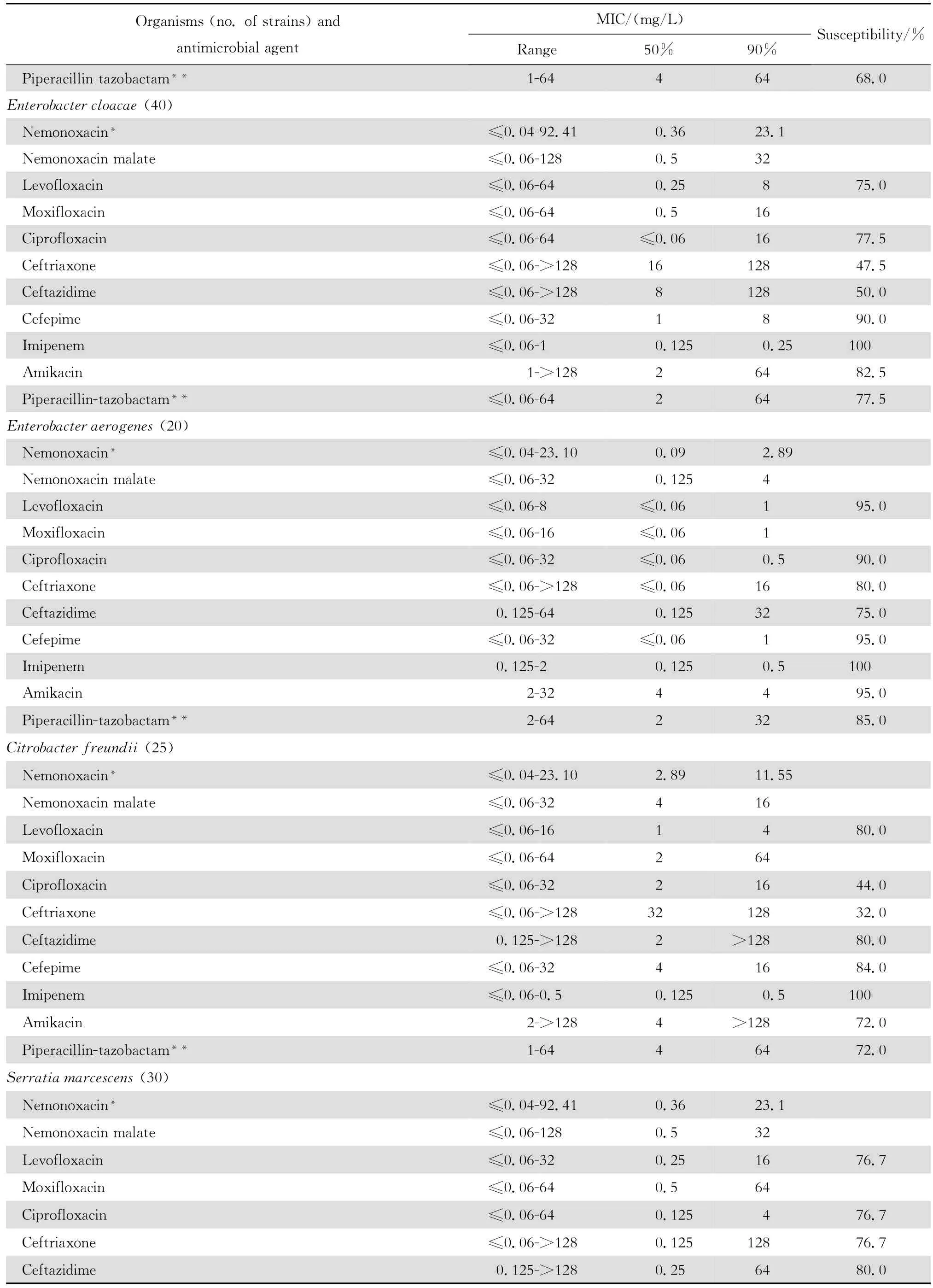
continued table2
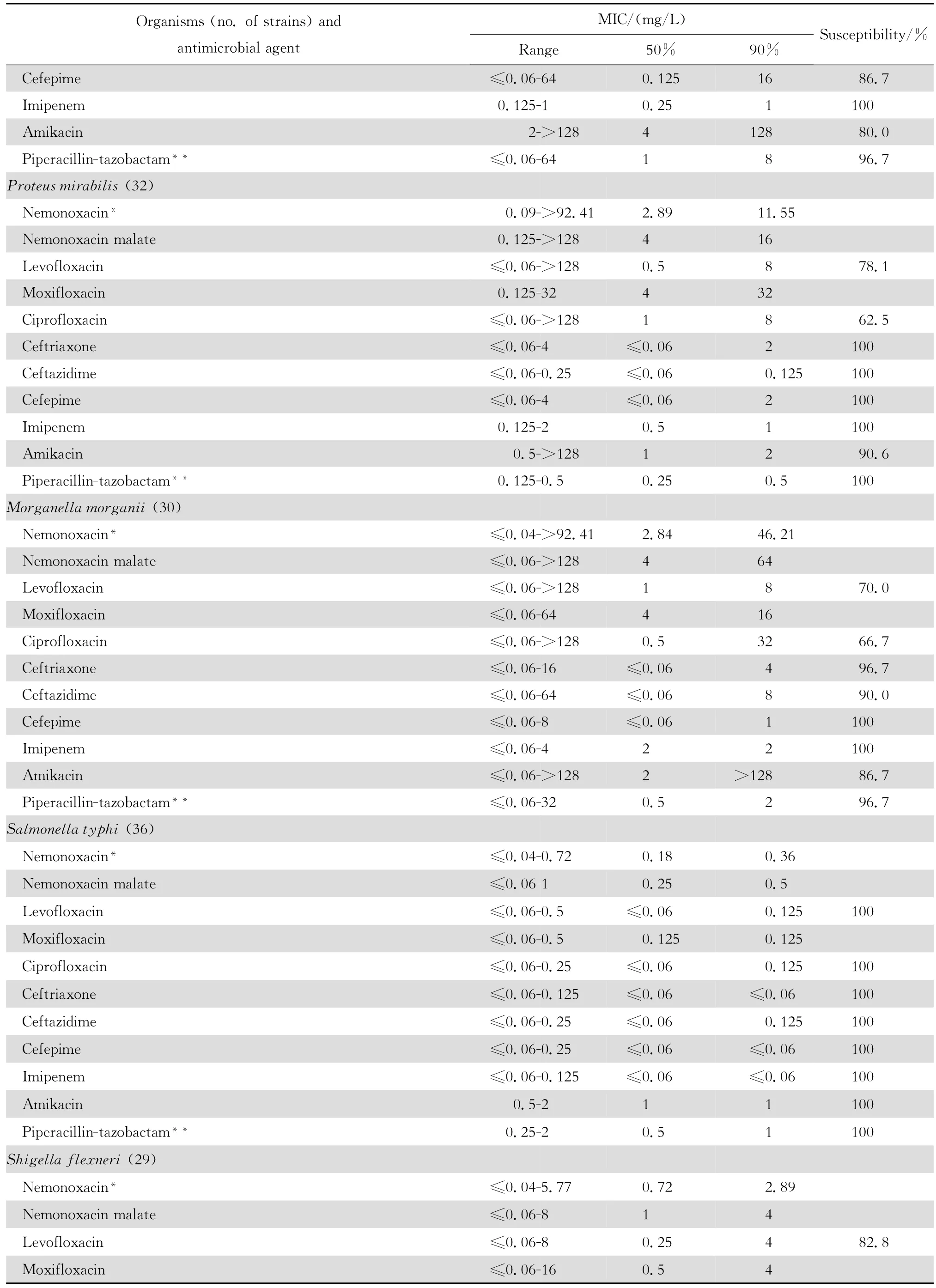
continued table2
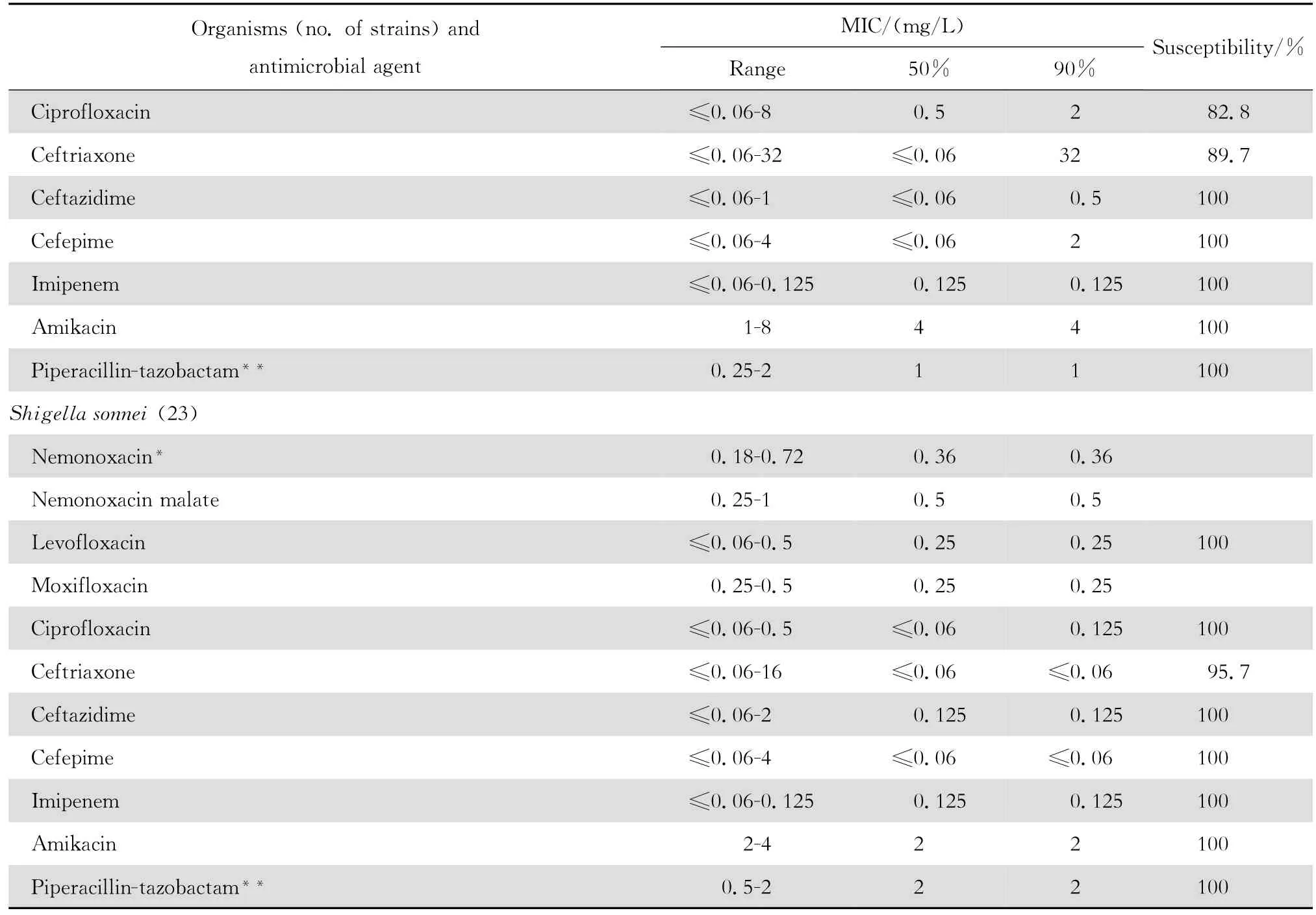
continued table2
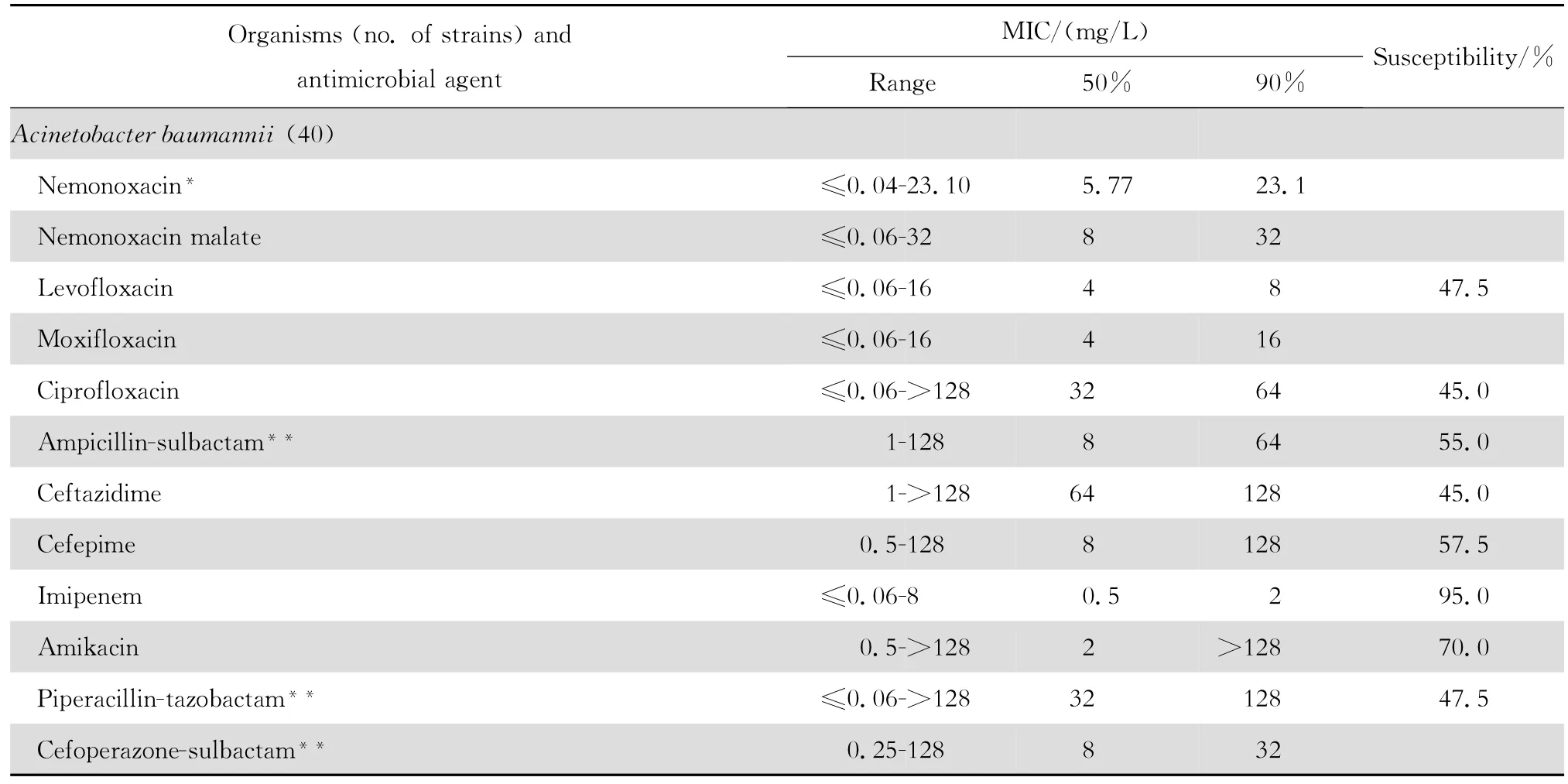
表3 奈诺沙星等抗菌药物对不发酵糖革兰阴性杆菌150株的体外药敏试验结果Table3 In vitro activities of nemonoxacin and comparative antimicrobial agents against non-fermenting gram-negative bacteria(n=150)
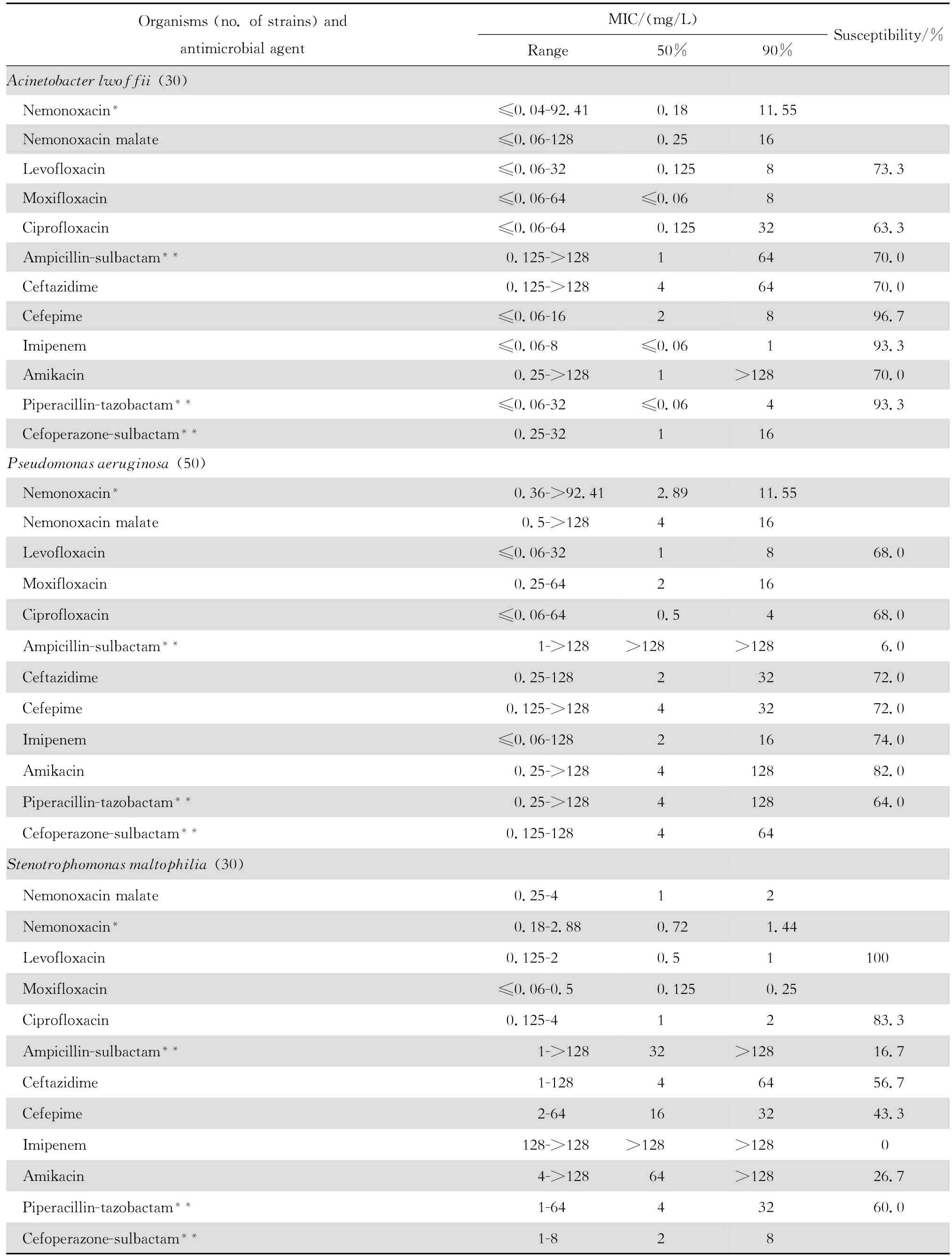
continued table3
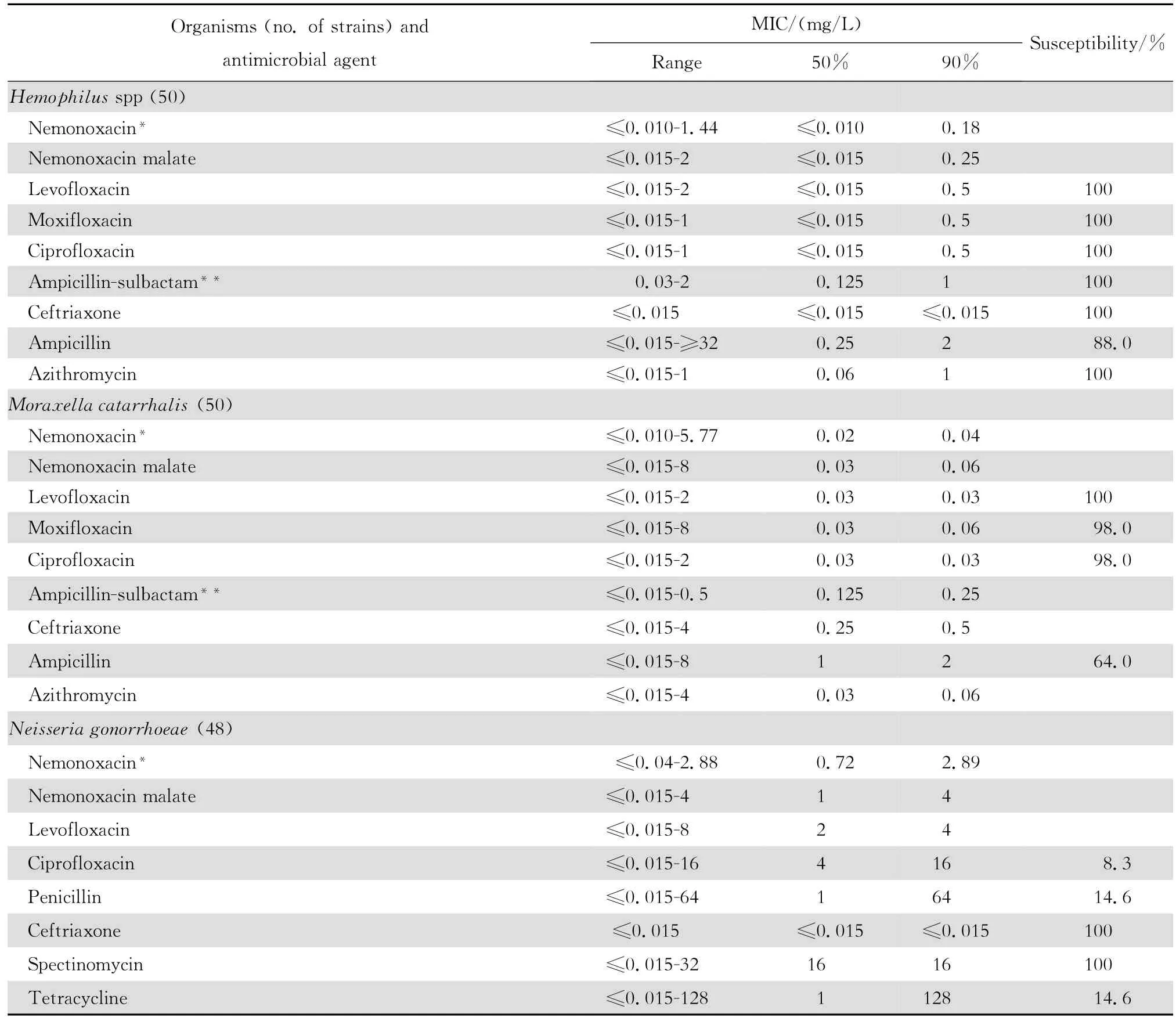
表4 奈诺沙星等抗菌药物对其他革兰阴性菌148株的体外药敏试验结果Table4 In vitro activities of nemonoxacin and comparative antimicrobial agents against other aerobic gram-negative microorganisms(n=148)
2.1.3 厌氧菌和非典型病原体 奈诺沙星对消化链球菌和痤疮丙酸杆菌有良好的抗菌活性,但对脆弱拟杆菌等拟杆菌属细菌作用差(表5)。奈诺沙星对肺炎支原体的抗微生物作用强,与受试的其他喹诺酮类相仿,但对解脲脲原体的抗微生物作用较差(表6)。
2.2 奈诺沙星的杀菌作用
奈诺沙星对194株临床分离菌的MBC显示其对MRSA,MSSA,青霉素敏感、中介和耐药肺炎链球菌(PSSP、PISP和PRSP)和粪肠球菌等多数革兰阳性菌、大肠埃希菌和肺炎克雷伯菌等肠杆菌科细菌具有较强的杀菌作用,其MBC90大多为MIC90的1~2倍。
杀菌试验结果显示本品对MSSA和MRSA有相同的杀菌作用,2 MIC、4 MIC和8 MIC浓度的奈诺沙星可分别在8 h、2~4 h和2 h达到99.9%或以上的杀菌率,优于对照药万古霉素。奈诺沙星对肺炎链球菌,在2 MIC、4 MIC和8 MIC的浓度分别可在2~4 h内达99.9%杀菌率,与万古霉素相仿。奈诺沙星对革兰阴性杆菌大肠埃希菌标准株和临床分离株的杀菌作用与环丙沙星基本相仿或略强,大多在2~4 h内达到99.9%杀菌率。
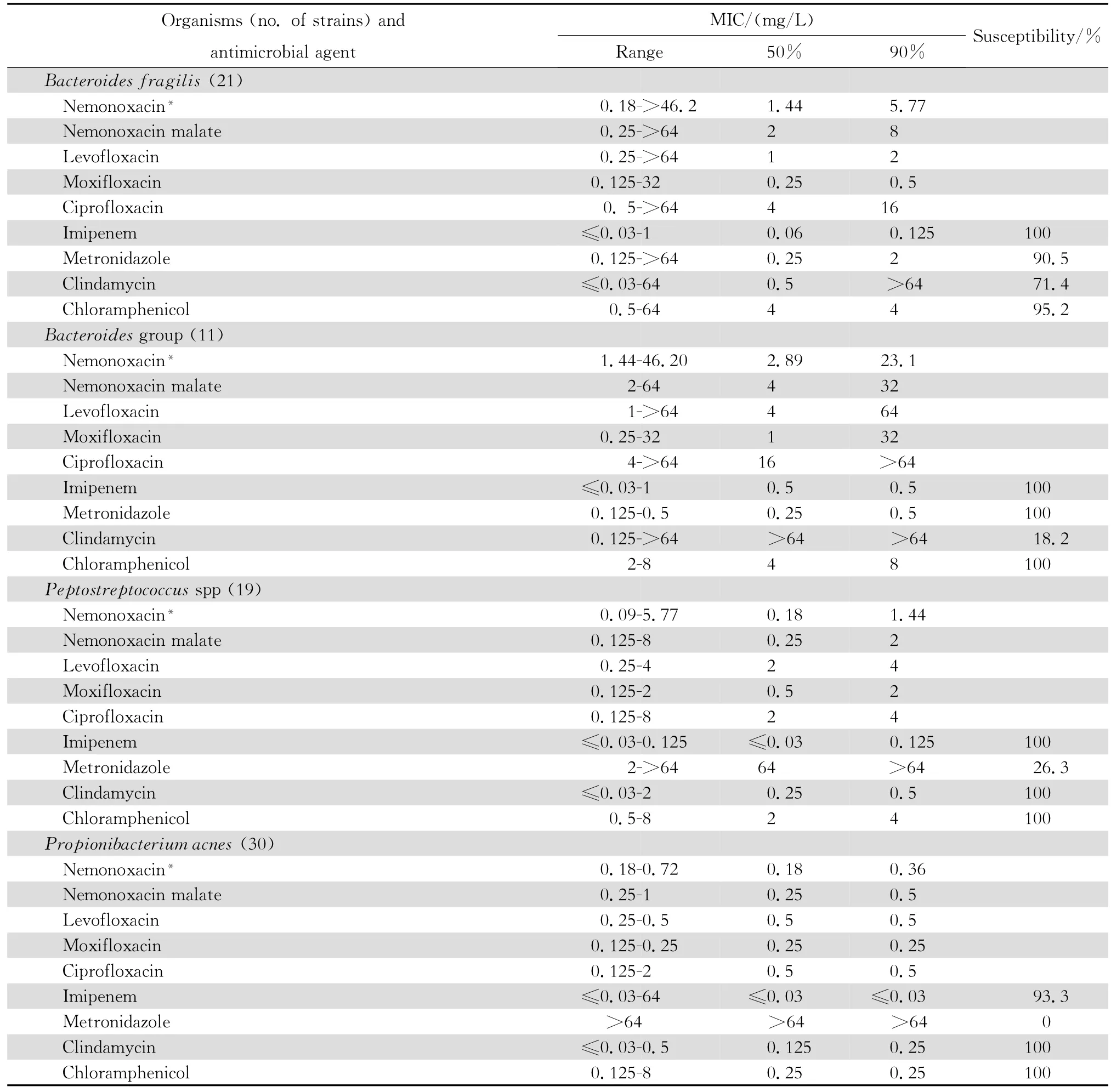
表5 奈诺沙星等抗菌药物对厌氧菌81株的体外药敏试验Table5 In vitro activities of nemonoxacin and comparative antimicrobial agents against anaerobes(n=81)
2.3 培养条件对奈诺沙星抗菌活性的影响
培养基的p H值为5或9时,奈诺沙星对60株临床分离株的MIC50和MIC90值为p H 7.0时的1/2 ~1倍,显示培养基p H的改变对奈诺沙星的抗菌作用影响不明显。
接种菌量为103CFU/mL时奈诺沙星的MIC50和MIC90值与接种量105CFU/mL时大致相仿;接种菌量为107CFU/mL时,其MIC50和MIC90均分别为105CFU/m L接种菌量时的1~2倍和1~4倍,显示细菌接种菌量的改变对奈诺沙星抗菌作用的影响不明显。
在含有25%、50%和75%血清的CAMHB培养基中测定奈诺沙星对临床分离菌60株的 MIC值。结果显示在含25%血清的CAMHB培养基中奈诺沙星对60株临床分离株的MIC90值多数为不含血清CAMHB培养基者的1~4倍,随着血清浓度的增加,含50%和75%血清的CAMHB培养基其MIC90值可分别增加为不含血清CAMHB培养基的2~8倍和>8倍,但对肺炎链球菌影响不明显。
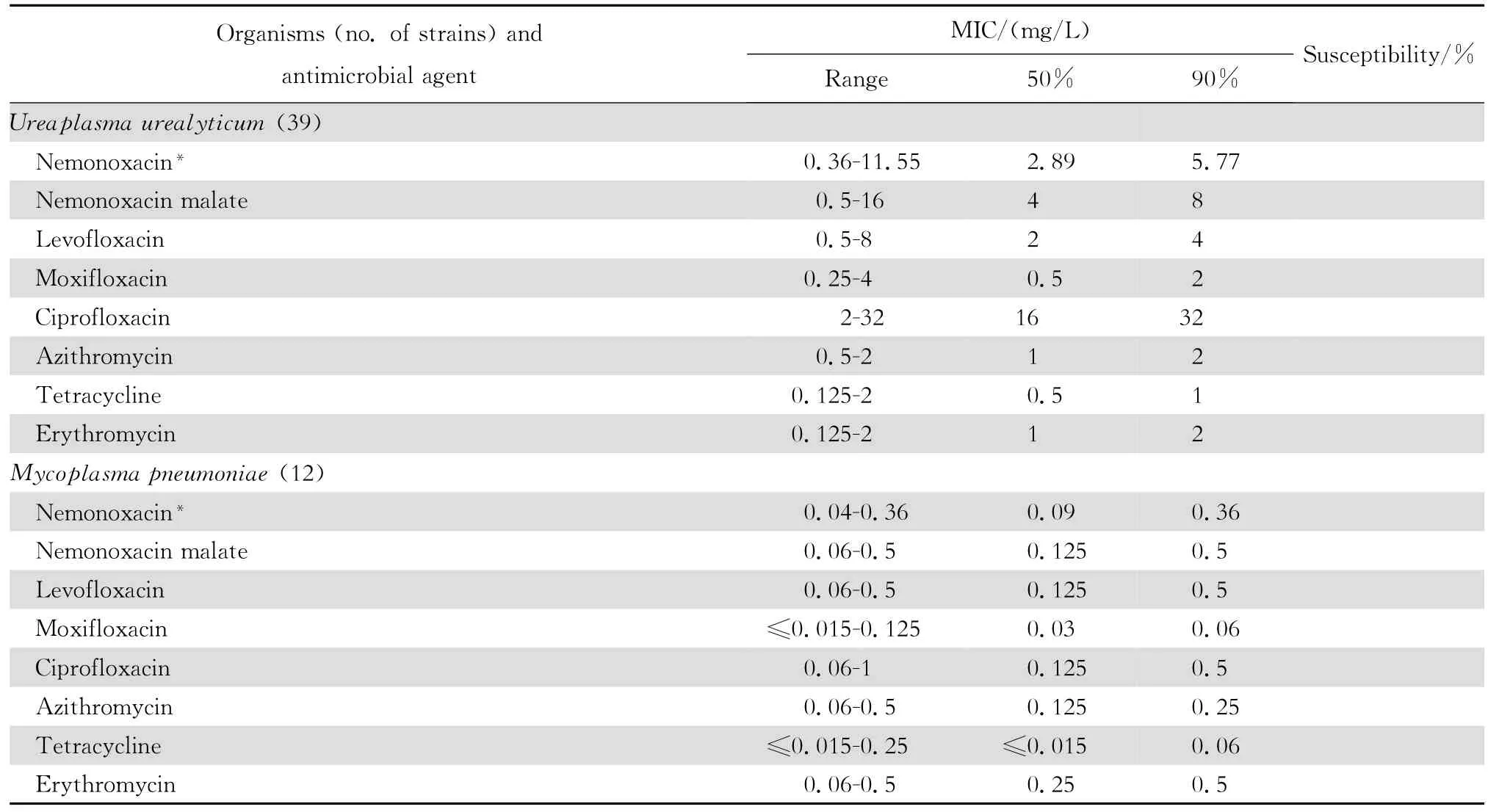
表6 奈诺沙星等抗菌药物对非典型病原体51株的体外药敏试验结果Table6 In vitro activities of nemonoxacin and comparative antimicrobial agents against Mycoplasma strains(n=51)
3 讨论
喹诺酮类抗菌药物因其抗菌谱广、抗菌作用强、与β内酰胺类、氨基糖苷类等抗生素无交叉耐药性等特性而被广泛、成功地用于治疗各种感染性疾病。但由于此类抗菌药物在临床的广泛应用,导致细菌耐药性亦随之日益增加[13-18]。奈诺沙星是一种无氟的C-8-甲氧基喹诺酮类新药,具有双重抑制细菌DNA旋转酶和拓扑异构酶Ⅳ的功能,而对革兰阳性菌和革兰阴性菌的广谱抗菌作用,并可降低耐药突变株的产生[1,19-21]。
本组资料显示奈诺沙星对革兰阳性球菌特别是PRSP、MRSA和MRCNS具有高度抗菌活性,对革兰阴性杆菌亦具良好的抗菌作用。与同类药相比,对革兰阳性球菌的抗菌活性显著较高。例如左氧氟沙星和环丙沙星对 MRSA几无抗菌活性;而奈诺沙星对MRSA的MIC50和MIC90值分别为0.36 mg/L 和0.72 mg/L,与万古霉素和利奈唑胺相仿,优于β内酰胺类、红霉素和克林霉素。奈诺沙星对MRSA的杀菌活性优于莫西沙星和环丙沙星,并随着奈诺沙星浓度的增加,其杀菌速率亦增加,提示本品为浓度依赖性杀菌剂。奈诺沙星对PRSP、PISP以及PSSP的MBC50和MBC90均分别与对上述细菌的MIC50和 MIC90值相等,显示其对多重耐药肺炎链球菌具有高度杀菌作用,与莫西沙星相仿,略优于环丙沙星和左氧氟沙星。
本研究结果与诸多文献报道相仿[2-4、22]。Chen等[4]报道奈诺沙星对喹诺酮类耐药MRSA和万古霉素不敏感MRSA均具高度抗菌作用。本资料虽未发现万古霉素不敏感的MRSA,但48株MRSA均为环丙沙星和左氧氟沙星耐药株,50株MRCNS中30株为环丙沙星和左氧氟沙星耐药株。奈诺沙星对上述耐药菌株的MIC50和MIC90分别为0.36 mg/L和0.72 mg/L以及0.18 mg/L和0.72 mg/L,显示奈诺沙星对甲氧西林耐药菌株具有高度抗菌活性,与其他喹诺酮类药物无交叉耐药。然而,奈诺沙星对50株环丙沙星耐药的大肠埃希菌的药敏试验结果显示该药的MIC90值>92.41 mg/L,其他3种受试的喹诺酮类的MIC90值为64~128 mg/L,呈明显的交叉耐药。
本资料显示奈诺沙星对革兰阳性消化链球菌和痤疮丙酸杆菌亦有良好的抗菌作用,其抗菌活性与莫西沙星相同,优于左氧氟沙星和环丙沙星;对肺炎支原体的抗微生物作用强,与左氧氟沙星和环丙沙星相同,显示奈诺沙星具有广谱的抗微生物作用。
以上结果显示奈诺沙星抗菌谱广、抗菌活性强;对革兰阳性菌的抗菌活性明显优于左氧氟沙星、环丙沙星和莫西沙星等同类药;对革兰阴性菌与其他受试喹诺酮类大致相仿,或略差于左氧氟沙星和环丙沙星。提示奈诺沙星可望用于治疗上述敏感细菌所致感染,特别是多重耐药肺炎链球菌、甲氧西林耐药葡萄球菌和肺炎支原体等感染。
[1] Arjona A,Castaner R,Bolos J.Nemonoxacin,Drugs of the future,2009,34(3);196-203.
[2] Adam HJ,Laing NM,King CR,et al.In vitroactivity of nemonoxacin,a novel nonfluorinated quinolone,against 2440 clinical isolates[J].Antimicrob Agents Chemother,2009,53 (11);4915-4920.
[3] Lauderdale TL,Shiau YR,Lai JF,et al.Comparativein vitroactivities of nemonoxacin(TG-873870),a novel nonfluorinated quinolone,and other quinolone against clinical isolates[J].Antimicrob Agents Chemother,2010,54(3);1338-1342.
[4] Chen YH,Liu CY,Lu JJ,et al.In vitroactivity of nemonoxacin (TG-873870),a novel non-fluorinated quinolone,against clinical isolates ofStaphylococcus aureus,enterococci andStreptococcus pneumoniawith various resistance phnotypes in Taiwan[J].J Antimicrob Chemother,2009,64(6);1226-1229.
[5] Guo B,Wu X,Zhang Y,et al.Safety and clinical pharmacokinetics of nemonoxacin,a novel non-fluorinated quinolone,in healthy Chinese volunteers following single and multiple oral doses[J].Clin Drug Investig,2012,32(7);475-486.
[6] Lin L,Chang LW,Tsai CY,et al.Dose escalation study of the safety,tolerability,and pharmacokinetics of nemonoxacin(TG-873870),a novel potent broad-spectrum nonfluorinated quinolone,in healthy volunteers[J].Antimicrob Agents Chemother,2010,54(1);405-410.
[7] Guo B,Zhang J,Yu J,et al.A liquid chromatographytanden mass spectrometry assay for the determination of nemonoxacin(TG-873870),a novel nonfluorinated quinolone,in human plasma and urine and its application to a single-dose pharmacokinetic study in healthy Chinese volunteers[J].Biomed Chromatogr,2012,26(11);1333-1340.
[8] Clinical and Laboratory Standards Institute.Performance standars for antimicrobial susceptibility testing;sixteenth informational supplement[S].2006,M100-S16,M7-A7-MIC testing.
[9] Lorian V.Antibiotics in Laboratory Medicine[M].Lippincott Williams&Wilkins,5th ed.2005;270-273.
[10] Lorian V.Antibiotics in Laboratory Medicine[M].Lippincott Williams&Wilkins,5th ed.2005;127-135.
[11] Clinical and Laboratory Standards Institute.Methodsfor antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidiouw bacteria,Approved guideline[S].2006,M45-A.
[12] National Committee for Clinical laboratory Standards.Methodsfor antimicrobial susceptibilitu testing of anaerobic bacteria,Approved standad-fifth edition[S].2001,M11-A5.
[13] Arias CA,Murray BE.Antibiotic-resistant bugs in the 21st century—a clinical super-challenge[J].N Engl J Med,2009,360;439-443.
[14] Andriole VT.The quinolones;past,present,and future[J].Clin Infect Dis,2005,41(Suppl 2);s113-s119.
[15] Low DE.Quinolone resistance among pneumococci;therapeutic and diagnostic implications[J].Clin Infect Dis,2004,38 (Suppl 4);s357-s362.
[16] 汪复,朱德妹,胡付品,等.2007年中国CHINET细菌耐药性监测[J].中国感染与化疗杂志,2008,8(5);325-333.
[17] Kim MJ,Yun HJ,Kong JW,et al.In vitrodevelopment to resistance to a novel fluoroquinolone,DW286,in methicilinresistanctStaphylococcus aureusclinical isolates[J].J Antimicrob Chemother,2003,51(4);1011-1016.
[18] Zhanel GG,DeCorby M,Laing N,et al.Antimicrobialresistant pathogens in intensive care unitis in Canada;results of the Canadian National Intensive Care Unit(CAN-ICU)study,2005-2006[J].Antimicrob Agents Chemother,2008,52(4);1430-1437.
[19] Abbanat D,Morrow B,Bush K.New agents in development for the treatment of bacterial Infections[J].Curr Opin Pharmacol,2008,8;582-592.
[20] Theuretzbacher U.Future antibiotics scenarios;is the tide starting to turn?[J].Int J Antimicrob Agents,2009,34 (1);15-20.
[21] Peterson LR.Quinolone molecular structure-activity relationships;what we have learned about improving antimicrobial activity [J].Clin Infect Dis,2001,33(Supple 3);s180-s186.
[22] Adam HJ,Laing NM,King CR,et al.In Vitroactivity of nemonoxacin,a novel nonfluorinated quinolone,against 2,440 clinical isolates[J].Antimicrob Agents Chemother,2009,53(11);4915-4920.
In vitro activities of nemonoxacin against clinical isolates from Shanghai,China
ZHU Demei,WU Peicheng,HU Fupin,WU Shi,YE Xinyu,ZHANG Yingyuan.(Institute of Antibiotics,Huashan Hospital,Fudan University,Key Laboratory of Clinical Pharmacology of Antibiotics,Ministry of Health,Shanghai 200040,China)
;ObjectiveTo evaluate thein vitroactivity of new fluoroquinolone nemonoxacin against clinical isolates.MethodsThe minimum inhibitory concentrations(MICs)of nemonoxacin were determined for 1 263 bacterial strains by means of agar dilution method and for 51Mycoplasmastrains by brouth microdilution.The test strains were recent clinical isolates collected from 16 hospitals in Shanghai,China.The MICs of nemonoxacin were compared with selected comparators.ResultsThe results showed that nemonoxacin had broad-spectrum antimicrobial activity.Nemonoxacin showed potent antibacterial activity against aerobic gram-positive cocci,including methicillin-resistantStaphylococcusand penicillin non-susceptibleStreptococcus pneumoniaewith the exception ofEnterococcus faecium.Its activity was slightly lower forEnterococcus faecalis.Thein vitroactivity of nemonoxacin was significantly better than that of levofloxacin,ciprofloxacin and moxifloxacin against gram positive cocci.Nemonoxacin also had good antibacterial activity against majority ofEnterobacteriaceaestrains,some glucose nonfermentative bacteria such asAcinetobacter lwoffiiandStenotrophomonas maltophilia.Nemonoxacin showed potent antibacterial activity againstHemophilusspp.andMoraxella catarrhalis.However,its activity was poor forNeisseria gonorrhoeae.Nemonoxacin was essentially comparable to or slightly poorer than the fluoroquinolones tested in the activity againstEnterobacteriaceaestrains,and most strains of non-fermentative bacteria excludingA.baumanniiandP.aeruginosa.Nemonoxacin also demonstrated good activity against anaerobic organisms suchasPeptostreptococcusandPropionibacterium acnes.Nemonoxacin had strong antimicrobial activity againstMycoplasma pneumoniae.Nemonoxacin had bactericidal activity against both the gram-positive cocci and gram-negative bacilli tested.In the test of culture conditions,p H of culture media and inoculum size did not show significant effect on thein vitroantibacterial activity of nemonoxacin.However,higher level of serum protein in the culture media reduced the antibacterial activity of nemonoxacin.ConclusionsNemonoxacin is a novel non-fluorinated quinolone with broad-spectrum antimicrobial activity against aerobic gram-positive cocci,gram-negative bacilli,some anaerobic bacteria andM.pneumoniae,especially its highly bacteriostatic and bactericidal activity against methicillin-resistantStaphylococcus aureusand multidrug-resistantS.pneumoniae,superior to all other quinolones tested.
; nemonoxacin; antimicrobial susceptibility testing; antibiotic; minimum inhibitory concentration
R978
A
1009-7708(2015)02-0097-16
2014-11-05
2014-11-14
国家科技部“重大新药创制”科技重大专项(2012ZX 09303004-001)。
复旦大学附属华山医院抗生素研究所,卫生部抗生素临床药理重点实验室,上海 200040。
朱德妹(1945—),女,教授,主要从事新抗菌药物药效学评价、细菌耐药性和临床重要病原菌基因诊断的研究。
张婴元,E-mail;yyzhang39®hotmail.com。
