氮硫双掺杂活性炭材料的制备和电容性能
2015-01-04李朝辉李仕蛟朱婷婷沈红龙禚淑萍山东理工大学化学工程学院山东淄博255049
李朝辉 李仕蛟 周 晋 朱婷婷 沈红龙 禚淑萍(山东理工大学化学工程学院,山东淄博255049)
氮硫双掺杂活性炭材料的制备和电容性能
李朝辉 李仕蛟 周 晋*朱婷婷 沈红龙 禚淑萍*
(山东理工大学化学工程学院,山东淄博255049)
以头发和蔗糖为原料,通过水热碳化和KOH活化两步法制备了氮硫双掺杂微孔炭材料.利用扫描电子显微镜,透射电子显微镜,氮气吸脱附,X射线光电子能谱,电子能谱和傅里叶交换红外光谱等手段系统表征了所制备活性炭材料的微观形貌,孔隙结构和表面化学性质.并在6 mol·L-1KOH溶液中研究了所制备活性炭材料的电容性能.氮气吸脱附测试表明,所制备活性炭材料的比表面积最高可达1849.4 m2·g-1,孔道以微孔为主.所制备活性炭材料氮元素含量为1.6%-2.5%(原子分数(x))),硫元素含量为0.2%-0.5%(x).由于N、O、S官能团的协同作用,所制备碳材料表现出明显的赝电容.活性炭材料的比电容值最高可达200 F·g-1,对应的能量密度为6.9 Wh·kg-1.功率密度达到10000 W·kg-1时,能量密度仍达到4.1 Wh·kg-1.本文的工作表明以生物质为原料可以方便制备氮硫双掺杂活性炭电极材料.
碳材料;超级电容器;人的头发;电化学性质;水热处理
©Editorial office ofActa Physico-Chimica Sinica
1 Introduction
Supercapacitors bridge the gap between batteries and dielectric capacitors on the Ragone plot describing the relation between energy and power.This electrochemical device has attracted more and more interest and attention due to its high power density,long cycle life,good reversibility,and broad energy storage application prospects.1According to the energy storage mechanism,supercapacitor can be defined into two kinds which are electrochemical double-layer capacitors(EDLCs)and pseudo-capacitors.In EDLCs,the electrical energy is achieved by electrostatic storage at the interface between the electrode surface and electrolyte solution.EDLCs require electrode materials with high effective surface area and pores adapted to the size of electrolyte ions, which is typical represented by nanostructured carbon materials. In pseudo-capacitors,the storage of electrical energy is achieved by the reversible Faradaic charge-transfer of electro-active species on the electrode.Electrode materials of pseudo-capacitors,mainly metal oxides and conductive polymers,have exhibit high specific capacitance.However,poor cycle stability,low conductivity,and high price have limited the practical application of these materials.2
Porous carbon materials have long been considered to be the most promising electrode materials for supercapacitors owing to their excellent physicochemical stability,good conductivity,and tailored nano-scale porosity.3Various carbon materials,including activated porous carbons,4carbide-derived carbons,5templated carbons,6hierarchical porous carbons,7graphenes,8carbon nanotubes9and so on,have been applied as electrode materials for EDLCs,and show high capacitive performance.Although many kinds of carbon materials with tailored porosity have been reported as electrode materials for EDLCs,activated carbons still dominate commercial supercapacitor applications due to their lowprice,easy preparation,and superior capacitive performance. Activated carbon electrodes not only exhibit predominantly electrochemical double-layer capacitance,but also exhibit pseudocapacitance.It has been widely proved that heteroatom-doped activated carbons exhibit excellent capacitive performance which is partly due to the fact that the heteroatom species in the carbon framework,such as N,O,S species usually induce additional pseudo-capacitance resulting in the improvement of the whole capacitive performance.10
Heteroatom-doping could be achieved via ammoxidationtreatment or using heteroatom-rich raw materials as carbon precursors.4a,11Post-ammoxidation of activated carbons11aor pyrolyzation under NH3atomosphere11binduces nitrogen-containing species,thus results in improvement of the capacitive performance of the activated carbons,but ammonia is a chemical agent with severe pungency and high toxicity.Heteroatom containing polymers,such as urea formaldehyde resins,4apolyaniline,11cpolypyrrole,11dand polythiophene,11ehave been used to prepared N or S-doped carbon electrode materials with high capacitive performance,while these heteroatom-containing polymers are always obtained from non-renewable fossil raw materials via complicated and time-consuming synthesis routes.Considering the broad scale of supercapacitor applications,the development of carbon materials from renewable biomass materials is of high application prospects.12Previous reports have proved that heteroatom-doped carbon materials could be easily prepared by selecting a specific biomass as carbon precursor.13Hair is a filamentous biomaterial that ceaselessly grows from follicles of dermis during the most of human lifetime.Human hair contains lots of N,S species in the form of keratin,a protein composed of cysteine and other amino acids.Therefore,N or S species may be induced into the target carbon product when using human hair as carbon precursor.In the present work,we prepare N,S co-doped activated carbon materials from sucrose and human hair via a combined method of hydrothermal carbonization and post-KOH activation.The capacitive performance of the prepared carbons is investigated in 6 mol·L-1KOH electrolyte.
2 Experimental
2.1 Material preparation
Hairs are collected from barbershop,and are thoroughly washed to remove the greases.All the chemical agents,including NaOH,KOH,KCl,polytetrafluoroethylene(PTFE)emulsion etc. are purchased from Sinopharm Chemical Reagent Co.,Ltd.10 g clean hairs were dissolved in 60 mL 2 mol·L-1NaOH solution. This solution was filtered to remove any insoluble matter.In a typical procedure,2 g sucrose and a certain volume of hair solution were mixed together,and subsequently were diluted to 20 mL using deionized water.The mixture was hydrothermally treated at 180°C overnight in a Teflon-lined stainless steel autoclave.The obtained dark-brown powders were further activated by double weight of KOH at 700°C with a heating ramp of 5°C· min-1for 3 h under N2flow.Finally,the resulting samples were washed with water until neutral pH was reached.In this way,three carbon samples were prepared by using different amount of hair solutions(2,4,8 mL),which were labeled as NSC-1,NSC-2, NSC-3,respectively.
2.2 Material characterization
A scanning electron microscope(SEM,Sirion 200 FEI, Netherlands)was used to observe the morphology of the carbon samples.The element composition and atom binding states were characterized by energy dispersive spectroscopy(EDS,INCA Energy spectrometer,Netherlands),X-ray photoelectron spectroscopy(XPS,Escalab 250,USA),and Fourier transform infrared spectroscopy(FTIR,Nicolet 5700,USA).The porosity of the prepared carbon materials was analyzed by an ASAP 2020 nitrogen sorption system(Micrometitics,USA).The carbon materials were degassed at 350°C overnight before sorption measurements.Brunauer-Emmett-Teller(BET)surface area(SBET)was calculated from the N2adsorption isotherm data within the relative pressure(p/p0)of 0.05 to 0.25.Total pore volume(VT)was obtained at a relative pressure of 0.995.Micropore volume(Vmicro) was calculated by t-plot method.Mesopore volume(Vmeso)was determined by subtracting the micropore volume from the total pore volume.Pore size distributions(PSDs)were obtained fromthe adsorption isotherms using the nonlocal density functional theory(NLDFT)model,assuming a slit-shape pore.
2.3 Electrochemical measurements
The working electrodes were prepared by mixing 95%(w) carbon materials and 5%(w)PTFE binder,pressing the mixture onto nickel foam at 15 MPa,and drying at 120°C for 10 h.All electrochemical measurements,including cyclic voltammetry (CV),galvanostatic charge/discharge test and electrochemical impedance spectroscopy(EIS),were carried on a CHI660D electrochemical testing station(Chenhua Instruments Co.Ltd., Shanghai,China).The galvanostatic charge/discharge test was performed in a two-electrode system to determine the specific capacitance of the prepared carbons at current densities ranging from0.2 to 20A·g-1.EIS test was performed with alternate current amplitude of 5 mV using a three-electrode system in 6 mol·L-1KOH electrolyte with a platinum plate electrode and a saturated calomel electrode(SCE)as the counter and reference electrodes, respectively.The potential range of CV and charge/discharge tests is-0.90-0.00 V,and the frequency range of EIS test is 10 mHz-100 kHz.
The specific capacitance is calculated by the following equation:

where Cm(F·g-1)is the gravimetric specific capacitance of the carbon samples,I(A)is the discharge current,t(s)is the discharge time,ΔV(V)is the potential window(0.9 V in this study), and m(g)is the mass of active material in two working electrodes.
Energy density(E)and powder density(P)could be calculated from the galvanostatic charge/discharge test using the equations of

where V is the discharge voltage(0.9 V in this case).
The Nyquist plot of NSC-2 is analyzed using the following equation:

where Z(ω)is complex impedance,Z'(ω)is real part of impedance,Z"(ω)is imaginary part of impedance,C'(ω)is real capacitance,C"(ω)is imaginary capacitance,ω is the angular frequency, f0is a frequency corresponding to peak value of C"(ω),and τ0is time relaxation constant.
3 Results and discussion
As shown in Fig.S1(Supporting Information),typically spherical shaped carbonaceous particles are obtained after hydrothermal treatment which is identical with the previous reports.14Fig.1 shows the microscopic morphologies of the prepared carbon materials imaged by SEM and TEM observation.The activated hydrothermal carbons are composed of smaller carbon fragments with large pores which are distinct with typically spherical shaped particles.That may be because the hydrothermal microspheres are oxidized and broken down during the severely chemical activation process.The prepared carbons have porous texture in the macroscopic scale,which makes ionic diffusion easy from bulk electrolyte into inner micropore surface(Fig.1(a-c)).No apparent nanopores are observed from high resolution SEM images indicating that the porosity of the prepared carbons mainly consisted of micropores(Fig.1(e-g)).High resolution TEM images clearly exhibit work-like micropores formed by stacking curved graphene micro-layers(Fig.1(d,h)).To determine the pore texture and specific surface area,we have performed N2sorption measurements(Fig.2),calculated PSDs by NLDFT model,and then summarized pore parameters in Table 1.It can be seen that all the carbons exhibit sorption isotherm of type I,rapidly achieve a high adsorption capacity at very low relative pressures(p/p0<0.05)and the sorption capacities of the prepared carbons retain almost unchanged with the increasing of relative pressures,indicating themicropore nature of these carbons.The PSD plots of the prepared carbons resemble each other,and the ultra-micropores(<1.5 nm) occupy a significant fraction in the total micropore volume.As shown in Table 1,the prepared carbons are highly microporous, and the specific surface area of the activated carbons is in the range of 1177.8 to 1849.4 m2·g-1.The similar porosity properties of the activated carbons demonstrate that the dosage of activated agents may be the main factor that determines the porosity of the carbon materials prepared in this work.

Fig.1 SEM images of(a,e)NSC-1,(b,f)NSC-2,(c,g)NSC-3,and TEM images of(d,h)NSC-2

Fig.2 (a)N2sorption isotherms and(b)pore size distributions of the prepared carbons
The heteroatom-containing surface functional groups,mainly N,S,O-containing groups,are considered important in charge storage ability given that the polar surface properties enable easy access of the electrolyte species which take part in either double layer formation or pseudo-Faradaic reactions.Hair is primarily composed of protein,notably keratin.Keratins have large amounts of cysteine.Therefore,N,S-containing groups could be induced into the prepared carbon materials when using human hair as carbon precursor.In another word,human hair acts as an N,S-supplier in this work,while the sucrose acts as the main carbon precursor.The surface chemical properties of the prepared carbons are analyzed by XPS and EDS measurements(Fig.3).It is seen that N,S-species are successfully induced into the prepared carbons according to the XPS spectra(Table 1,Fig.3(a)).The nitrogen contents are 2.5%,2.1%,and 1.6%(atomic fraction,x),and the sulfur contents are 0.3%,0.5%,and 0.2%(x)for NSC-1,NSC-2,and NSC-3,respectively.It should be noted that the oxygen content of the prepared carbons is very high,more than 5%(x), which is a common result for the KOH-activated carbons.The S-doping is also proved by EDS measurement.About 0.4%,0.7%, and 0.3%(x)of S-doping were determined by EDS for NSC-1, NSC-2,and NSC-3,respectively,which are very close to the values determined by XPS measurement.Element mapping demonstrates that the distributions of N and S species in the carbon are homogeneous(Fig.3(b)).The heteroatom-doping is further confirmed by FTIR spectroscopy(Fig.3(c)).The absorption bands at 1000-1150 and 1550 cm-1are assigned to C―O groups in ethers or phenols and the C―N groups in pyridinic N or pyrrolic N groups,respectively.Amoderate absorption peak at about 620 cm-1for NSC-2 reflects the existance of thiophenic groups,further indicating the success of sulfur doping.Together with the results of EDS,XPS,and FTIR,N,S co-doped activated carbons are successfully prepared by using human hair as precursor in this work.
In order to further investigate the atom binding states of the prepared carbons,the high resolution XPS spectra were performed (Fig.4).The peak assignments for high resolution XPS of the prepared carbons are summarized in Table S1(Supporting Information).C 1s scans show several peaks with varying contributions.In general,the peak at around 284.7 eV can be assigned to sp2hybridized carbon in an aromatic environment.The shoulder peak at 285.4 eV corresponds to carbon atoms single bonded to sulfur,nitrogen or oxygen in the form of thioether,thiophene, pyrrolidonic,phenol or ether.Weak peaks at 286.4 eV(assigned to carbonyl or amide groups)and 289.0 eV(assigned to ester or carboxylic groups)are also observed.In the region of O 1s XPS response,peaks at binding energies of 531.2,532.3,and 533.5 eV are attributed to quinone groups(O-I),phenol groups and/or C―O―C ether groups(O-II),and carboxyl groups(O-III),respectively(Fig.4(b)).15As shown in N 1s spectra,four types of nitrogen species including pyridinic N(N-6),pyrrolic N(N-5), quaternary N(N-Q),and pyridinic N-oxide group(N-X),could be determined by the peaks at 398.2,400.4,401.2,and 402.5-403.4 eV,respectively(Fig.4(c)).16In the case of sulfur(Fig.4(d)),the majority of sulfur species are defined as thiophenic species corresponding to the peaks of 163.8-164.7 eV,and a few oxidized sulfur species(sulfone or sulfoxide)are also determined by the peaks of 168.0-169.5 eV.11eBesides,a little disulfide species can also be found(peaks of 162.1-162.3 eV).
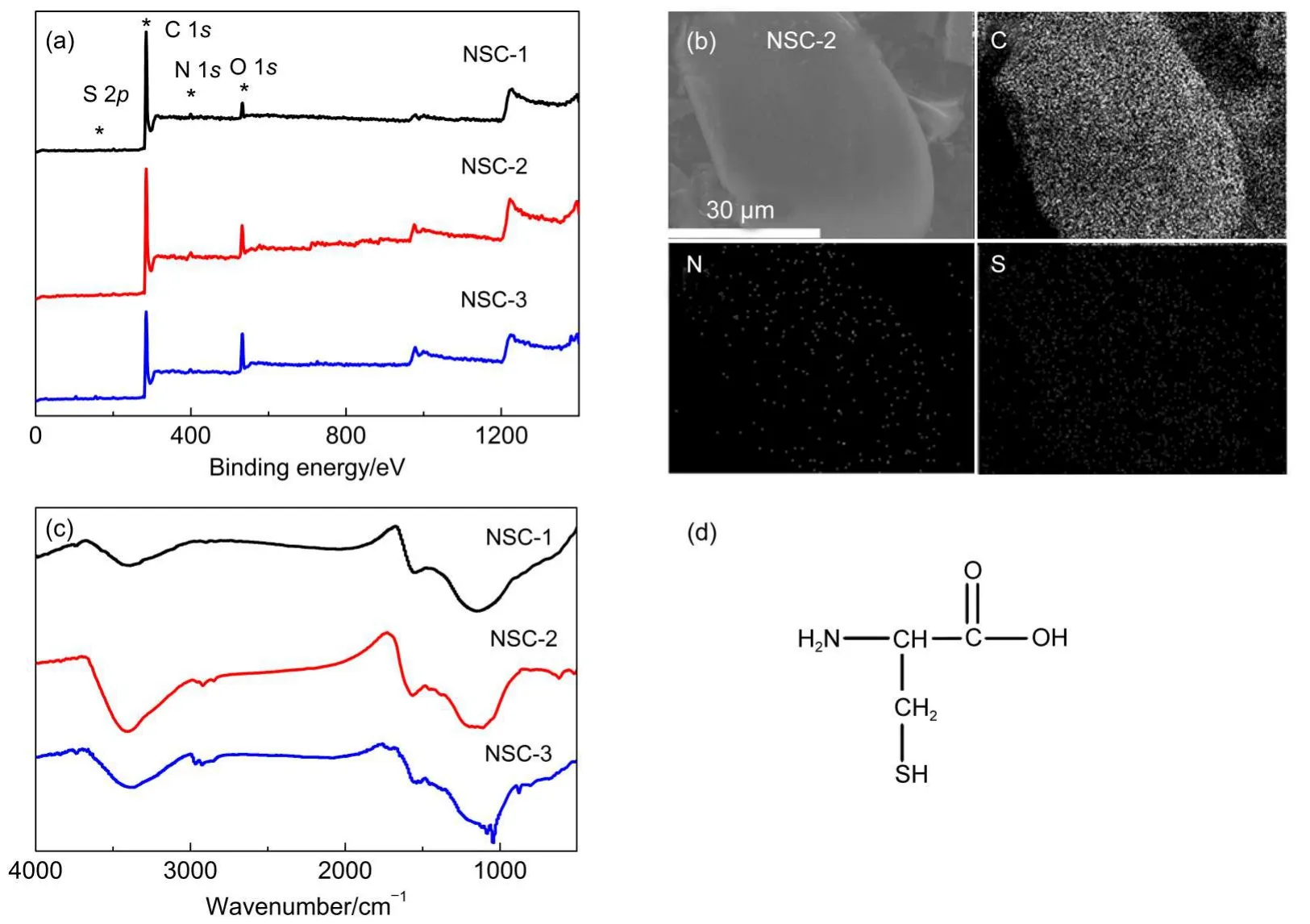
Fig.3 (a)XPS spectra of NSC-1,NSC-2,and NSC-3;(b)EDS mapping of NSC-2;(c)FTIR spectra of NSC-1, NSC-2,and NSC-3;(d)molecular structure of cysteine
The electrochemical behaviors of the prepared carbon materials as supercapacitor electrodes were firstly investigated by cyclic voltammetry using a three-electrode system in 6 mol·L-1KOH electrolyte with a platinum plate electrode and SCE as the counter and reference electrodes,respectively(Fig.5(a)).It is seen that the CV curves apparently deviate from the standard rectangle shape, and a visible increase of response current in the potential range of-0.4--0.9 V is observed for the CV curves,indicating that the prepared carbons possess a large pseudo-capacitance.No visible redox peaks are observed on the CV curves which may be due to the fusion of the complicated redox reactions induced by the various electrochemical active N-,O-,and S-species.17It has been widely accepted that the heteroatom-containing groups could improve the capacitive performance in aqueous electrolyte by introducing noticeable pseudo-capacitance via reversible redox reaction.The contribution of O-containing groups to pseudocapacitance should be prior considered because all the prepared carbons possess high amount of oxygen species(more than 10% (x)).Aredox mechanism for a carbonyl or quinone-type group has been proposed18

where―CxOK represents a phenol-or hydroquinone-type groups. This reaction should make large contribution to the pseudo-capacitance of the prepared carbons.As very electrochemical active sites,the nitrogen-containing groups can induce extra pseudocapacitance due to Faradaic redox reactions of N-6 and N-5 groups.10a,19Recently,it is reported that the electron-rich sulfur dopants deliver more polarized surface as well as reversible pseudo-sites and thus result in superior performance(equations (8),(9)).17b

Although the S-doping may play a positive role in the whole capacitance,it should be noted that the S-doping of the prepared carbons is low,and the electroactive oxidized sulfur species is less (equations(8),(9)and Table S1(Supporting Information)).Thus the pseudo-capacitance is mainly attributed to the nitrogen and oxygen-containing groups.Furthermore,the hydrophilic nitrogen, oxygen or sulfur species promote the wettability of carbon pore surface to the aqueous electrolyte,and the conductivity of the carbons could be improved by doping nitrogen into the carbon skeleton.14bThese facts may be also positive for the capacitive performance.
CV curves for NSC-2,given by a two-electrode test,with sweep rates ranging from 5 to 200 mV·s-1are shown in Fig.5(b). CV curve at a high sweep rate of 200 mV·s-1is still close to rectangle-like shape,indicating an excellent power capability of this carbon.The gravimetric specific capacitances of the prepared carbons at current densities ranging from 0.2 to 20 A·g-1are determined by galvanostatic charge/discharge test(Fig.5(c,d)).At a low current density of 0.2A·g-1,the specific capacitances of the prepared carbons are 185,200,and 190 F·g-1for NSC-1,NSC-2, and NSC-3,respectively.The specific capacitance of the prepared carbons decreases with the current density increases,because the ions of electrolyte have not enough time to reach all the pore surface of the electrode materials at high current density.The NSC-2 carbon still remains a high specific capacitance of 117 F· g-1even when the current density increases by 100 times,up to 20A·g-1,indicating the good power capability of this carbon(Fig.5 (d)).Generally,the larger the specific surface area,the higher the capacitance is.Alarge amount of heteroatom doping could induce large pseudo-capacitance,thus enhances the whole capacitance of the carbon materials.Compared with NSC-1,NSC-2 possesses much larger specific surface area and more O-,S-species.That may be why NSC-2 shows higher capacitance than NSC-1 although the latter one possesses more N-species.Compared with NSC-3,NSC-2 possesses large amount of N-,S-species which will induce large pseudo-capacitance.Thus,we could explain that NSC-2 exhibits the highest capacitance among the prepared carbons.Additionally,the conductivity also plays an important role in the capacitive performance,which will be discussed next.

Fig.4 High resolution XPS spectra of NSC-1,NSC-2,NSC-3(a-d)and structure of N,O,S-containing groups(e)
We also prepared two carbon materials denoted as SC and HC which were prepared by using only hydrothermal treatment sucrose or human hair as precursor,respectively.The specific capacitances determined by galvanostatic charge/discharge test are shown in Fig.S2(Supporting Information).It could be seen that the HC shows lower capacitance and much poorer power capability than the NSC-2.Surprisingly,the SC shows slight larger capacitance and power capability than NSC-2.That may have two reasons:(1)SC carbon possesses very high specific surface area up to 1700 m2·g-1and abundant microporosity;(2)the N/S species on the pore surface of carbon may be obstacles for the diffusion of ions.Anyhow,we should face seriously with this fact, and will further investigate it in future.
Energy density and power density of all the investigated carbon materials are calculated by Equations(2),(3),and the Ragone plots are shown in Fig.6(a).It could be seen that all the Ragone plots gradually slope down as the increase of power density, which means that less energy can be released at higher power output.Among the three samples,NSC-2 delivers the highest energy density of 6.9 Wh·kg-1at a power density of 100 W·kg-1. At a high power density of 10000 W·kg-1,the energy density of NSC-2 still reaches up to 4.1 Wh·kg-1.The long-term cyclic stability of the prepared carbons was measured by using galvanostatic charge/discharge test up to 10000 cycles at 1A·g-1(Fig.6 (b)).It could be seen that the prepared carbons show excellent characteristic of recycling charge/discharge performance with a high capacitance retention ratio up to 98%based on the dischargecapacitance of the initial cycle.

Fig.5 Electrochemical behavior of NSC-1,NSC-2,and NSC-3 in 6 mol·L-1KOH electrolyte

Fig.6 (a)Ragone plots and(b)cycle tests of NSC-1,NSC-2,and NSC-3 at 1A·g-1
Nyquist plots,simulated plot from ZView software,and the equivalent circuit are illustrated in Fig.7.Apparently,the Nyquist plots could be divided into several distinct parts,an uncompleted semicircle part at high frequency,an inclined portion of the curve (about 45°)at middle frequency,and a linear part at low frequency.At very high frequencies,the imaginary part(Zʺ)of the impedance is near to zero and the real part of resistance(Z') measured is the ohmic resistance derived from the electrolyte and the contact between the electrode and the current collector(Rs). The low values of Rs(about 0.4 Ω)indicate the good conductivity of the prepared carbons.It could be also observed that the ohmic resistances of the carbons are very close to each other indicating that the conductivity of the prepared carbon is close.Thus we can conclude that the conductivity may be not the major reason for the difference of capacitive performance.The uncompleted semicircle loop observed in the range of medium-high frequencies stands for charge transfer resistance(Rct)at the interface between the electrolyte and electrode.The Rctvalues of the prepared carbons are 2.2,1.7,and 2.5 Ω for NSC-1,NSC-2,and NSC-3,respectively. The values of Rctmay be related to the heteroatom-doping of the prepared carbons.According to XPS results,large amounts of hydrophilic oxygen and nitrogen species exist on the carbon surface,increase the polarity of the carbon surface and facilitate the contact between the carbon surface and the aqueous electrolyte.NSC-2 reaches an optimum value of Rct,which may be dueto the synergistic effect of multi N,O,S-doping.Although the NSC-3 carbon contains much more polar oxygen species than the other carbons,the Rctof NSC-3 is much larger than that of NSC-2.For NSC-3,larger O-doping will result in more strain and defect sites in the carbon materials,which may lead to the increasing of charge transfer resistance for this carbon.The 45°slope regions in the middle frequencies are ascribed to the Warburg impedance (W),responding to the frequency dependence of ion diffusion/ transport from electrolyte to the micropore surface of carbon electrode.The short length of this slope for NSC-2 indicates that the electrolyte ions diffuse fast in the pores of this carbon which may be due to the hetero-doped polar pore surface.The almost vertical line represents the dominance of ideal double-layer charge/ discharge(Cdl)at low frequencies.The existence of a limit pseudocapacitor(CL)demonstrates the redox reaction of heteroatomcontaining groups.The relation plots between normalized real capacitance(C'(ω))and imaginary capacitance(Cʺ(ω))with frequency are illustrated in Fig.7(d).The real capacitance values decrease with the increasing of test frequency since only exterior pore surface could be reached at higher frequencies.The time constant of the cells could be calculated according to the peak frequency of the normalizedCʺ(ω)plot.According to Equation (6),a low time constant is determined to be 0.53 s for NSC-2 further suggesting that the carbon is a promising electrode material for high power application.

Fig.7 (a)EIS spectra of NSC-1,NSC-2,and NSC-3;(b)simulation of the EIS spectra of NSC-2;(c)equivalent circuit; and(d)dependence of normalized real and imaginary capacitance on frequencies of NSC-2
4 Conclusions
In summary,N,S co-doped activated microporous carbons are successfully prepared from renewable human hair and sucrose via a combined method of hydrothermal carbonization and chemical activation.Visible pseudo-capacitances are given by the N,O,S-doped species.The carbons show high specific capacitance of up to 200 F·g-1in KOH electrolyte.The preparation method employed in this work is facile and efficient,and shows the significance of developing heteroatom-doped carbon functional materials from renewable biomass.
Supporting Information:available free of charge via the internet at http://www.whxb.pku.edu.cn.
(1) (a)Conway,B.;Birss,V.;Wojtowicz,J.J.Power Sources 1997, 66(1),1. (b)Simon,P.;Gogotsi,Y.Nature Materials 2008,7(11),845. (c)Candelaria,S.L.;Shao,Y.;Zhou,W.;Li,X.;Xiao,J.; Zhang,J.G.;Wang,Y.;Liu,J.;Li,J.;Cao,G.Nano Energy 2012,1(2),195. (d)Chen,S.;Xing,W.;Duan,J.;Hu,X.;Qiao,S.Z.J.Mater. Chem.A 2013,1(9),2941.
(2) (a)Yu,G.;Xie,X.;Pan,L.;Bao,Z.;Cui,Y.Nano Energy 2013, 2(2),213.doi:10.1016/j.nanoen.2012.10.006 (b)Chen,C.J.;Hu,Z.A.;Hu,Y.Y.;Li,L.;Yang,Y.Y.;An,N.; Li,Z.M.;Wu,H.Y.Acta Phys.-Chim.Sin.2014,30(12),2256. [陈婵娟,胡中爱,胡英瑛,李 丽,杨玉英,安 宁,李志敏,吴红英.物理化学学报,2014,30(12),2256.]doi:10.3866/PKU.WHXB201409302
(3) Zhang,L.L.;Zhao,X.Chem.Soc.Rev.2009,38(9),2520.doi: 10.1039/b813846j
(4) (a)Chen,X.Y.;Chen,C.;Zhang,Z.J.;Xie,D.H.;Deng,X.; Liu,J.W.J.Power Sources 2013,230,50.doi:10.1016/j. jpowsour.2012.12.054 (b)Kim,T.;Jung,G.;Yoo,S.;Suh,K.S.;Ruoff,R.S.ACS Nano 2013,7(8),6899. (c)Calvo,E.;Lufrano,F.;Staiti,P.;Brigandì,A.;Arenillas,A.; Menéndez,J.J.Power Sources 2013,241,776. (d)Chen,M.;Kang,X.;Wumaier,T.;Dou,J.;Gao,B.;Han,Y.; Xu,G.;Liu,Z.;Zhang,L.J.Solid State Electrochem.2013,17 (4),1005. (e)Ma,G.F.;Mu,J.J.;Zhang,Z.G.;Sun,K.J.;Peng,H.;Lei, Z.Q.Acta Phys.-Chim.Sin.2013,29(11),2385.[马国富,牟晶晶,张稚国,孙看军,彭 辉,雷自强.物理化学学报,2013, 29(11),2385.]doi:10.3866/PKU.WHXB201309051
(5) (a)Tsai,W.Y.;Gao,P.C.;Daffos,B.;Taberna,P.L.;Perez,C. R.;Gogotsi,Y.;Favier,F.Electrochem.Commun.2013,34,109. doi:10.1016/j.elecom.2013.05.031 (b)Pérez,C.R.;Yeon,S.H.;Ségalini,J.;Presser,V.;Taberna, P.L.;Simon,P.;Gogotsi,Y.Adv.Funct.Mater.2013,23(8), 1081.
(6) (a)Nishihara,H.;Kyotani,T.Adv.Mater.2012,24(33),4473. doi:10.1002/adma.v24.33 (b)Xing,W.;Qiao,S.;Ding,R.;Li,F.;Lu,G.;Yan,Z.;Cheng, H.Carbon 2006,44(2),216. (c)Wei,J.;Zhou,D.;Sun,Z.;Deng,Y.;Xia,Y.;Zhao,D.Adv. Funct.Mater.2013,23(18),2322.
(7)(a)Huang,W.;Zhang,H.;Huang,Y.;Wang,W.;Wei,S.Carbon 2011,49(3),838.doi:10.1016/j.carbon.2010.10.025 (b)Lv,Y.;Zhang,F.;Dou,Y.;Zhai,Y.;Wang,J.;Liu,H.;Xia, Y.;Tu,B.;Zhao,D.J.Mater.Chem.2012,22(1),93. (c)Wang,Q.;Yan,J.;Wang,Y.;Wei,T.;Zhang,M.;Jing,X.; Fan,Z.Carbon 2014,67,119.
(8) (a)Bai,Y.;Rakhi,R.;Chen,W.;Alshareef,H.J.Power Sources 2013,233,313.doi:10.1016/j.jpowsour.2013.01.122 (b)Xu,Y.;Lin,Z.;Huang,X.;Liu,Y.;Huang,Y.;Duan,X.ACS Nano 2013,7(5),4042. (c)Wang,J.D.;Peng,T.J.;Sun,H.J.;Hou,Y.D.Acta Phys.-Chim.Sin.2014,30(11),2077.[汪建德,彭同江,孙红娟,侯云丹.物理化学学报,2014,30(11),2077.]doi:10.3866/ PKU.WHXB201409152
(9) Niu,Z.;Dong,H.;Zhu,B.;Li,J.;Hng,H.H.;Zhou,W.;Chen, X.;Xie,S.Adv.Mater.2013,25(7),1058.doi:10.1002/adma. v25.7
(10) (a)Ra,E.;Raymundo-Piñero,E.;Lee,Y.;Béguin,F.Carbon 2009,47(13),2984;doi:10.1016/j.carbon.2009.06.051 (b)Seredych,M.;Bandosz,T.J.J.Mat.Chem.A 2013,1(38), 11717. (c)Okajima,K.;Ohta,K.;Sudoh,M.Electrochimica Acta 2005,50(11),2227.
(11) (a)Jurewicz,K.;Babeł,K.;Źiółkowski,A.;Wachowska,H. Electrochimica Acta 2003,48(11),1491.doi:10.1016/S0013-4686(03)00035-5 (b)Wang,X.;Liu,C.G.;Neff,D.;Fulvio,P.F.;Mayes,R.T.; Zhamu,A.;Fang,Q.;Chen,G.;Meyer,H.M.;Jang,B.Z. J.Mater.Chem.A 2013,1(27),7920. (c)Han,J.;Xu,G.;Ding,B.;Pan,J.;Dou,H.;MacFarlane,D. R.J.Mater.Chem.A 2014,2(15),5352. (d)Wei,L.;Sevilla,M.;Fuertes,A.B.;Mokaya,R.;Yushin,G. Adv.Funct.Mater.2012,22(4),827. (e)Gu,W.;Sevilla,M.;Magasinski,A.;Fuertes,A.B.;Yushin, G.Energy&Environmental Science 2013,6(8),2465.
(12) (a)Wei,L.;Gleb,Y.Nano Energy 2012,1(4),552.doi:10.1016/ j.nanoen.2012.05.002 (b)Rufford,T.E.;Hulicova-Jurcakova,D.;Zhu,Z.;Lu,G.Q. Electrochem.Commun.2008,10(10),1594.
(13) (a)Braghiroli,F.;Fierro,V.;Izquierdo,M.;Parmentier,J.;Pizzi, A.;Celzard,A.Carbon 2012,50(15),5411.doi:10.1016/j. carbon.2012.07.027 (b)Raymundo-Piñero,E.;Cadek,M.;Beguin,F.Adv.Funct. Mater.2009,19(7),1032.
(14) (a)Sevilla,M.;Fuertes,A.B.Carbon 2009,47(9),2281.doi: 10.1016/j.carbon.2009.04.026 (b)Zhao,L.;Baccile,N.;Gross,S.;Zhang,Y.;Wei,W.;Sun,Y.; Antonietti,M.;Titirici,M.M.Carbon 2010,48(13),3778.
(15) Hulicova-Jurcakova,D.;Seredych,M.;Lu,G.Q.;Bandosz,T.J. Adv.Funct.Mater.2009,19(3),438.doi:10.1002/adfm.v19:3
(16) Jansen,R.;Van Bekkum,H.Carbon 1995,33(8),1021.doi: 10.1016/0008-6223(95)00030-H
(17) (a)Raymundo-Piñero,E.;Leroux,F.;Béguin,F.Adv.Mater. 2006,18(14),1877. (b)Zhao,X.;Zhang,Q.;Chen,C.M.;Zhang,B.;Reiche,S.; Wang,A.;Zhang,T.;Schlögl,R.;Sheng,S.D.Nano Energy 2012,1(4),624.
(18) Sun,G.;Long,D.;Liu,X.;Qiao,W.;Zhan,L.;Liang,X.;Ling, L.Journal of Electroanalytical Chemistry 2011,659(2),161. doi:10.1016/j.jelechem.2011.05.017
(19) Li,W.;Chen,D.;Li,Z.;Shi,Y.;Wan,Y.;Huang,J.;Yang,J.; Zhao,D.;Jiang,Z.Electrochem.Commun.2007,9(4),569. doi:10.1016/j.elecom.2006.10.027
Preparation and Supercapacitive Performance of N,S Co-Doped Activated Carbon Materials
LI Zhao-Hui LI Shi-Jiao ZHOU Jin*ZHU Ting-Ting SHEN Hong-Long ZHUO Shu-Ping*
(School of Chemical Engineering,Shandong University of Technology,Zibo 255049,Shandong Province,P.R.China)
In this work,N,S co-doped microporous carbon materials were successfully prepared using human hair and sucrose as carbon precursors via a two-step method that combined hydrothermal treatment and post-KOH activation.The morphology,pore texture,and surface chemical properties of the activated carbon materials were investigated by scanning electron microscopy,transmission electron microscopy,N2adsorption/desorption, X-ray photoelectron spectroscopy,energy dispersive spectroscopy,and Fourier transform infrared spectroscopy. The electrochemical capacitive behavior of the prepared carbons was systematically studied in 6 mol·L-1KOH electrolyte.The maximum specific surface area of the prepared carbons was found to be 1849.4 m2·g-1with a porosity that mainly consisted of micropores.Nitrogen and sulfur contents varied from 1.6%to 2.5%and from 0.2%to 0.5%(atomic fraction(x)),respectively.The synergistic-positive effect of N,O,and S-containing groups caused the prepared carbons to exhibit a large pseudo-capacitance.High specific capacitances of up to 200 F·g-1at 0.2A·g-1were observed,response to an energy density of 6.9 Wh·kg-1.At a power density of 10000 W·kg-1,the energy density was found to be 4.1 Wh·kg-1.The present work highlights the significance of this new strategy to prepare N,S co-doped carbon materials from renewable biomass.
Carbon materials;Supercapacitor;Human hair;Electrochemical property; Hydrothermal treatment
O646
10.3866/PKU.WHXB201501281www.whxb.pku.edu.cn
Received:November 13,2014;Revised:January 23,2015;Published on Web:January 28,2015.
∗Corresponding authors.ZHOU Jin,Email:zhoujsdut@gmail.com.ZHUO Shu-Ping,Email:zhuosp_cademic@yahoo.com; Tel/Fax:+86-533-2781664.
The project was supported by the National Natural Science Foundation of China(51302156).
国家自然科学基金(51302156)资助项目
