Acid Dye Adsorption Properties of Ethylenediamine-Modified Magnetic Chitosan Nanoparticles
2014-10-14ZHOULiMinSHANGChaoLIUZhiRong
ZHOU Li-Min SHANG Chao LIU Zhi-Rong
(1State Key Laboratory Breeding Base of Nuclear Resources and Environment,East China Institute of Technology,Nanchang 330013,P.R.China; 2Key Laboratory of Radioactive Geology and Exploration Technology Fundamental Science for National Defense,East China Institute of Technology,Fuzhou 344000,Jiangxi Province,P.R.China)
Acid Dye Adsorption Properties of Ethylenediamine-Modified Magnetic Chitosan Nanoparticles
ZHOU Li-Min1,2,*SHANG Chao1LIU Zhi-Rong1
(1State Key Laboratory Breeding Base of Nuclear Resources and Environment,East China Institute of Technology,Nanchang 330013,P.R.China;2Key Laboratory of Radioactive Geology and Exploration Technology Fundamental Science for National Defense,East China Institute of Technology,Fuzhou 344000,Jiangxi Province,P.R.China)
Abstract: Ethylenediamine-modified magnetic chitosan nanoparticles(EMCN)were prepared and used for the adsorption of Acid Orange 7(AO7)and Acid Orange 10(AO10)from aqueous solutions.Magnetic chitosan nanoparticles were prepared by adding a basic precipitant NaOH solution to a W/O microemulsion system containing cyclohexane/n-hexanol,chitosan and ferrous salt.This was then modified with ethylenediamine to increase the amine content and to improve the adsorption capacity.Transmission electron microscopy showed that the EMCN was essentially monodispersed and had a main particle size distribution of 15-40 nm.Adsorption experiments indicated that the maximum adsorption capacity was at a pH of 4.0 for AO7 and a pH of 3.0 for AO10.Because of the small diameter and the high surface reactivity of EMCN,the adsorption equilibrium for both dyes was reached very quickly.The equilibrium experiments fitted the Langmuir isotherm model well and the maximum adsorption capacities of 3.47 and 2.25 mmol·g-1were obtained for AO7 and AO10,respectively.We estimated the thermodynamic parameters and accordingly the adsorption process was found to be spontaneous and exothermic.Additionally,we regenerated EMCN with an NH4OH/NH4Cl solution(pH 10.0)and the regenerated material was used to readsorb the dyes.
Key Words:Adsorption;Chitosan;Magnetic nanoparticle;Ethylenediamine;Acid dye
1 Introduction
Most of dyes released during textiles,clothing,printing,and dyeing processes are considered as hazardous and toxic to some organisms and may cause allergic dermatitis,skin irritation,carcinogenic,and mutagenic to human and aquatic organisms.1Several techniques are available for the treatment of dyes such as a electrochemical technique,2a bio-degradation process,3homogeneous and heterogeneous photocatalytic oxidation.4,5Among the many techniques for dye removal,adsorption is the procedure of choice as it can be used to remove different types of dyes.6,7Various adsorbents have been developed until now and chitosan is one of them which is being greatly exploited because it is relatively cheap and exhibit higher adsorption capacities.8,9The use of chitosan resins for the removal of dyes from aqueous solutions was recently reported by several authors.6,9-12
Chitosan is usually needed to be cross-linked to improve its chemical stability in acid media.Although the crosslinking method may enhance the resistance of chitosan against acids,the process may reduce its adsorption capacity of dyes,especially when the crosslinking procedure involves in the reaction of amino groups,which are expected to play a great part in the adsorption process.In order to improve the adsorption capacity and selectivity of dyes,a number of chitosan derivates have been obtained by grafting functional groups such as acrylic and acrylamide,9poly(methylmethacrylate),10poly(alkyl methacrylate),11and vinyl acetate12through a crosslinked chitosan back bone.
Most of the chitosan-based adsorbents were submicron to micron-sized and need large internal porosities to ensure adequate surface area for adsorption.Compared to the traditional micron-sized supports used in separation process,nano-sized adsorbents possess quite good performance due to high specific surface area and the absence of internal diffusion resistance.13However,the nano-adsorbents could not be separated easily from aqueous solution by filtration or centrifugation.Magnetic nano-adsorbents can be manipulated by an external magnetic field and hence facilitate phase separation.
Several studies have indicated that―NH2groups in chitosan are the main groups for the adsorption of dyes containing sulfonate groups through the ionic interactions of the colored dye ions with the protonated amino groups on the chitosan.6,8,9In this work,the magnetic chitosan nanoparticles(MCN)were prepared and then modified with ethylenediamine(EMCN)to increase the―NH2active groups and thus enhance the adsorption capacity for acid dyes.The adsorption behaviour of the EMCN toward Acid Orange 7(AO7)and Acid Orange 10(AO10)was studied.The equilibrium isotherms and thermodynamic were discussed.
2 Experimental
2.1 Chemicals and reagents
Chitosan with 40 mesh,90%degree of deacetylation and relative molecular mass of 1.3×105was purchased from Yuhuan Ocean Biology Company(Zhejiang,China).Glutardialdehyde,epichlorohydrine,ethylenediamine,Acid Orange 7 and Acid Orange 10 were purchased from Aldrich and Sigma Chemical,and were used without any further purification.All the other reagents used in this work were of analytical grade.
2.2 Preparation and characterization of the adsorbents
The preparation of magnetic chitosan nanoparticles(EMCN)in a W/O microemulsion system containing chitosan and ferrous salt was in accordance with the previous work.14Grafting of ethylenediamine using epichlorohydrin as a crosslinking agent was carried out similar to the procedure described by Atia et al.15with chitosan resin.The magnetic chitosan nanoparticles(2.5 g)were suspended in 35 mL isopropyl alcohol to which 2.5 mL epichlorohydrine(31.25 mmol)dissolved in 50 mL acetone/water mixture(volume ratio 1:1)was added.The contents were stirred for 24 h at 333 K.The solid was isolated and then were transfered in 50 mL ethanol/water mixture(volume ratio 1:1),then ethylenediamine(2.5 mL)was added.The reaction mixture was stirred at 333 K for 12 h,then the solid products(ethylenediamine-modified magnetic chitosan nanoparticles,EMCN)were isolated and washed with ethanol followed by water,and finally dried in a vacuum oven at 333 K.
The dimension and morphology of the EMCN were observed by transmission electron microscopy(TEM)(Hitachi,H-800).X-ray diffraction(XRD)data were collected on a XRD-2000X-ray diffractometer with Cu Kαradiation.Thermalgravimetric analysis was conducted on Shimadzu TGA-50H with heating rate of 10 K·min-1.The concentration of the amine active sites in the obtained resins was estimated using the volumetric method.16
2.3 Batch adsorption
Batch adsorption were performed at controlled pH and temperature by shaking 50 mg of EMCN with 50 mL dye solution for 1.5 h at 200 r·min-1.The parameter ranges for the experiments are:pH 2-10;temperature 298-318 K;initial concentration for the dyes 0.5-6 mmol·L-1.The solution pH was adjusted to the desired value by adding either nitric acid or sodium hydroxide standardized solutions.After mixing,the aqueous phase was separated from the solid phase by magnetic settlement and centrifugation at 12000 r·min-1.The residual concentration of dyes was determined at the maximum wavelength(484 nm for AO7 and 475 nm for AO10)using a Cary 50 UVVis spectrophotometer(Varian,USA).
For the desorption studies,the dye-loaded EMCN were collected and washed with distilled water to remove any unadsorbed dyes,and then were agitated with NH4OH/NH4Cl(pH 10.0,5.6 mol·L-1NH4OH/1.0 mol·L-1NH4Cl)for 2 h.To investigate the reusablity of the adsorbents,the EMCN after desorption was reused in adsorption experiments and the process was repeated for three times.
3 Results and discussion
3.1 Characterization of EMCN
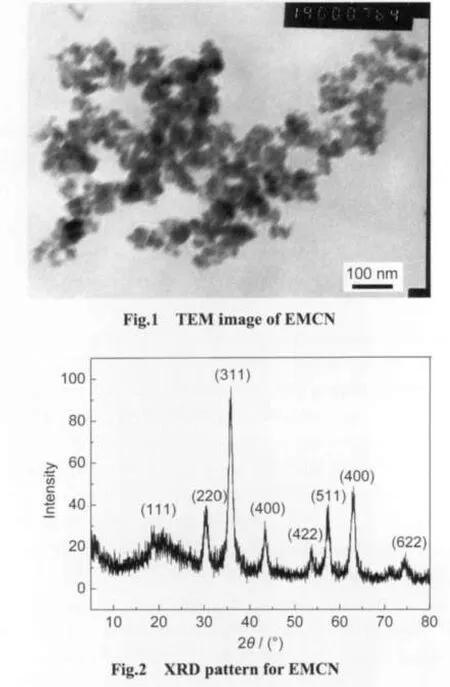
The TEM image of EMCN is shown in Fig.1.It can be observed that EMCN were essentially monodispersed and had a particle size distribution of 15-40 nm.Fig.2 shows the XRD pattern for EMCN.Eight characteristic peaks for Fe3O4marked by their indices((111),(220),(311),(400),(422),(511),(440),and(622))were observed for the sample.These peaks are consistent with the database in JCPDS file(PDF No.65-3107)and reveal that the resultant nanoparticles are pure Fe3O4with a spinel structure.
The average mass content of Fe3O4in the EMCN by TGA was about 33.5%,as calculated from the TGA data at 873 K.The magnetization measurement performed with vibrating sample magnetometer(VSM)(Fig.3)indicated that the saturation magnetization of the EMCN was 25.6 A·m2·kg-1.As mentioned in a previous report,this magnetic susceptibility value is sufficient for this resin to be used in wastewater treatment.14The concentration of the amine active sites of the MCN and EMCN was determined to be 2.4 mmol·g-1and 3.8 mmol·g-1,respectively.
3.2 Effect of pH
The influence of pH on the adsorption capacity(Q)of AO7 and AO10 onto the EMCN for pH 2-10 are illustrated in Fig.4.It can be seen that the maximum uptake values for AO7 and AO10 were obtained at pH 4.0 and pH 3.0,respecitively(the optimum pH values selected for the further experiments).The observed decrease in the uptake value at low pH(less than the optimum pH values)may be attributed to the decrease in dye dissociation which leads to a lower concentration of the anionic dye species available to interact with the resin's active sites.Above the optimum pH values,the EMCN displays a sharp decrease in the uptake value as pH increases.This behaviour can be explained on the basis of the lower extent of protonation of amino groups at high pH value.
The mechanisms of the adsorption process of the acid dyes(AO7 and AO10)on the EMCN are likely to be the ionic interactions of the colored dye ions with the amino groups of the EMCN.In aqueous solution,the acid dyes are first dissolved and the sulfonate groups of acid dye(D―SO3Na)dissociate and are converted to anionic dye ions.


Also,in the presence of H+,the amino groups of the EMCN(R―NH2)became protonated.

The adsorption process then proceeds due to the electrostatic attraction between these two oppositely charged ions.
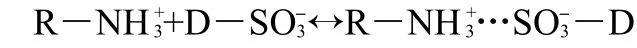
The point of zero charge for the EMCN was found to be 4.8 using standard potentiometric method.Therefore,the surface charge of the EMCN is positively charged at pH<4.8.It seems that at pH 3-4,most of―NH2groups are protonated,which are favorable for the adsorpion of anionic dyes.However,at high pH,the number of protonated―NH2groups will decrease and more OH-ions will be available to compete with the anion-ic sulfonic groups,therefore the adsorption capacity for the acid dyes decreases.
3.3 Effect of contact time
Fig.5 shows the effect of contact time on the adsorption capacity of AO7 and AO10 by the EMCN.The results demonstrate that the adsorption for both dyes is rapid.In the case of AO7,the maximum adsorption is attained in 40 min,while for AO10 it takes 60 min,after which the change in the removal percentage is insignificant.The contact time of 90 min was found to be sufficient to reach equilibrium,and so it was selected in further experiments.
It was reported that more than 2 h is needed to attain equilibrium for dyes with several adsorbents,such as glutaraldehyde and H2SO4-crosslinked chitosan resin,6chitosan/kaolin/γ-Fe2O3composites,7and chitosan derivatives.9In contrast,in the present study,AO7 and AO10 are adsorbed in a short time(no more than 1 h).This can be attributed to the large surface area,the sufficient exposure of active sites and the high surface reactivity of the magnetic chitosan nanoparticles.
3.4 Adsorption isotherms
Fig.6 shows the adsorption isotherms of AO7 and AO10 on the EMCN at different temperatures.The equilibrium adsorption capacity of the dye(Qe)increased with increasing of dye concentration.The adsorption curves indicate that the uptake of both dyes decreases with increasing temperature.The adsorption isotherms were studied using three isotherm models:
Langmuir isotherm equation:

Freundlich isotherm equation:

and Dubinin-Radushkevich(D-R)isotherm equation17:

where Ceis the equilibrium concentration of the dye(mmol·L-1);Qeis the adsorbed value of dyes at equilibrium concentration(mmol·g-1);KLis the Langmuir binding constant which is related to the energy of adsorption(L·mmol-1);KFand bFare the Freundlich constants related to the adsorption capacity and intensity,respectively.QDRis D-R maximum adsorption capacities of the dye(mmol·g-1);K is the D-R constants;ε is the Polanyi potential given as Eq.(4)18:



where R is the gas constant(8.314 J·(K.·mol)-1),and T is the temperature(K).The D-R constant(K)can give the valuable information regarding the mean energy of adsorption by Eq.(5)19:

where E is the mean adsorption energy.The results were listed in Table 1.The Langmuir isotherm was found to fit quite well with the experimental data for both AO7 and AO10 in comparison with the linear correlation coefficients(r2).This indicates the homogeneity of active sites on the surface of the EMCN.It is notable that the EMCN is a composite adsorbent which is composed of chitosan and Fe3O4.However,chitosan was mainly responsible for the adsorption of the adsorption of the dyes,therefore,it is reasonable that the EMCN can provide the homogeneity of active sites.
The Langmuir maximum adsorption capacity(Qm)of AO7(2.82-3.47 mmol·g-1)at different temperatures(298-318 K)was much higher than that of AO10(1.79-2.25 mmol·g-1).The difference in the degree of adsorption may be attributed to the size and chemical structure of the dye molecule.AO7 has only one sulfonate acid group(monovalent)and has the smaller molecular size which implies smaller surface area and protonated amino groups of the adsorbent being occupied by each dye molecule.The small molecular size not only increases the concentration of dye on the surface of the chitosan particle but also enables a deeper penetration of dye molecules into the internal pore structure of the EMCN.The monovalent nature of AO7 dye molecules makes more protonated amino groups on the chitosan particle available for the adsorption of dye molecules.It also reduces the electrostatic repulsion of adjacent dye molecules on the adsorbent surface when compared with divalent dye molecule such as AO10,enabling dye molecules to be packed more closely on the adsorbent surface.
The maximum adsorption capacities(Qm)of the EMCN obtained by Langmuir isotherm for AO7 and AO10 adsorption at 298 K were 3.47 and 2.25 mmol·g-1,respectively,which were higher than that of the unmodified magnetic chitosan microspheres(2.65 mmol·g-1for AO7 and 1.76 mmol·g-1for AO10)obtained in the same conditions.These results indicated that chemical modification with ethylenediamine improved the adsorption capacity for both AO7 and AO10 due to the higher concentration of active sites of the EMCN.
It was notable that only 66.5%of chitosan was responsible for the adsorption of the AO7 and AO10 dyes.The maximum adsorption capacity based on the weight of chitosan were 5.12 mmol·g-1(1827 mg·g-1)for AO7 and 3.38 mmol·g-1(1530 mg·g-1)for AO10,respectively,which were higher than those of other adsorbents.6,9The high adsorption capacity of the EMCN for the dyes might be reasonably referred to the high specific surface area of magnetic chitosan nanoparticles with a much smaller diameter,leading to almost all active sites available.
In addition,the mean adsorption energy(E)from the D-R isotherm means the free energy of one mole of solute from infinity(in solution)to the adsorbent's surface.The adsorption behavior could be the physical adsorption in the range of 1-8 kJ·mol-1and the chemical adsorption in more than 8 kJ·mol-1.18TheEvalues(Table 1)of 8.53-9.29 kJ·mol-1for AO7 and 8.41-9.66 kJ·mol-1for AO10 indicated that the adsorption of both dyes onto the EMCN might be predominant on the chemisorption process.
The degree of suitability of the obtained resins towards dyes was estimated from the values of the separation factor(RL)using the following relation.20

whereKLis the Langmuir equilibrium constant andC0is the initial concentration of dye.Values of 0<RL<1 indicates the suitability of the process.The values ofRLfor the EMCN toward the adsorption of AO7 and AO10 for all concentration ranges(0.5-6.0 mmol·L-1)at 298-318 K lie between 0.012-0.162 and 0.020-0.231,respectively.This indicates the suitability of the EMCN for bothAO7 andAO10 adsorption.

Table 1 Langmuir,Freundlich and Dubinin-Radushkevich isotherm constants and correlation coefficients
3.5 Thermodynamic of AO7 and AO10 adsorption
The thermodynamic parameters of the adsorption process are obtained from experiments at various temperatures(298-318 K).The values ofKL(Table 1)at different temperature were processed according to the following van't Hoff equation21to obtain the thermodynamic parameters.

where ΔH⊖and ΔS⊖are enthalpy and entropy changes,respectively,Ris the universal gas constant(8.314 J·mol-1·K-1)andTis the absolute temperature(K).Plotting lnKLagainst 1/Tgives a straight line with slope and intercept equal to ΔH⊖/Rand ΔS⊖/R,respectively.The values of ΔH⊖and ΔS⊖were calculated from Fig.7 and reported in Table 2.The negtive values of ΔH⊖indicate the exothermic nature of adsorption process.The positive values of ΔS⊖suggest the increased randomness during the adsorption of AO7 and AO10.The source of this entropy gain is due to liberation of water molecules from the hydrated shells of the sorbed species.18Gibbs free energy of adsorption(ΔG⊖)was calculated from the following relation andaslo given in Table 2.
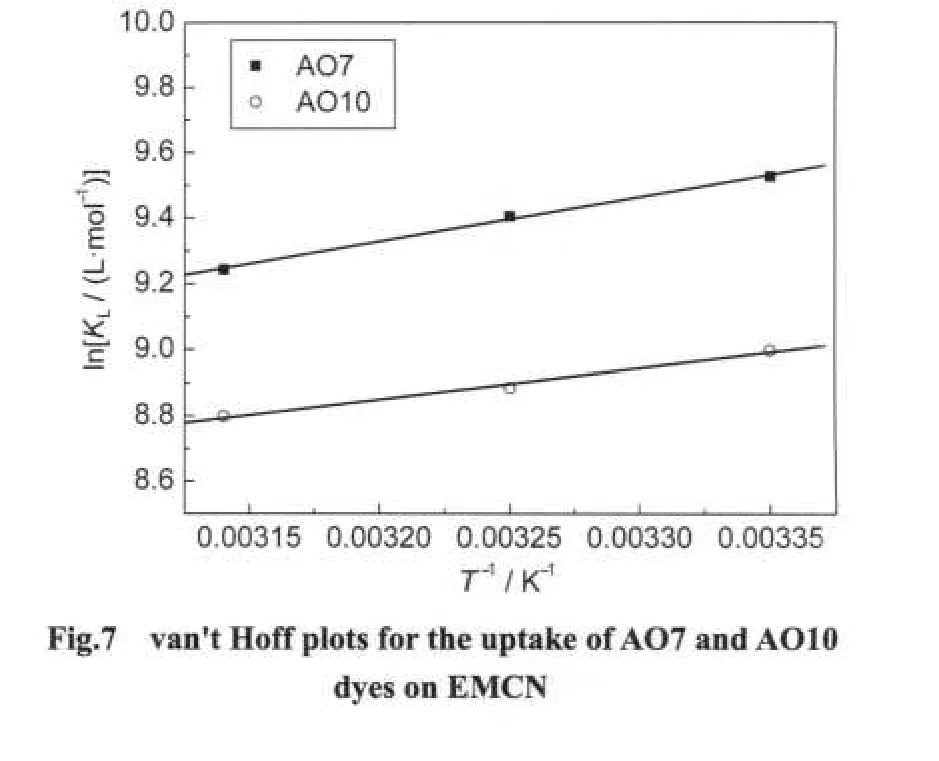

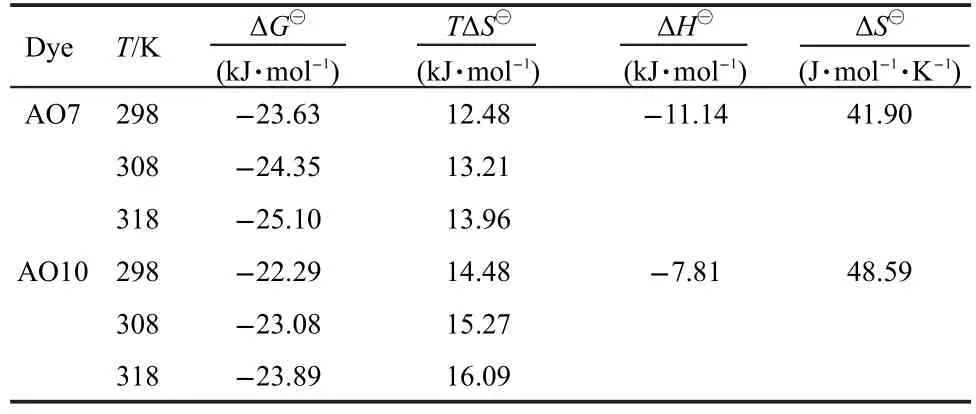
Table 2 Thermodynamic parameters ofAO7 and AO10 adsorption by EMCN
The negative values of ΔG⊖for both dyes indicate that the adsorption on the EMCN is a spontaneous process,whereby no energy input from outside of the system is required.However,the values of ΔG⊖decreased with increasing temperature,suggesting that adsorption of AO7 and AO10 onto the EMCN became less favourable at higher temperature.6As the temperature increases,the mobility of dye molecules increases,causing the molecules to escape from the solid phase to the liquid phase.Therefore,the amount of AO7 and AO10 that can be adsorbed will decrease.The increase mobility of dye molecules at elevated temperature may also be reflected in the values ofKL(Table 2).The values ofKLfor both dyes decrease as the temperature increases,indicating lower affinity of the resins towards the dyes at higher temperature.
3.6 Desorption and reuse
It was observed that at the first adsorption step,the adsorption capacities for the AO7 and AO10 dyes reached the values of 3.16 and 2.13 mmol·g-1,respectively.After the desorption step,the adsorbed AO7 and AO10 dyes were removed about 81.1%and 87.8%,respectively,by NH4OH/NH4Cl solution at pH 10.This could be ascribed to the fact that,in the basic solution,the positively charged amino groups were deprotonated and the electrostatic interaction between chitosan and dye molecules became much weaker.After one cycle of adsorption and desorption of the acid dyes,about 1.2%of Fe3O4on the EMCN was dissolved and 95%of the saturation magnetization was remained.The results of thermogravimetric tests showed that the average mass content of Fe3O4on the EMCN decreased from 33.5%before adsorption to 32.3%after desorption.No obvious leakage of resin materials and change of the EMCN were observed in the experimental process.The EMCN becomes more resistant to acidic and alkaline mediums compared to their parent chitosan due to the cross-linking reaction in the preparation process.The total adsorption capacities of both AO7 and AO10 dyes for the second and the third step maintain more than 90%of those for the first adsorption step.Therefore,the EMCN can be reused for further dye adsorption.
4 Conclusions
This research has demonstrated that the EMCN can be used for the effective adsorption ofAO7 andAO10 from aqueous solution.The EMCN exhibited good kinetic characteristics(equilibrium time are 40 min for AO7 and 60 min for AO10)and high adsorption loading capacities for AO7 and AO10(i.e.,3.47 and 2.25 mmol·g-1at 298 K,respectively).The EMCN also showed good improvements in the uptake properties of AO7 and AO10 compared to unmodified ones.Equilibrium experiments fitted well the Langmuir isotherm model and the adsorption capacity decreases with increasing temperature.The EMCN showed higher adsorption capacity for AO7 than for AO10 due to the size and chemical structure of the dye molecule.In addition,the mean adsorption energy from the Dubinnin-Radushkevich isotherms revealed that the adsorption process was predominant on the chemisorption process.Thermodynamic calculation indicated that the adsorption process was spontaneous and exothermic.Furthermore,the EMCN could be regenerated using NH4OH/NH4Cl solution at pH10 and could be reused to adsorb the dyes.
(1) Zollinger,H.Colour Chemistry-Synthesis,Properties of Organic Dyes and Pigments;VCH Publishers:New York,1987;pp 28-35.
(2) Lin,S.H.;Peng,C.F.Water Res.1994,28,277.
(3)McMullan,G.;Meehan,C.;Conneely,A.;Kirby,N.;Robinson,T.;Nigam,P.;Banat,I.M.;Marchant,R.;Smyth,W.F.Appl.Microbiol.Biotechnol.2001,56,81.
(4) Liu,D.;Xu,Y.M.Acta Phys.-Chim.Sin.2008,24,1584.[刘 鼎许宜铭.物理化学学报,2008,24,1584.]
(5) Jiang,R.;Zhu,H.Y.;Li,X.D.;Xiao,L.Chem.Eng.J.2009,152,537.
(6)Kamari,A.;Wan,N.W.;Chong,M.Y.;Cheah,M.L.Desalination2009,249,1180.
(7) Zhu,H.Y.;Jiang,R.;Xiao,L.Appl.Clay Sci.2010,48,522.
(8) Crini,G.;Badot,P.M.Prog.Polym.Sci.2008,33,399.
(9) Kyzas,G.Z.;Lazaridis,N.K.J.Colloid Interface Sci.2009,331,32.
(10) Singha,V.;Sharma,A.K.;Tripathi,D.N.;Sanghi R.J.Hazard.Mater.2009,161,955.
(11) Konaganti,V.;Kota,R.;Patil,S.;Madras,G.Chem.Eng.J.2010,158,393.
(12) Elkholy,S.;Khalil,K.D.;Elsabee,M.Z.;Ewels,M.J.Appl.Polm.Sci.2007,103,1651.
(13)Chang,Y.C.;Chang,S.W.;Chen,D.H.React.Funct.Polym.2006,66,335.
(14) Zhi,J.;Wang,Y.;Lu,Y.;Ma,J.;Luo,G.React.Funct.Polym.2006,66,1552.
(15)Atia,A.A.Hydrometallurgy2005,80,13.
(16)Latha,G.A.;George,K.B.;Kannan,G.K.;Ninan,N.K.J.Appl.Polm.Sci.1991,43,1159.
(17) Ramnani,S.P.;Sabharwal,S.React.Funct.Polym.2006,66,902.
(18)Chen,A.H.;Chen,S.M.J.Hazard.Mater.2009,172,1111.
(19)Varma,A.J.;Deshpande,S.V.;Kennedy,J.F.Carbohydr.Polym.2004,55,77.
(20) Qi,L.;Xu,Z.Colloids Surf.A2004,251,186.
(21) Tellinghuisen,J.Biophys.Chem.2006,120,114.
乙二胺改性磁性壳聚糖纳米粒子对酸性染料的吸附特性
周利民1,2,*尚 超1刘峙嵘1
(1东华理工大学核资源与环境国家重点实验室培育基地,南昌330013;2东华理工大学放射地质与勘探国防基础重点实验室,江西抚州344000)
利用乙二胺改性磁性壳聚糖纳米粒子(EMCN)吸附酸性橙7(AO7)和酸性橙10(AO10).EMCN制备时先通过在由环已烷/正已醇、壳聚糖和铁盐组成的反相微乳体系中加NaOH溶液沉淀剂,得到磁性壳聚糖纳米粒子,再经乙二胺改性以增加氨基含量和提高吸附容量.透射电镜表明,EMCN分散良好,粒径15-40 nm.吸附实验表明,AO7和AO10最佳吸附分别在pH 4.0和pH 3.0.EMCN具有粒径小和高表面活性,因此吸附速率快.吸附平衡符合Langmuir模型,AO7和AO10的最大吸附容量分别为3.47和2.25 mmol·g-1.热力学分析表明吸附过程放热,且能自发进行.EMCN可用NH4OH/NH4Cl(pH 10.0)溶液再生并可重复使用.
吸附;壳聚糖;磁性纳米粒子;乙二胺;酸性染料
O647.3
Received:December 8,2010;Revised:December 22,2010;Published on Web:January 28,2011.
∗Corresponding author.Email:minglzh@sohu.com;Tel:+86-794-8829625;Fax:+86-794-8258320.
The project was supported by the Science&Technology Pillar Program of Jiangxi,China(2009BSB08600)and Scientific Research Fund from the Education Bureau of Jiangxi,China(GJJ10494).
江西省科技支撑项目(2009BSB08600)和江西省教育厅科技项目(GJJ10494)资助
猜你喜欢
杂志排行
物理化学学报的其它文章
- Effect of the Ionic Liquid Additive-[BMIM]HSO4on the Kinetics of Oxygen Evolution during Zinc Electrowinning
- Catalytic Decomposition of Cellulose in Cooperative Ionic Liquids
- Electronic Structures and Optical Properties of Ilmenite-Type Hexagonal ZnTiO3
- Effects of Substrate-Target Distance and Si Co-Doping on the Properties of Al-Doped ZnO Films Deposited by Magnetron Sputtering
- Molecular Dynamics Simulations ofα-Tocopherol in Model Biomembranes
- Pt-Ni Catalyst Supported on CMK-5 for the Electrochemical Oxidation of Methanol
