Full length cDNA cloning and expression analysis of annexinA2 gene from deer antler tissue
2014-09-06LiHaoXianghongXiaoHepingLi
Li Hao · Xianghong Xiao · Heping Li
ORIGINAL PAPER
Full length cDNA cloning and expression analysis of annexinA2 gene from deer antler tissue
Li Hao · Xianghong Xiao · Heping Li
Received: 2013-10-09; Accepted: 2013-12-03
© Northeast Forestry University and Springer-Verlag Berlin Heidelberg 2014
ANXA2(AnnexinA2), a calcium-dependent phospholipid binding protein, is involved in various Ca2+-related biological activities. In the present study, full-length cDNA of ANXA2 was isolated from the velvet antler tip tissue of sika deer (Cervus nippon hortulorum); the amino acid sequence and gene expression was analyzed by using bioinformatics and real-time reverse transcriptase polymerase chain reaction (RT-PCR) techniques. Nucleotide sequence analysis reveals that the full-length cDNA of the ANXA2 gene was 1372 bp, of which 1020 bp was in the open-reading frame (ORF) encoding 339 amino acids; its relative molecular weight was 38.3 kDa; and isoelectric point was 6.72. Sequence analysis indicates that the protein includes four conserved tandem-duplication ANX domains. The gene-accession nucleotide sequence number in GenBank is JX315571. Expression analysis by RT-PCR reveals that ANXA2 gene expression has a significant positive correlation with the antler-tissue mineralization process, indicating that this gene may play an important role in the regulation of antler-tissue mineralization.
velvet antler, AnnexinA2 (ANXA2) gene, cDNA library, clone, real-time quantitative RT-PCR
Introduction
Velvet antler is the dense fuzzed, immature horn of male deer (excluding reindeer) (Brockes and Kumar 2002; Price and Allen2004), it has attracted the attentions of many scientists due to its incomparable growth rate, regeneration mechanism, and perceived magical medicinal value (Li et al. 1995; Odelberg 2005).
Velvet antler has a long growth period of about 90−120 days every year, and the fastest growth rate has been recorded at 1−2 cm per day (Brockes and Kumar 2005; Han et al. 2005). Some researchers divide the development of antler into two phases: growth period and ossification period (Gerke et al. 2005; Markoff and Gerke 2005). In the first phase, growth is dominant, with a slow ossification rate. In contrast, during in the second phase, antler can be ossified fast, being accompanied with the accumulation of large amounts of mineral matters; however, this process produces an inhibitory effect on antler growth. Antler with a high level of ossification contains a high concentration of mineral matter and a declining ratio of organic components. Therefore, ossification degree indexes the old and young degree of velvet antler and the changes of components, further decides the rank of velvet antler. Improvement in the yield of velvet antler and the economic benefits of the deer industry should not only inhibit or reduce antler ossification but also not influence antler growth rate. Illuminating the relationship between the growth rate and ossification is the basis to achieve this objective (Benaud et al. 2004; Rescher and Gerke 2004).
ANXA2 is a calcium/phospholipids-binding multi-functional protein (Filipenko et al. 2004; Yamada et al. 2005; Mussunoor and Murray 2008). It plays important roles in the formation of biomembranes, endocytosis/exocytosis, osteoblast and osteal reabsorption, membrane transport, ion channels, DNA synthesis, and cell proliferation (Chiang et al. 1999; Gerke and Moss 2002; Okuse et al. 2002). In the present study, the ANXA2 gene in the constructed velvet antler tip tissue of sika deer belongs to this high-abundance expression gene, indicating that it may be an important factor regulating antler development.
To date, there is no record of deer-derived ANXA2 gene sequences in GenBank. In this paper, the full-length cDNA of ANXA2 genes were cloned successfully from the full-length cDNA library of velvet antler tip tissues of sika deer. Further, the gene structure and the expression levels of different tissue layers of the antler tip were studied using bioinformatics and real-timequantitative RT-PCR technique. These results provide an important basis for further study of the biological functions of ANXA2 genes, molecular mechanism of regulating antler development, and velvet antler production.
Materials and methods
Velvet antler tissue
Antler grown for 80 days were collected from anaesthetized adult sika deer (Cervus nippon hortulorum) stags. The distal 4 cm of the tips was removed. Different tissue layers (skin, reserve mesenchyme, precartilage, and cartilage) of the growing antlers were determined as described by Li et al. (2002). After being harvested, all samples were immediately preserved in liquid nitrogen and kept at -80°C until they were used for isolating the RNA.
Methods for obtaining full length cDNA of ANXA2 genes
Total RNA extraction and cDNA library construction were based on the protocols of SV Total RNA Isolation System reagent (Promega) and CreatorTMSMARTTMcDNA Library Construction Kit (Clontech), respectively. Using M13 primer, single colonies randomly selected from velvet antler tip tissue in the original library were put through large-scale 5’-end EST sequencing, by using a 3730XL sequencer (Applied Biosystems, Foster City, CA, USA). High-quality ESTs were clustered and spliced, using Phrap software. The consensus sequence splice was programmed by BLASTX and BLASTN; from there, the sequence homology comparison and function notes of the obtained genes were conducted with non-redundant proteins and nucleic acid database in GenBank. ANXA2 genes with corresponding positive colonies were selected to carry out PCR identification, bacterial amplification, and plasmid extraction, and then two-way sequencing for the plasmid DNA.
Nucleotide sequence analysis
The ANXA2 gene sequence was analyzed and compared using the BLAST P and ORF search programs with GenBank database search. The multiple sequence alignment of ANXA2 gene was carried out, using the Clustal W analysis program; the signal-peptide site was predicted by Signal P3.0; and the ANXA2 protein MW and pI were computed by using the ProtParam tool. A phylogenetic tree, based on evolutionary distances, was constructed from amino-acid sequences using the njplotWIN95 program.
Quantification of the ANXA2 gene expression by real-time RT-PCR
Total RNAs using SV Total RNA Isolation System reagent (Promega) were isolated from different tissues including skin, mesenchyme, precartilage, and cartilage. The DNA residue was removed by DNase Ι digesting, at 37°C for 30 min. Four microgram of the total RNA were used in each lane and electrophoresed in a 0.8% agarose gel, at 100 V/12 cm for 15 min. First-strand cDNA synthesis was performed using M-MLV reverse transcriptase (TaKaRa Biotechnology (Dalian) Co., Ltd. Japan) to transcribe poly (A)+RNA with oligo-d(T)18 and random six as the primers; reaction conditions were set as the manufacturer’s instructions. The cDNA was used for the assay of quantitative real-time PCR. The SYBR Green I real-time RT-PCR assay was carried out in an Option-II Sequence Detection System (MJ Research, U.S.).
In a real-time RT-PCR study, specific primers (ANXA2-F: 5'-AGCTGCAGGAAATCAACAG-3' and ANXA2-R: 5'-ATCAATGACAGAGCCATCC-3') were used to amplify a 146 bp fragment with cDNA from skin, reserve mesenchyme, precartilage and cartilage, and organs using 18S as a positive control. Quantitative real-time PCR primers were designed on the basis of EST sequences of the ANXA2 gene and 18S rRNA gene from deer-antler tip tissue by using Primer 5.0 and Oligo 6.0 software. The EST sequences of the two genes were obtained by large-scale EST sequencing of the full-length cDNA library from deer-antler tip tissue constructed by our lab. The amplifications were performed in a 96-well plate, in a 25 μL reaction volume containing 12.5 μL of 2 × SYBR Green Master Mix (TARAKA), 2.5 μL (each) of ANXA2-F and ANXA2-R primers (10 mM), 1 μL of template, and 9 μL of DEPC-water. The thermal profile for SYBR Green real-time PCR was 95°C for 2 min, followed by 45 cycles of 94°C for 12 s, and 55°C for 30 s. In the 96-well plate, each sample was amplified in triplicate. DEPC-water for the replacement of template was used as negative control. The relative expression was calculated as 2−ΔΔCt(Livak and Schmittgen 2001).
Statistical analysis
A multiple comparisons (Duncan’s) test was conducted to compare significant differences in the ANXA2 expression between skin, mesenchyme, precartilage, and cartilage, using the SPSS13.0 software. A significance level of p = 0.05 was chosen.
Results and discussion
Sequencing and bioinformatics analysis of ANXA2
Computer analysis, using the BLAST algorithm, confirmed that the selected sequence corresponded to ANXA2. The full-length ANXA2 cDNA contains 1372 bp, of which 21 bp in the 5'-untranslated region (UTR); 1020 bp in the open reading frame (ORF); and 331 bp in 3'-UTR with poly (A) tail (Fig. 1). The ORF encodes a polypeptide of 339 amino acids. The calculated molecular mass of the mature protein (339 amino acids) is 38.3 kDa, with an estimated pI of 6.72. ANXA2 is in shortage of signal peptides, so they belong to non-secretory proteins and can not be excreted out cells by the classical protein excretion pathway. However, in a temperature stress state, these substances can betransferred from the cytoplasm to the membrane surface (Deora et al. 2004). The cDNA sequence has been submitted to the NCBI GenBank as accession number JX315571.
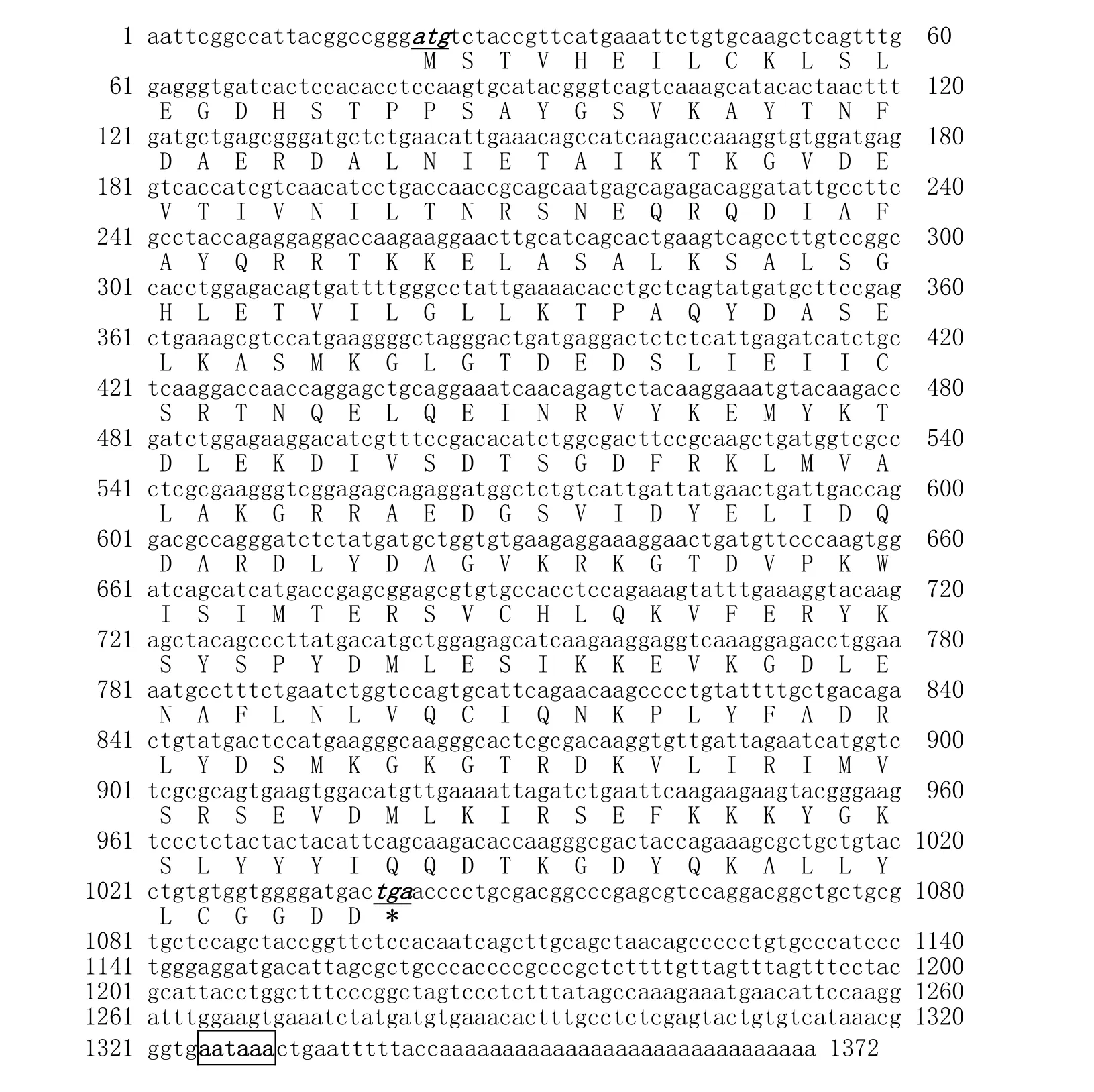
Fig. 1: Nucleotide and deduced amino acid sequences of ANXA2 cDNA of velvet antler from sika deer
The C'-end of ANXA2 gene has four homologous duplication sequences (ANX domains) that are from the amino acids 37 to 102, 122 to 174, 207 to 259, and 282 to 334 (Fig. 2). They can be bound with Ca2+, phospholipids, and F-actin, thus having important significance in cytoskeleton recombination and various membrane-related changes (Hayes et al. 2006).
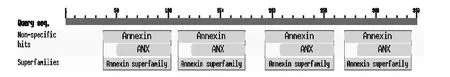
Fig. 2: Search for the conserved domains in deduced amino acid sequence of ANXA2 cDNA
The N'-end of the gene contains three loci, namely Tyr23, Ser11, and Ser25. Among them, Ser1 and Ser25 can be phosphorylated by the action of protein kinase; Tyr23 can be modified by some membrane-binding kinases such as the IGF-1 and PDGF receptors. Therefore, the three protein kinases play important roles in cellular signal transduction and cell differentiation (Babbin et al. 2007).
Homology comparison of ANXA2
The comparison of the ORFs with other known ANXA2s indicated that the ANXA2 shows a homology of Identities = 338/339 (99%) with Bos Taurus, Identities = 333/339 (98%) with Homo sapiens, Identities = 323/339 (95%) with Rattus sp., Identities = 304/339 (89%) with Gallus gallus, Identities =275/339 (81%) with Xenopus laevis, Identities =215/339 (63%) with Monopterus albus. Homological analysis indicated that ANXA2 gene encoding regions of different genus show a high homology. Multiple alignment of ANXA2 protein sequences showed large variation in the beginning of C'-end, but in general, ANXA2 mature protein is highly conservative (Fig. 3). The evolutionary tree can be divided into two branches: one branch is mammals; the other branch contains birds, amphibians, and fish. The mammal graph showed that in the gene locus, sika deer has the closest evolutionary relationship with cattle (Bos Taurus) and the furthest genetic relationship with fish such as Monopterus albus (Fig. 4). This result is in agreement with their traditional taxonomic position for the tested species, according to animal evolution relationships.

Fig. 3: Multiple alignment of ANXA2 protein sequences
Tissue expression of ANXA2
ANXA2 expressed in each tissue of velvet antler tip is shown in Fig. 5. The real-time RT-PCR showed that the ANXA2 gene was detected in skin, reserve mesenchyme, precartilage, and cartilage. However, the gene has a low expression level in the skin layer and reserve mesenchyme layer of velvet antler, but a high expression level in the precartilage and cartilage layers. The gene expression has a significant positive correlation with antler tissue mineralization process, indicating an important role of the gene in the process of antler tissue mineralization.
ANXA2 genes belong to protein super-family members thatcan be combined to negative-charge membrane phospholipids by Ca2+mediated pathway, thus these proteins are involved in various Ca2+-related biological activities. Francis and Suttie (1998) detected ANXA2 gene expression in red deer antler by using the Western blotting technique, and found that ANXA2 proteins were located in the edge of osteoblastic membrane. Based on our findings and previous results, it is assumed that ANXA2 genes play a partial role in antler-tissue mineralization by participating in the formation of a Ca2+-ion channel.
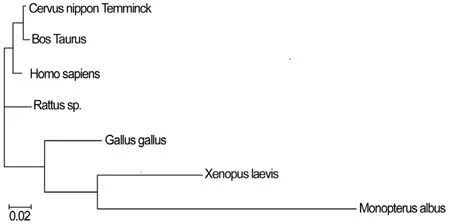
Fig. 4: The phylogenetic tree of ANXA2 from animals. Clustal W was used to establish the phylogenetic tree, and the result was displayed using Treeview software.
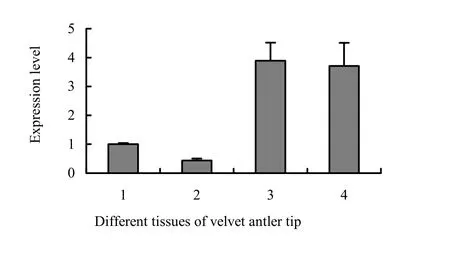
Fig. 5: Expression of ANXA2 in different tissues of velvet antler tip, 1-skin; 2-reserve mesenchyme; 3-precartilage; 4-cartilage
Velvet antler, as the most active growing point, stores large numbers of cell factors; these substances are the biochemical basis for clinical functions of velvet antler. Currently, our laboratory is conducting studies on the biological functions of ANXA2 genes. Research on antler ossification can not only reveal the old and young degree of velvet antler and component changes, which directly decide the optimal picking time and rank, but it also has significance for the construction of antler bone development models. Moreover, the related researches are helpful to improve velvet antler yield and quality, and provide the theoretical foundation for new drug development.
Babbin BA, Parkos CA, Mandell KJ, Winfree LM, Laur O, Ivanov AI, Nusrat A. 2007. Annexin 2 regulates intestinal epithelial cell spreading and wound closure through Rho-related signaling. Am J Pathol, 170: 951−966.
Benaud C, Gentil BJ, Assard N, Court M, Garin J, Delphin C, Baudier J. 2004. AHNAK interaction with the annexin 2/S100A10 complex regulates cell membrane cytoarchitecture. J Cell Biol, 164: 133–144.
Brockes JP, Kumar A. 2002. Plasticity and reprogramming of differentiated cells in amphibian regeneration. Nat Rev Mol Cell Biol, 3: 566–574.
Brockes JP, Kumar A. 2005. Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science, 310: 1919–1923.
Chiang Y, Rizzino A, Sibenaller ZA, Wold MS, Vishwanatha JK. 1999. Specific down-regulation of annexin expression in human cells interferes with cell proliferation. Mol Cell Biochem, 199: 139–147.
Deora AB, Kreitzer G, Jacovina AT, Hajjar KA. 2004. An annexin 2 phosphorylation switch mediates p11-dependent translocation of annexin 2 to the cell surface. J Biol Chem, 279: 43411–43418.
Filipenko NR, MacLeod TJ, Yoon CS, Waisman DM. 2004. Annexin A2 is a novel RNA-binding protein. J Biol Chem, 279: 8723–8731.
Francis SM, Suttie JM. 1998. Detection of growth factors and proto-oncogene mRNA in the growing tip of red deer (Cervus elaphus) antler using reverse-transcriptase polymerase chain reaction (RT-PCR). J Exp Zool, 281: 36–42.
Gerke V, Moss SE. 2002. Annexins: From structure to function. Physiol Rev, 82: 331–371.
Gerke V, Creutz CE, Moss SE. 2005. Annexins: Linking Ca2+signalling to membrane dynamics. Nat Rev Mol Cell Biol, 6: 449–461.
Han M, Yang X, Taylor G, Burdsal CA, Anderson RA, Muneoka K. 2005. Limb regeneration in higher vertebrates: Developing a roadmap. Anat Rec B New Anat, 287: 14–24.
Hayes MJ, Shao D, Bailly M, Moss SE. 2006. Regulation of actin dynamics by annexin 2. EMBO J, 25: 1816–1826.
Li C, Clark DE, Lord EA, Stanton JA, Suttie JM. 2002. Sampling technique to discriminate the different tissue layers of growing antler tips for gene discovery. Anat Rec, 268: 125–130.
Li C, Waldrup KA, Corson ID, Littlejohn RP, Suttie JM. 1995. Histogenesis of antlerogenic tissues cultivated in diffusion chambers in vivo in red deer (Cervus elaphus). J Exp Zool, 272: 345–355.
Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods, 25: 402–408.
Markoff A, Gerke V. 2005. Expression and functions of annexins in the kidney. Am J Renal Physiol, 289: 949–956.
Mussunoor S, Murray GI. 2008. The role of annexins in tumour development and progression. J Pathol, 216: 131–140.
Odelberg SJ. 2005. Cellular plasticity in vertebrate regeneration. Anat Rec B New Anat, 287: 25–35.
Okuse K, Malik-Hall M, Baker MD, Poon WY, Kong H, Chao MV, Wood JN. 2002. Annexin II light chain regulates sensory neuron-specific sodium channel expression. Nature, 417: 653–656.
Price J, Allen S. 2004. Exploring the mechanisms regulating regeneration of deer antlers. Philos Trans R Soc Lond B Biol Sci, 359: 809–822.
Rescher U, Gerke V. 2004. Annexins-unique membrane binding proteins with diverse functions. J Cell Sci, 117: 2631–2639.
Yamada A, Irie K, Hirota T, Ooshio T, Fukuhara A, Takai Y. 2005. Involvement of the annexin II-S100A10 complex in the formation of E-cadherin-based adherens junctions in Madin-Darby canine kidney cells. J Biol Chem, 280: 6016–6027.
DOI 10.1007/s11676-014-0542-2
Project funding: This research was funded by the General Program of the National Natural Science Foundation of China (31271324), and the Fundamental Research Funds for the Central Universities (2572014EA05-03), and the Fundamental Research Funds for the Central Universities (DL10BA08).
The online version is available at http://www.springerlink.com
Li Hao, Xianghong Xiao, Heping Li ()
College of Wildlife Resource, Northeast Forestry University, 26# Hexing Road, Harbin 150040, China. Email: 89187762@qq.com
Corresponding editor: Hu Yanbo
杂志排行
Journal of Forestry Research的其它文章
- Growth and yield of two grain crops on sites former covered with eucalypt plantations in Koga Watershed, northwestern Ethiopia
- Carbon stock in Korean larch plantations along a chronosequence in the Lesser Khingan Mountains, China
- Biomass accumulation and nutrient uptake of 16 riparian woody plant species in Northeast China
- Cloning and sequence analysis of nine novel MYB genes in Taxodiaceae plants
- Genetic and morphological variation in natural teak (Tectona grandis) populations of the Western Ghats in Southern India
- Improved salt tolerance of Populus davidiana × P. bolleana overexpressed LEA from Tamarix androssowii
