Nest site selection of white-naped crane (Grus vipio) at Zhalong National Nature Reserve, Heilongjiang, China
2014-09-06QingmingWUHongfeiZOUJianzhangMA
Qing-ming WU · Hong-fei ZOU · Jian-zhang MA
ORIGINAL PAPER
Nest site selection of white-naped crane (Grus vipio) at Zhalong National Nature Reserve, Heilongjiang, China
Qing-ming WU · Hong-fei ZOU · Jian-zhang MA
Received: 2013-03-15; Accepted: 2013-07-09
© Northeast Forestry University and Springer-Verlag Berlin Heidelberg 2014
White-naped crane (Grus vipio) is a globally threatened species. It is very important to analyze its nest site selection in circumstances where there are multiple disturbances, and also helpful to accumulate valuable information about this threatened species and supply scientific suggestions for conservation and management. We studied nest site selection and the effects of environmental variables on nesting habits of white-naped crane at Zhalong National Nature Reserve, Qiqihar City, Heilongjiang, China, during March-May of 2002−2008. White-naped crane responded and adapted to changes in the quality of the spatial environments of landscape and microhabitat under multiple environmental disturbances. Nest site selection included two scales and two choices, namely the choice of nest site habitat type within the macro-habitat scale and nest site micro-habitat selection within the micro-habitat scale. Nest sites were recorded only in reed marshes. The choice of nest site micro-habitat included three basic elements and six factors, namely incubation element (nest parameters factor, incubation temperature factor and incubation humidity factor), safety element (protection factor and concealment factor), and food element (water factor). Water, remnant reed clusters, and fire were major resource management challenges during the breeding period for the white-naped crane in this Reserve.
factor analysis, environmental variable, Grus vipio, nest site selection pattern, Zhalong Nature Reserve
Introduction
The breeding period is an important stage in avian life history that directly affects changes in avian populations (Lack 1933). The nest is the location for avian incubation and environmental variables around a nest are important factors affecting breeding success (Dalley et al. 2009; Rangel-Salazar et al. 2008). Thus, nest site selection is an important choice that influences natural selection (Cody 1981). Identification and conservation of nest site habitat features that can influence reproduction and survival are also essential for management and long-term viability of bird populations (Svardson 1949).
Various scholars have described patterns of habitat selection by birds. Hilden (1965) and Svardson (1949) considered that habitat selection should include two processes: rough selection to the general features of the environment versus differing environments, and then selection in terms of more subtle features of that environment. Johnson (1980) defined four scales of habitat selection: the geographical range of a species, the home range of an individual or colony within the geographic range, the different habitat types used by the animal within the home range, and the securing of food items or the selection of nest sites from those available within the different habitat types. Chu and Zheng (1993) considered that avian habitat selection involved three levels: the avian geographic region, living environment within the geographic region, and sites for life activities within the living environment. Yang et al. (2000) considered that avian breeding habitat selection, especially nest site selection, depended primarily on vegetation structure at the small scale, for instance, vegetation coverage around the nest site and vegetation height. Many reviews of avian habitat selection conclude that spatial scale influences the perception of habitat availability and the sense of habitat selection (Clark and Shutler 1999; Jones 2001; Zhang and Li 2005).
White-naped crane (Grus vipio) is a globally threatened species (VU, IUCN 2009). The literature includes reports on white-naped crane migration routes (Fujita et al. 2004), stopoversites (Guan 2001; Higuchi et al. 1994), wintering habitat, feeding habitat, and habitat preference during the breeding period (Wu & Zou 2009; Zou and Wu 2006; Zou et al. 2005; Fujita et al. 1994). However, information on nesting, particularly nest site selection, nest site habitat type, and nest site micro-habitat is unavailable.
Zhalong National Nature Reserve (ZNNR) is listed as an internationally important wetland under the Ramsar Convention and is a major breeding area for white-naped crane (Su et al. 2000). Since 1997, various severe environmental disturbances such as drought, fire, and water replenishing have occurred here. In particular, fires typically occur in ZNR every year. All of these disturbances have made great changes in the habitat quality at ZNNR. White-naped crane has, however, continued to nest here. In China, the breeding area of red-crowned crane overlaps that of white-naped crane. Zou and Wu (2009) reported that red-crowned crane at ZNNR adapts to changes in the spatial pattern of landscape and microhabitat during the breeding season. The capacity of white-naped crane to adapt to these changes was previously unreported.
It is important to understand the physical features of nest sites and the nesting micro-habitat preferences of white-naped crane to design management strategies for conservation of the species and, more importantly, to conserve the required wetland resources. In this study, we report on nest site selection of white-naped crane and assessed the effects of environmental variables on nest site habitat selection in the face of multiple environmental disturbances at ZNNR in northeast China.
Materials and methods
Study site description
ZNNR is located southeast of the city of Qiqihar in Heilongjiang province, northeast China (46°52′−47°32′ N, 123°47′−124°37′ E), and was listed as a Wetland of International Importance, especially as Waterfowl Habitat in 1992 by the Ramsar Convention Secretariat. The region has a continental, semiarid, and monsoon climate. ZNNR covers a total area of 2,100 ha at an average of 144.0 m above mean sea level. The vegetation types consist of reed marsh (Phragmites australis), covering 80−90% of the reserve, carex swamp (Carex pseudoeuraica), meadow (Leymus chinensis), and grassland. The reeds in the many natural lakes became major economic resources of the local villagers, and also served as the main nesting material, respectively, for many waterfowl species, and particularly for the cranes. Fires are set every year to deal with reeds of low economic-value that grow at low densities and heights. These factors influence the reproduction and survival of all kinds of waterfowl (Zou et al. 2007).
Our surveys took place throughout the nature reserve, and our main observations were made at five sites (Fig. 1): (1) Zhalong, (2) Laomachang, (3) Dangnai, (4) Tumutai, and (5) Shijiadian.
Data collection
First, we sought breeding pairs of white-naped crane by using 8×20 binoculars and a 20×60 spotting scope near the five main observation sites within the reserve during March-May of 2002-2008. Then, we differentiated various breeding pairs by behavioral characteristics such as territorial behavior and incubating behavior, and observed them repeatedly until nesting. Nest site locations were determined on foot by natural markers on the ground and a range finder, a 20× telescope, and positioning by GPS. We measured and recorded environmental variables by plot sampling. We also sampled contrasting plots by the same method outside white-naped crane nesting territories (Li and Li 1998).

Fig. 1: Map of observation sites at Zhalong National Nature Reserve
Plot sampling was undertaken as follows. The nest site was deemed to be the center point O, through which two perpendiculars were aligned in arbitrary directions, to delimit one 1 m × 1 m square centered on point O and at 5 m and 10 m distances from point O. Measured environmental variables (Zou et al. 2005; Zou and Wu 2006; Zou et al. 2007) included (1) nest site habitat type, (2) distance from an interference region (km, X1, the distance between the nest site and the nearest man-made movement area, such as a settlement, road, hut for fish management, dyke, or large driveway), (3) distance from a lake (m, X2, the distance between the nest site and the nearest water body more than 1 m2in area) and the area of that lake (m2, X3), (4) distance from a fire zone (km, X4, the distance between a nest site and a fire zone within 2.5 km) and the area of the fire zone (m2, X5), (5) distance from remnant reeds (m, X6, the distance between a nest site and the nearest remnant reeds) and remnant reed area (m2, X7), remnant reed density (stems/m2, X8), remnant reed height (cm, X9,), (6) water depth (cm, X10, the average natural water depth in the sampling plot), (7) vegetation height (cm, X11, the average natural height of vegetation in the sampling plot), (8) vegetation density (stems/m2, X12, the average density of vegetation in the sampling plot), (9) vegetation coverage (%, X13, the proportion of base area of vegetation in the sampling plot), and (10) elevation (m, X14, the average elevation of the sampling plot).
Data analysis
A univariate analysis of environmental variables was performed to test the specificity and significance of nest site habitat selection of the white-naped cranes using one-way analysis of variance (ANOVA) and an Independent-Samples T Test.
The influence factors on nest site habitat selection were subsequently analyzed by the program Data Reduction Factor Analysis. The standard of factor selection was that eigenvalues of the factors after Data Reduction be greater than 1.
All statistical analyses were carried out using SPSS software (ver. 11.5) and presented as means ± standard error. P values less than 0.05 were considered significant.
Results
Nest site habitat types
By repeated observation of white-naped crane foraging activity and natural markers (e.g., surplus reed cluster, irrigation ditch, fire zone), 33 white-naped crane nests were positioned and subsequently confirmed by GPS. The habitat type of all nest sites was reed marsh. The same breeding cranes were found to build nests at different sites within the same nesting territory each year. Randomness of nest site micro-habitat selection
The measured environmental variables significantly influenced nest site selection (F =24.099, p <0.01), indicating that nest site selection was specific and not random. The Independent-Samples T Test showed notable differences between environmental variables at nest sites and those at contrasting plots. Distance from a fire zone, distance from remnant reeds, remnant reed area, remnant reed height, vegetation height, and vegetation coverage showed marked differences (p <0.01) between nest site habitat and contrasting plots (Table 1). Distance from an interference region, area of the nearest lake, and remnant reed density also showed significant differences (p <0.05); while other factors such as distance from a lake, fire zone area, water depth, vegetation density, and elevation did not (Table 1).
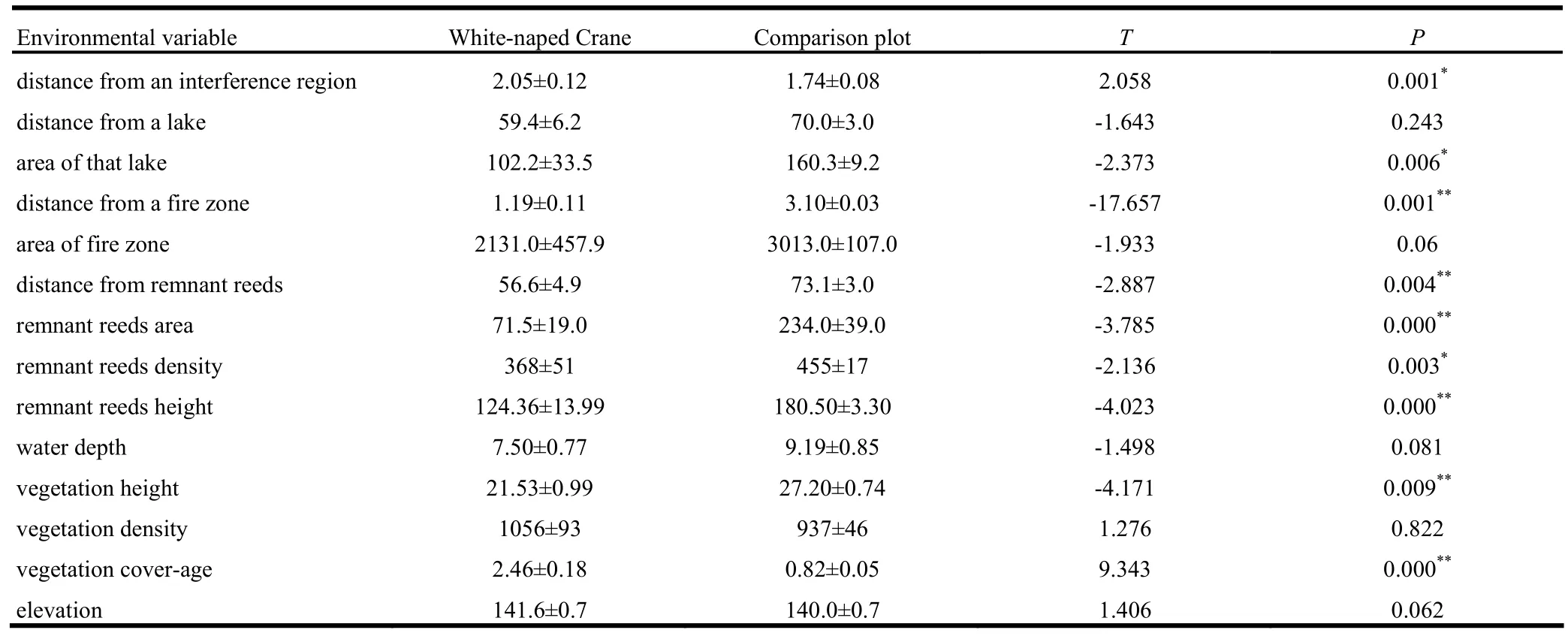
Table 1: Significance test of environment variables between nest site habitat and comparison plot sampled.
Factor analysis of nest site micro-habitat selection
Data Reduction Factor Analysis indicated that eigenvalues of the first six factors were greater than 1, with less relativity, and eigenvalues of all other factors were less than 1, with more relativity (Fig. 2). Therefore, the first six factors were selected. The cumulative contribution rate of the first six factors was 83%, which meant 83.486% of the information contained in all environment variables was covered by the first six factors (Table 2). The first six factors were used to calculate the relevant eigenvectors (Table 2), and finally defined as (1) nest parameters, (2) protection factor, (3) food, (4) incubation temperature, (5) concealment, and (6) incubation humidity. The newly defined six factors represented the primary information related to nest site selection by white-naped cranes.
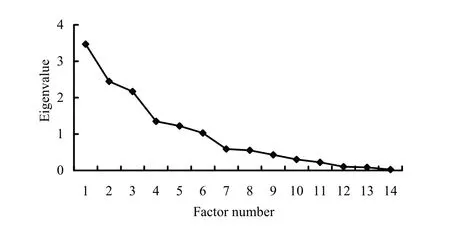
Fig. 2: Eigenvalues from the factor analysis of nest site habitat factors of breeding white-naped cranes

Table 2: Principal component analysis of nest site habitat factors of white-naped cranes.
The first factor, nest parameters, included distance from a lake, fire zone area, remnant reed area, and water depth. Their coefficients were larger than those of other factors so these were defined as the nest parameter factor. The nest is an important location for white-naped crane to lay and incubate eggs. Environmental qualities that directly affect nest quality also inevitably affect egg incubation. For the white-naped crane, the nest material comes mostly from reeds. Thus, remnant reeds not harvested by villagers because of their limited economic value provided most of the nesting material for cranes. Large areas of remnant reeds than can be used by cranes to build nests can benefit egg incubation. All burned regions had large areas of remnant reeds but white-naped crane used only smaller areas (p <0.01). The larger areas had higher probability of being burned than did the smaller areas of remnant reeds and this increased nesting success for white-naped crane. We also found that the regions near remnant reeds, ice and snow thawed earlier and lakes formed earlier. Larger areas of remnant reeds, larger lakes, deeper water, and a lower probability of being burned, such an environment would be expected to be more favorable for white-naped cranes to nest. Thus, distance from a lake, fire zone area, remnant reed area, and water depth will affect the area of remnant reeds, which indirectly affect the availability of the white-naped crane’s nesting materials.
The second factor, protection, included density and height of remnant reed. Adult white-naped cranes guard the nest and eggs during incubation and hatching. The density of remnant reeds was significantly lower than that of harvested reeds (p <0.05). In addition to reeds, there was also guinea grass (Genus species) growing to 1 m height within remnant reed areas. The grass height was slightly higher than the height of a white-naped crane sitting in the nest (nest height 12−16 cm, neck length 30−50 cm, and body height 20−30 cm, for a total of 62−96 cm). The height of remnant reeds (124.36±13.99 cm) was greater than the body height of incubating white-naped cranes. If a crane stretched its neck when sitting on the nest or stood on the nest, the reeds concealed both the nest and the incubating adult. Thus the density and height of remnant reeds were also favorable as protection for cranes during the nesting period.
The third factor, food, included the area of a lake, distance from a fire zone, and vegetation coverage. Vegetation coverage was related to vegetation density, but they made different contributions to nest site selection by cranes. When one member of the adult pair was foraging and had not returned to take over incubation, the incubating adult would leave the nest and forage nearby in the lake. When the lake dried reed coverage declined to zero and the habitat became a carex marsh or guinea grass marsh. Cranes were unlikely to select such habitats for nesting. At the other extreme, when reed coverage was 100%, cranes had nowhere to forage and would leave instead of continuing to nest. Ideal nesting habitats were those that provided favorable supplies of nearby food during the hatching and pre-fledging periods. The germination of reeds in a fire region occurred earlier than in an unburned region. New reeds in a fire region often grew very fast and vigorously, and this increased the food supply for young fledglings. Thus, these three components of factor three, lake area, distance from burn, and vegetation coverage, were related to short-term food supply for adult and fledgling crane foraging, and were defined as the food factor.
The fourth factor, incubation temperature, included distance from remnant reeds and vegetation height. Temperature and humidity are two important influence on bird egg incubation. Vegetation of adequate height sheltered nests by forming a windbreak that moderated temperature extremes. But in tall,dense stands of reeds, ground temperature was lower in spring because of shading. These two variables affected the temperature of eggs in nests, and this factor was defined as the incubation temperature factor.
The fifth factor, concealment, included the distance from an interference region and elevation. White-naped crane is sensitive to disturbances caused by man and domestic animals because of its timid nature, especially during incubation. To reduce extraneous disturbances and increase nesting success, nesting away from an interference region (2.05±0.12 km) was an adaptive strategy for white-naped cranes. In addition, the elevation of the nest site (141.6±0.7 m) was slightly lower than the 144-m average elevation of the reserve, which might have contributed to nest concealment. These two factors reduced nest disturbance and increased nest concealment, and were defined as the concealment factor.
The sixth factor, incubation humidity, included vegetation density. Reed stands of low density and height had little economic value to villagers, and were difficult to harvest. Thus, they became remnant reeds. Reed stands of high density, height, and value were usually harvested. Thus, the density of reeds was a key factor in determining their economic value and whether they would be harvested. Where water levels were suitable, reeds grew well and had higher economic value, increasing the chance they would be harvested. However, where water was too low, reeds grew poorly, had little economic value, and became remnant reeds. To some extent, if a site had dense reed vegetation, it meant there was water. In the nesting period, water is important for hatching white-naped crane eggs. Vapor from ground water can wet the eggs through the nest and provide humidity required for hatching. Sunlight can also affect the water-evaporating capacity and humidity. Furthermore, the micro-environment formed by the body of the incubating adult, the nest, and the ground water can adjust the humidity. Thus, we defined this factor as the incubation humidity factor.
Discussion
Nest site habitat type
Reed marsh was the major vegetation type at ZNNR, and its distribution was related to the distribution of water. Existence of large water bodies influenced local agricultural production because areas with little or no standing water were converted to farmland. There were some areas with standing water that retained natural vegetation and had limited human disturbance. These were distant from farms and villages. Reed marshes with these features had less disturbance during nesting and incubation, and higher nesting success. Remnant reeds and other related habitat features at ZNNR not only provided abundant nest material for white-naped cranes, but also enhanced incubation conditions, including temperature and humidity. Additionally, water and food of reed marshes at ZNNR supplied energy for nesting white-naped cranes, and also met the demand for brooding of nestlings. To some extent, if a region has dense reed vegetation, there must be sufficient water. In the nesting period, water is particularly important for successful hatching of white-naped crane. Sunlight affects the rate and capacity for evaporation, and thus influenced nest humidity. Vapor from ground water increased the humidity of the eggs through the nest, and this enhanced hatching. So, the natural habitat conditions of reed marsh at ZNNR provided white-naped cranes the need of nesting, incubation and brooding.
Nest site micro-habitat selection
Environmental qualities directly affected nest quality, and the success of incubation. When white-naped cranes selected nest sites, the factors such as nest material availability, incubation humidity, and incubation temperature were important. The main factors influencing nest site micro-habitat selection were described as: nest parameters factor, protection factor, food factor, incubation temperature factor, concealment factor, and incubation humidity factor.
Nesting material provides the platform for eggs and their incubation, and therefore its parameters directly affect hatching success. Incubation humidity and temperature were two important factors for egg incubation. Reeds of suitable height provided distance between the nest and the ground, and formed a windbreak that moderated temperature and humidity of the eggs in the nest. These factors directly affect the success of incubation (Qiao et al. 2002; Wu and Zou 2009). Human activities such as reed harvest and others at ZNNR directly affected egg incubation success of white-naped crane. At ZNNR, reed stands of high density and height were harvested for their high economic value. The main factor promoting reed growth was water availability.
Protection factors and concealment factors were related to the successful incubation. Protecting is an instinctive behavioral response of animals to enhance reproduction and survival (Feng et al. 2005; Hou et al. 2007). Nest protecting during incubation was independent of the degree of concealment. Concealment factors were directly related to both nest site selection and hatching of white-naped cranes. This was a safeguard for white-naped crane nesting success.
The food factor included both food and water (Dai et al. 2007). Brooding adult cranes would leave the nest to forage in the immediate vicinity of the nest when the foraging mate did not return to the nest. In the prophase of brooding, adult cranes led the young cranes to feed around the nest.
Nest site selection pattern
Habitat selection theories distinguish between macro-habitat and micro-habitat features. Nest site habitat selection by white-naped cranes nesting at ZNNR showed a similar pattern of habitat selection on macro- and micro-habitat scales. Macro-habitat selection at ZNNR was defined as the choice of nest site habitat type: nests were only in reed marsh. Micro-habitat selection enhanced hatching success. Successful incubation should have three conditions: no or little disturbance, proper incubation conditions, and proper brooding conditions. Our analysis of the main factorsinfluencing nest site micro-habitat selection was consistent with this: incubation element (nest parameters factor, incubation temperature factor, and incubation humidity factor), safety element (protection factor and concealment factor), and food element (namely food factor).
In summary, nest site selection by white-naped cranes was consistent with the general rules of avian habitat selection, despite environmental disturbances. White-naped cranes responded and adapted to changes in micro-habitat quality under multiple environmental disturbances. Nest site selection ZNNR occurred on two scales: the macro-habitat scale and the micro-habitat scale, the former was the choice of nest site habitat type, and the latter was realized as three elements through six factors defined as incubation element (nest parameters, incubation temperature and incubation humidity), safety element (protection and concealment), and food element (food).
Cody ML. 1981. Habitat selection in birds: The roles of vegetation structure, competitors and productivity. BioScience, 31, 107−113.
Dalley KL, Taylor PD, Shutler D. 2009. Success of migratory songbirds breeding in harvested boreal forests of northwestern Newfoundland. The Condor, 111(2): 314−325.
Feng J, Gao W, Sheng LX. 2005. Animal Ecology. Beijing: Science Press, 39−59. (In Chinese)
Fujita G, Harris J, Bold A, Tveenmayadag N, Chuluunbatar S. 1994. Habitat preference of Demoiselle and White-naped cranes breeding in Mongolia. In: Higuchi, H., Minton, J. (eds), The Future of Cranes and Wetlands. Tokyo: Wild Bird Society of Japan, pp.93−96.
Go FJ, Guan HL, Mutsuyuki U, Oleg G, Vladimir K, Kiyoaki O, Nagahisa M, Hiroyoshi H. 2004. Comparing areas of suitable habitats along traveled and possible shortest routes in migration of White-naped cranes in East Asia. Ibis, 146: 461−474.
Guan H. 2001. Identification of potential stopover sites in China for migrating White-naped cranes by satellite imageries. MSc thesis, University of Tokyo.
Gunnar S. 1949. Competition and habitat selection in birds. Oikos, 1(2): 157−174.
Hilden O. 1965. Habitat selection in birds: A review. Annales Zoologici Fennici, 2: 53−75.
Hiroyoshi H, Kiyoaki O, Golovushkin K. 1994. The migration routes and important rest-sites of cranes satellite tracked from south-central Russia. In: Higuchi, H., Minton, J. (eds), The Future of Cranes and Wetlands. Tokyo: Wild Bird Society of Japan, pp.15−25.
Hou YX, Zhou LZ, Yang C, Wang QS. 2007. Disturbance to the oriental white stork (Ciconia boyciana) breeding in the wintering area. Zoological Research, 28(4): 344−352. (In Chinese)
Jason J. 2001. Habitat selection studies in avian ecology: a critical review. The Auk, 118(2): 557−562.
Ji X, Du WG. 2001. The effects of thermal and hydric environments on hatching success, embryonic use of energy and hatchling traits in a colubrid snake, Elaphe carinata. Comparative Biochemistry and Physiology Part A, 129: 461−471.
Li FM, Li PX. 1998. A comparative study on territories of White-naped crane and red-crowned crane. Current Zoology, 44(1): 109−111. (In Chinese)
Lu X. 2004. Anti-predation vigilance of individual Tibetan eared pheasants temporarily separated from the flocks. Acta Zoologica Sinica, 50(1): 32−36.
Qiao JF, Yang WK, Gao XY. 2002. A preliminary study on the egg temperature and female incubation behaviour in Houbara bustard population in Mulei Xinjiang. Zoological Research, 23(3): 210−213. (In Chinese)
Rangel-salazar JL, Kathy M, Peter M, Robert E. 2008. Influence of habitat variation, nest-site selection, and parental behavior on breeding success of Ruddy-capped nightingale thrushes in Chiapas, Mexico. The Auk, 125(2): 358−367.
Su HL, Lin YH, Li DQ, Qian FW. 2000. Status of Chinese cranes and their conservation strategies. Chinese Biodiversity, 8(2): 180−191. (In Chinese)
Wu QM, Zou HF. 2009. Forage habitat selection of White-naped crane during its incubation period in Zhalong wetland. Chinese Journal of Applied Ecology, 20(7): 1722−1728. (In Chinese)
Yang WK, Zhong WQ, Gao XY. 2000. A review of studies on avian habitat selection. Arid Zone Research, 17(3): 71−78. (In Chinese)
Yang Y, Chen WH, Jiang WG, Yang SJ, Peng GH, Huang TF. 2006. Effects of group size on vigilance behavior of wintering Common cranes. Zoological Research, 27(4): 357−362. (In Chinese)
Zhang MH, Li YK. 2005. The temporal and spatial scales in animal habitat selection research. Acta Theriological Sinica, 25(4): 395−401. (In Chinese)
Zou HoF, Wu QM. 2009. Internal distribution pattern of the nests and home ranges of Red-crowned cranes in Zhalong nature reserve. Acta Ecologica Sinica, 29(4): 1710−1718. (In Chinese)
Zou HF, Wu QM. 2006. Feeding habitat of Red-crowned crane and White-naped crane during their courtship period in Zhalong wetland. Chinese Journal of Applied Ecology, 17(3): 444−449. (In Chinese)
Zou HF, Wu QM, Jiao WY. 2007. Comparison of feeding habitat selection between semi-domestic and wild Red-crowned crane during the breeding period in Zhalong nature reserve. Journal of Northeast Forestry University, 35(11): 56−59. (In Chinese)
Zou HF, Wu QM, Niu MG. 2005. Comparison of feeding habitat selection between the wild and semi-domestic White-naped crane during the pre-breeding period in Zhalong wetland. Chinese Journal of Zoology, 40(4): 45−50. (In Chinese)
DOI 10.1007/s11676-014-0541-3
Project funding: The project was financially supported by the Fundamental Research Funds for the Central Universities (No. 2572014CA05 and DL12EA04), National Natural Science Foundation of China (No. 31401978 and 31070345), China Postdoctoral Science Foundation (No. 2011M500631), and Heilongjiang Postdoctoral Foundation (No. 520-415268).
The online version is available at http://www.springerlink.com
Qing-ming WU, Hong-fei ZOU (), Jian-zhang Ma
College of Wildlife Resource, Northeast Forestry University, Harbin 150040, P. R. China. Email: hongfeizou@163.com
Corresponding editor: Hu Yanbo
杂志排行
Journal of Forestry Research的其它文章
- Growth and yield of two grain crops on sites former covered with eucalypt plantations in Koga Watershed, northwestern Ethiopia
- Carbon stock in Korean larch plantations along a chronosequence in the Lesser Khingan Mountains, China
- Biomass accumulation and nutrient uptake of 16 riparian woody plant species in Northeast China
- Cloning and sequence analysis of nine novel MYB genes in Taxodiaceae plants
- Genetic and morphological variation in natural teak (Tectona grandis) populations of the Western Ghats in Southern India
- Improved salt tolerance of Populus davidiana × P. bolleana overexpressed LEA from Tamarix androssowii
