Effects of submergence in water on seed germination and vigor of the Copaifera lucens (Fabaceae) seedlings
2014-09-06DanielaBaldezVidalIsisLeiteAndradeEusiniaLouzadaPereiraAndradeMarceloSchrammMielke
Daniela Baldez Vidal · Isis Leite Andrade · Eusinia Louzada Pereira Andrade Marcelo Schramm Mielke
ORIGINAL PAPER
Effects of submergence in water on seed germination and vigor of the Copaifera lucens (Fabaceae) seedlings
Daniela Baldez Vidal · Isis Leite Andrade · Eusinia Louzada Pereira Andrade Marcelo Schramm Mielke
Received: 2013-03-01; Accepted: 2013-12-03
© Northeast Forestry University and Springer-Verlag Berlin Heidelberg 2014
We analyzed the tolerance of Copaifera lucens seeds to submersion in water to assess the use of this species for direct seeding in riparian forest restoration programs. Seeds were submerged in water for 2, 4, 8, 16 and 32 days or not submerged (control = 0 days of submergence). For the control and at the end of each period of submersion, germination and seedling vigor tests were carried out. For germination tests, seeds were sown in plastic pots containing sand and kept in laboratory conditions. The percentage of seed germination, the germination rate and the average germination time were analyzed. For seedlings, total biomass, leaf area, leaf mass per area and leaf area ratio were analyzed. Submersion time drastically affected the dissolved oxygen content and seed germination. Between 4 and 8 days of submersion there was a decrease from 83.8% to 15.6% in the germination percentage. No seed germination occurred after 16 days of submersion. Although there was a significant decrease in the percentage of seed germination between 4 and 8 days of submersion, seedling vigor was not affected. Seeds of this species were partially tolerant to submersion in water, suggesting that C. lucens is a promising species for direct seeding in riparian forest restoration projects.
Copaiba, Brazilian Atlantic Forest, forest restoration, germination, vigor
Introduction
Riparian forests occur along waterways and function as regulators of water flow, sediment and nutrients between the upper parts of the watershed and the aquatic ecosystems (Kozlowski 2002). Because riparian forests are located near watercourses, these ecosystems are highly unstable forest formations and susceptible to periodic flooding (Budke et al. 2008). Although protected by law, most of the riparian forests of the Brazilian Atlantic Rainforest are degraded and require the results of scientific studies to provide basic information applicable to forest restoration programs (Mielke et al. 2005; Lavinsky et al. 2007).
Knowledge of the environmental requirements for tree species to be used for restoration of degraded riparian forests can assist in developing strategies that enhance the establishment of seedlings, reduce costs and increase the success of restoration. Considering expense, convenience, and ease of planting, direct seeding is a promising technique that has been tested for restoration of forest ecosystems (Engel and Parrotta 2001; Camargo et al. 2002; Woods and Elliot 2004; Doust et al. 2008; Bonilla-Moreno and Holl 2010; Cole et al. 2011), including riparian forests (Santos Jr et al. 2004; Ferreira et al. 2009). Studies of the viability of seeds of forest species subjected to submersion in water can be useful for the planning and success of projects to recover degraded riparian forests.
Seed germination is one of the main factors affecting the management of native forests, both for natural regeneration and for production of seedlings in forest nurseries (Schmidt 2007). The effectiveness of seed germination is closely related to seed requirements such as water availability, gas composition, and temperature (Bewley and Black 1994). Water is usually the limiting factor for the germination of non-dormant seeds, and affects the rate, speed and uniformity of the process. Adequate supply of water to the seed is associated with the mobilization of reserves and release of energy through respiration, and stimulates theactivity of enzymes and plant hormones (Baskin and Baskin 1998).
Even though most seeds do not require oxygen concentrations above 10% for germination, lower levels can cause problems (Bewley and Black 1994; Baskin and Baskin 1998). In case of flooding, respiration of aerobic organisms associated with the low diffusion of oxygen in water leads to a rapid decline in available oxygen resulting in hypoxia or anoxia (Bailey-Serres and Voeseneck 2008). Limited oxygen supply to seeds during all stages of germination induces changes in aerobic respiratory rates, causing increased fermentation, or anaerobic pathway, producing lactic acid and ethanol (Crawford 1992; Lobo and Joly 1998; Kolb and Joly 2010), which are toxic and can lead to cell death and loss of seed viability.
Plants of the genus Copaifera are popularly known as ‘copaibeiras’ or ‘pau-d'óleo’, as they produce an oil-resin of medicinal and commercial importance (Veiga-Junior and Pinto 2002; Rigamonte-Azevedo et al. 2004). Copaibeiras grow to 10-40 meters tall and have dense foliage consisting of composite pinnate leaves, in alternate form, with coriaceous leaflets of 3-6 cm in length (Pio Corrêa 1984). Costa (2007) reported 38 species in the genus Copaifera, 22 of which are restricted to Brazil. These species occur naturally in a variety of ecosystems, such as upland forests, wetlands, lake shores and streams of the Amazon Basin, in the forests of Central Brazilian vegetation and Atlantic Forest (Rigamonte-Azevedo et al. 2004). Costa (2007) reported that Copaifera lucens Dwyer occurs exclusively in the eastern portion of Brazil, in areas of the Atlantic Rainforest in the states of Bahia, Espirito Santo, Minas Gerais, Rio de Janeiro and São Paulo. In southern Bahia, this species has been found in rainforests and semideciduous forests, primary and secondary formations, and riparian forests, and is recommended for use in forest restoration programs because the seed aril provides food for animals (Sambuichi 2009).
Despite the potential of direct seeding (Campbell et al. 2002; Santos Jr et al. 2004; Moreno-Bonilla and Holl 2010) and the indications for the use of species in the genus Copaifera for the restoration of degraded riparian forests (Sambuichi 2009), to date no information is available in the literature about the viability of seeds of these species after submersion in water. Thus, this study was carried out to investigate the effect of submersion in water on seed germination and seedling vigor of C. lucens.
Materials and methods
Fruits of C. lucens were collected in August 2010 from trees located on ‘Terra Vista’ Settlement, Arataca, Bahia, Brazil. After collection, the fruits were taken to the laboratory of Plant Science, Universidade Estadual de Santa Cruz (UESC), Ilhéus, Bahia, Brazil. Seeds were manually extracted and those damaged in the mechanical process or by pests, diseases or impurities were discarded. Seeds were then sterilized in 70% ethanol for 1 minute, rinsed in distilled water, submerged in a commercial bleach solution for 10 minutes, washed again with distilled water and treated with the fungicide Nystatin for one minute. After sterilization, seeds were incubated for one hour on paper towels to remove surface water. After drying, measurements were taken for length, width, thickness, the weight of 1,000 seeds and the seed moisture content. Biometrics (length, width and thickness) were measured for 200 seeds using a caliper. The weight of 1,000 seeds was determined by eight replicates of 100 seeds (Brazilian Ministry of Agriculture and Food Supplies 2009). Seed moisture content was determined by drying the mass of seeds in an oven at 105 °C for 24 hours, with four replications of 10 seeds (Brazilian Ministry of Agriculture and Food Supplies 2009).
Seeds were submerged in distilled water for 2, 4, 8, 16, and 32 days or not submersed (0 days of submergence for controls). Six 1,000 ml beakers containing 186 seeds and 400 ml of distilled water each were used. For the submersion treatments, the seeds were incubated in a germinator at 25 °C in the dark. During incubation periods, dissolved oxygen (DO) was monitored using a multiparameter water quality analyzer HI 9828 (Hanna, Woonsocket, Rhode Island, USA) at 0, 1, 2, 3, 4, 5, 6, 7, 8, and 24 hours, and 2, 4, 8, 16, 21, and 32 days after the beginning of treatments. For the control, seeds were sown immediately after biometric measurements were carried out. At the end of each submergence period, four replications of 40 seeds were subjected to germination tests. Seeds were sown in plastic containers (19 cm × 45 cm × 14 cm) with washed and sterilized sand as substrate and kept under laboratory conditions. A Hobo H8 Pro Series (Onset Computer, Bourne, Massachusetts, USA) sensor was used to monitor the environmental conditions inside the laboratory, and the average temperature and relative humidity during the experiment were 26.3 °C and 66%, respectively. Germinated seeds were counted every two days from the beginning of each germination test. The germination rate was expressed by the germination velocity index (GVI) (Maguire 1962) and average germination time (AGT) was calculated according to Edmond and Drapala (1958).
To evaluate the effects of seed submersion on the vigor of seedlings, at 23 days after the end of each germination test three normal seedlings per container were collected to have leaf area (LA), total biomass, leaf mass per area (LMA) and the leaf area ratio (LAR) measured. LA was estimated using an automatic leaf area meter LI-3100 (LI-COR, Lincoln, Nebraska, USA). Seedling biomass was obtained after drying plant material at 70°C until a constant mass was reached. LMA and LAR were calculated by the ratios between dry mass of leaves and leaf area, and between leaf area and total biomass per plant, respectively.
The experimental design was completely randomized with six treatments (submersion periods) and four replications. For germination tests, each experimental unit consisted of 40 seeds and for seedling vigor analysis each experimental unit consisted of three seedlings. The results expressed as percentages were transformed into arcsin (x/100)0.5. The results were analyzed using ANOVA followed by Tukey's test at a 5% probability level.
Results
The seeds used in this study had small variations in length, width and thickness (CV<10%), (Table 1), with a tendency to be longer than wide or thick. The results also indicated that 1,000 seeds of C. lucens weighed approximately 2.5 kg with a moisture content above 30%.
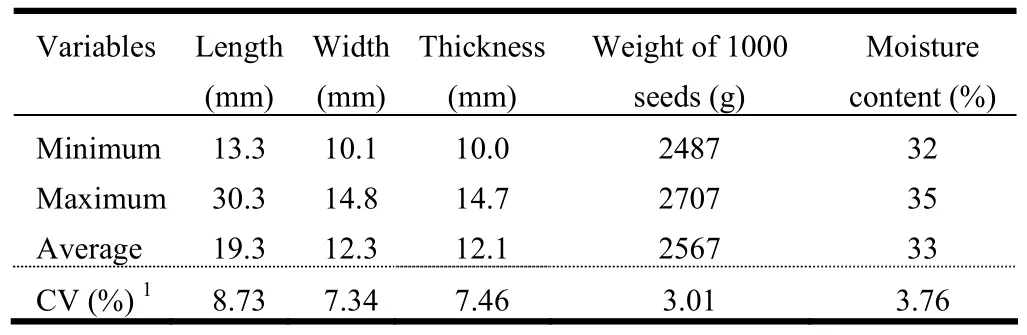
Table 1: Biometric variables, weight of 1,000 seeds and moisture content of C. lucens seeds.
There was a dramatic decrease in DO within the first hours after immersing the seeds in water, falling from approximately 70% to 20% within 8 hours of immersion (Fig. 1a). Approximately 16 hours after immersing seeds in water, DO reached 0% and remained around this value until 768 hours (32 days) after the beginning of the experiment (Fig. 1b). Thus, only the seeds of the control treatment (0 days of submergence) were in an environment with adequate oxygen levels to start the germination process.
For the control and for the treatments of submersion for 2, 4 and 8 days, seed germination began at 9, 7, 5, and 5 days after sowing, respectively (Fig. 2). No seed germination occurred after 16 days of submersion. Although only the control was at adequate levels of oxygen availability to start the germination process, anoxia did not affect seed germination for up to 4 days of submersion in water, since no significant differences were observed for the control and treatments of 2 and 4 days of submersion in water for G40 and AGT (Table 2).
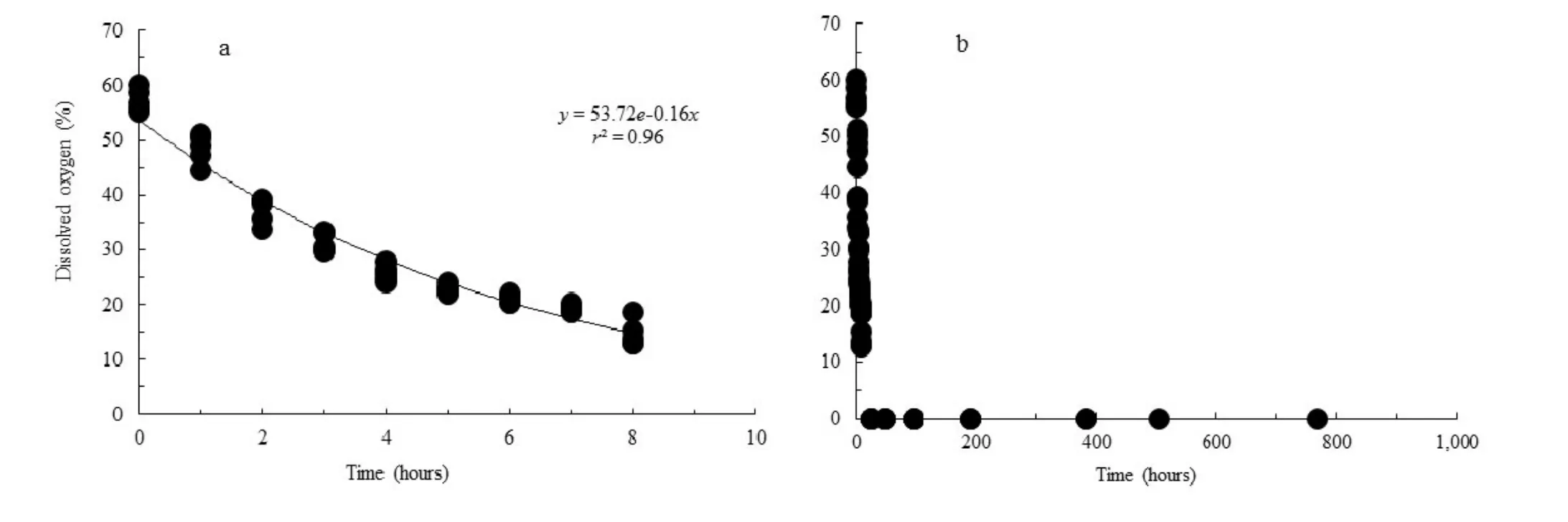
Fig. 1: Dissolved oxygen content in water as a function of submersion time of C. lucens seeds. Measurements taken every hour until 8 hours (a) and 24, 48, 96, 192, 384, 504 and 768 hours (1, 2, 4, 8, 16, 21 and 32 days, respectively) after the sinking of the seeds (b).

Fig. 2: Germination of C. lucens seeds as a function of the time of submersion in water.
GVI after 4 days of submersion was significantly higher than for the other treatments. The control seeds and seeds submerged for 2 days did not differ significantly in terms of GVI, but GVI was lower for the 8 day submergence treatment. There was no significant difference in AGT between untreated seeds and all other treatments. Although the mean AGT values did not differ between submersion periods, radicle protrusion for submersion of 4 and 8 days started at the same time (5 days after sowing), but immersing the seeds for 8 days significantly affected germination (Fig. 2). Submersion of seeds for 8 days led to significant increase in the number of dead seeds at end of the test (82%) and reduced the germination rate (Table 2, Fig. 2).
At 23 days after the end of germination tests (Table 3), when the seedlings had two fully expanded leaves, there were no significant differences between treatments for total dry biomass, LA, LMA and LAR.
Discussion
In a study of Copaifera multijuga, Brum et al. (2009) reported moisture in seeds of about 27.6%, a value approximating that observed in this study. However, the weight of 1,000 seeds measured in our study was over three times higher than the value obtained by Guerra et al. (2006) for C. langsdorffii. This difference may have been caused by seed biometrics since according to Guerra et al. (2006) seeds of C. langsdorffii were 13.23 mm long, 8.46 mm wide and 8.81 mm thick, smaller than C. lucens seeds (Table 1). Moreover, Brum et al. (2009) reported that seeds of C. multijuga were on average 20 mm long, 13 mm wide and 11 mm thick, very close to the results of our study for C. lucens. Information on seed biometrics and weight of 1000 seeds is important for direct seeding in forest restoration projects, providing information on the amount of seeds that each worker can take to the planting areas, as well as to calculate the amount of seed needed for the planting area.
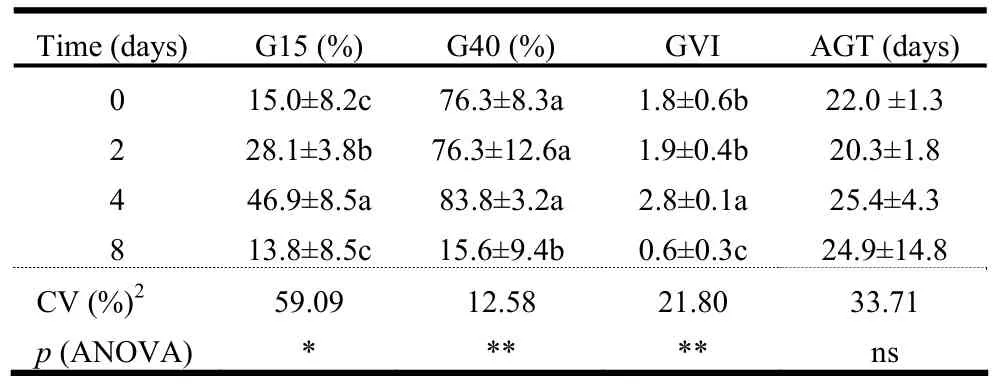
Table 2: Percentages of germinated seeds at fifteen (G15) and forty days (G40) after starting germination tests, germination speed index (GVI) and average seed germination time (AGT) at the end of the germination test (40 days) of C. lucens a function of time of submersion in water. Values are means ± standard deviation. (n =4).
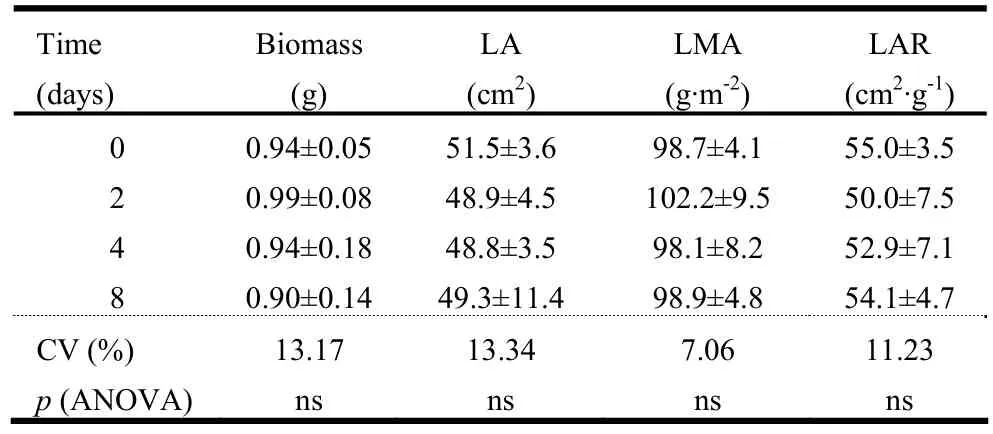
Table 3: Biomass, leaf area (LA), specific leaf mass (LMA), leaf area ratio (LAR) of C. lucens seedlings originated from the untreated seeds (0 days of submergence) and for the treatments of submersion for 2, 4 and 8 days. Values are means ± standard deviation. (n =4).
The beginning of the imbibition of water by the seed occurs due to the formation of the water potential gradient between the seed and the substrate, followed by a rapid increase in respiratory activity. In dry seeds, the amount of adenosine triphosphate (ATP) is extremely low but increases rapidly during imbibition due to aerobic respiration that is the main source of ATP before the emergence of the radicle. However, when seeds are deprived of oxygen, ATP is consumed rapidly, with no replacement due to the block of oxidation in the mitochondria terminal (Baskin and Baskin 1998). Thus, the rapid decline of dissolved oxygen, characterizing hypoxia and anoxia around 8 and 16 hours after immersing the seeds, respectively, may have been a consequence of their high respiration rate.
Noleto et al. (2010) reported that germination of C. langsdorffii began six days after sowing, approximating our results. However, for the controls, the values of G40 were lower than those obtained by Noleto et al. (2010) for C. langsdorffii, who reported germination rates of 83% 25 days after sowing.
Custódio et al. (2002) reported a decrease of approximately 50% in germination and vigor of Phaseolus vulgaris L. (Fabaceae) seeds after 8 hours of immersion in water. Moreover, an increase in the number of dead seeds and inhibition of root growth was observed as the submersion time increased. After 48 hours 80-90% of the seeds died and after 16 hours of immersion the length of the root and hypocotyl and the dry weight of the shoot decreased more than 90%. On the other hand, Okamoto and Joly (2000) found that seeds of Inga sessilis (Vell.) Mart. (Fabaceae) when subjected to anoxia for four days showed a decrease of about 30% in germination. When seeds were subjected to anoxia for 10 days there was practically no germination. Considering that P. vulgaris is intolerant to flooding (Schravendijk and van Andel 1985) and I. sessilis is partially tolerant, the results obtained in our study indicate that seeds of C. lucens can tolerate short periods of flooding.
LA, LMA and LAR are very important attributes associated with photosynthetic activity and growth in tropical tree seedlings (Poorter and Bongers 2006; Houter and Pons 2012). Also, a higher LMA is related to greater allocation of carbon to support and defense (Castro-Díez et al. 2000), enhancing seedling survival and growth when subjected to environmental stress (Poorter and Bongers 2006). Therefore, the lack of significant differences between treatments for total dry biomass, LA, LMA and LAR at 23 days after the end of the germination tests showed that, although there were differences between treatments for the germination tests, the submersion of seeds in water did not affect seedling vigor. Even though field tests are still necessary, these results have shown that seedlings of C. lucens from seeds submerged in water could have the same chance of survival and establishment as seedlings from non-submerged seeds.
Studies of temperate and tropical trees have shown that seed weight is an important trait associated with seedling survival and establishment (Khan 2004; Baraloto et al. 2005; Metz et al. 2010; Pérez-Ramos et al. 2010). Seed weight can also be related to seedling vigor after relatively long periods of submergence in water (Pérez-Ramos and Marañón 2009). Thus, the similar vigor of seedlings of control seeds in comparison with treatments of 2, 4 and 8 days of submersion may be related to the size and weight of C. lucens seeds. Even though immersing seeds in water reduced germination, under natural conditions germination andseedling establishment of this species are possible even after the occurrence of occasional flooding. This supports the option of using direct sowing as a technique (Santos Jr. et al. 2004) for the use of C. lucens in restoration projects of degraded riparian forests.
We conclude that the time of submergence drastically affected the dissolved oxygen content and the germination of C. lucens, and that between 4 and 8 days of immersion subsequent germination declined from 83.8 to 15.6%. On the other hand, although there was a significant decrease in the percentage of seed germination between 4 and 8 days of submersion, seedling vigor was not affected. Although further studies on the metabolism of C. lucens seeds under anoxic conditions and field tests must still be performed, our results indicate that seeds of this species are partially tolerant to submersion in water, suggesting that C. lucens is a promising species for direct seeding in riparian forests restoration projects.
Acknowledgements
Daniela B. Vidal was master’s degree scholarship student at the Foundation for Research Support of the State of Bahia (FAPESB). The authors acknowledge the Terra Vista settlement and biologists Jose Lima da Paixão and Michaele Pessoa for providing the seed lot used in this study. Marcelo S. Mielke thanks the Brazilian National Council for Scientific and Technological Development (CNPq) for a productivity fellowship. We also thank Dr. Carter R. Miller, Dr. Janisete G. Silva and the anonymous reviewers for constructive comments to help improve the manuscript.
Baskin CC, Baskin JM. 1998. Seeds: ecology, biogeography, and evolution of dormancy and germination. San Diego: Academic Press, p. 666.
Bailey-Serres J, Voeseneck LACJ. 2008. Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol, 59: 313−339.
Baraloto C, Forget PM, Goldberg DE. 2005. Seed mass, seedling size and neotropical tree seedling establishment. J Ecol, 93: 1156−1166.
Bewley DD, Black M. 1994. Seeds. Physiology of development and germination. New York: Plenum Press, p. 445.
Bonilla-Moheno M, Holl K. 2010. Direct seeding to restore tropical mature-forest species in areas of slash-and-burn agriculture. Restor Ecol, 18: 438−445.
Brazilian Ministry of Agriculture and Food Supplies. 2009. Rules for Seed Analysis. Brasília: Brazilian Ministry of Agriculture and Food Supplies, p. 399. (Portuguese)
Brum HD, Camargo JLC, Ferraz IDK. 2009. Copaíba-roxa, Copaifera mutlijuga Hayne. In: Ferraz, IDK, Camargo JLC. (eds), Manual of Amazonian Seeds. Manaus: National Institute of Amazonian Research (INPA), p. 12. (Portuguese with abstract in English)
Budke JC, Jarenkow JA, Oliveira-Filho AT. 2008. Tree community features of two stands of riverine forest under different flooding regimes in Southern Brazil. Flora, 203: 162−174.
Camargo JLC, Ferraz IDK, Imakawa AM. 2002. Rehabilitation of degraded areas of Central Amazonia using direct sowing of forest tree seeds. Restor Ecol, 10: 636−644.
Castro-Díez P, Puyravaud JP, Cornelissen JHC. 2000. Leaf structure and anatomy as related to leaf mass per area variation in seedlings of a wide range of woody plant species and types. Oecologia, 124: 476−486.
Cole RJ, Holl KD, Keene CL, Zahawi RA. 2011. Direct seeding of late-successional trees to restore tropical montane forest. Forest Ecol Manag, 261: 1590−1597.
Costa JAS. 2007. Taxonomic, biosystematic and phylogenetic studies in Copaifera species with emphasis on the extra-Amazonian Brazil. Feira de Santana: State University of Feira de Santana, p. 266. (Portuguese with abstract in English)
Crawford RMM. 1992. Oxygen availability as an ecological limit to plant distribution. Adv Ecol Res, 23: 93−185.
Custódio CC, Machado Neto NB, Ito HM, Vivan MR. 2002. The flooding effects on the bean seed germination and vigor of bean seeds. Rev Bras Sementes, 24: 49−54. (Portuguese with abstract in English)
Doust SJ, Erskine PD, Lamb D. 2008. Restoring rainforest species by direct seeding: Tree seedling establishment and growth performance on degraded land in wet tropics of Australia. Forest Ecol Manag, 256: 1178−1188.
Edmond JB, Drapala WJ. 1958. The effects of temperature, sand and soil, and acetone on germination of okra seed. P Am Soc Hortic Sci, 71: 428−434.
Engel VL, Parrotta JA. 2001. An evaluation of direct seeding for reforestation of degraded lands in central São Paulo state, Brazil. Forest Ecol Manag, 152: 169−181.
Ferreira RA, Santos PL, Aragão AG, Santos TIS, Santos Neto EM, Rezende AMS. 2009. Direct sowing for the implantation of a riparian forest at the lower San Francisco River in Sergipe State, Brazil. Scientia Forestalis, 37: 37−46. (Portuguese with abstract in English)
Guerra, MEC, Medeiros Filho, S, Gallão, MI. 2006. Seed, seedlings and germination morphology of Copaifera langsdorfii Desf. (Leguminosae-Caesalpinioideae). Cerne, 12: 322−328. (Portuguese with abstract in English)
Houter NC, Pons TL. 2012. Ontogenetic changes in leaf traits of tropical rainforest trees differing in juvenile light environment. Oecologia, 169: 33−45.
Khan ML. 2004. Effect of seed mass on seedling success in Artocarpus heterophyllus L., a tropical tree species of north-east India. Acta Oecol, 25: 103−110.
Kolb RM, Joly CA. 2010. Germination and anaerobic metabolism of seeds of Tabebuia cassinoides (Lam.) DC subjected to flooding and anoxia. Flora, 205: 112−117.
Kozlowski TT. 2002. Physiological-ecological impacts of flooding on riparian forest ecosystems. Wetlands, 22: 550−561.
Lavinsky AO, Sant’Ana, CS, Mielke, MS, Almeida, A-AF, Gomes, FP, França, S, Silva, DC. 2007. Effects of light availability and soil flooding on growth and photosynthetic characteristics of Genipa americana L. seedlings. New For, 34: 41−50.
Lobo PC, Joly CA. 1998. Tolerance to hypoxia and anoxia in neotropical tree species. Oecologia Brasiliensis, 4: 137−156.
Maguire J. 1962. Speed of germination-aid in selection and evaluation for seedling emergence and vigor. Crop Sci, 2: 176–177.
Metz J, Liancourt P, Kigel J, Harel D, Sternberg M, Tielbörger K. 2010. Plant survival in relation to seed size along environmental gradients: along-term study from semi-arid and Mediterranean annual plant communities. J Ecol, 98: 697−704.
Mielke MS, Almeida AAF, Gomes FP, Mangabeira PAO, Silva DC. 2005. Effects of soil flooding on leaf gas exchange and growth of two neotropical pioneer tree species. New For, 29: 161−168.
Noleto LG, Pereira MFR, Amaral LIV. 2010. Structural and physiological changes in seeds and seedlings of Copaifera langsdorffii Desf. (Leguminosae – Caesalpinioideae) submitted to sodium hypochlorite treatment. Rev Bras Sementes, 32: 45−52. (Portuguese with abstract in English)
Okamoto JM, Joly CA. 2000. Ecophysiology and respiratory metabolism during the germination of Inga sessilis (Vell.) Mart. (Mimosaceae) seeds subjected to hypoxia and anoxia. Rev Bras Bot, 23: 51−57.
Pérez-Ramos IM, Marañón T. 2009. Effects of waterlogging on seed germination of three Mediterranean oak species: Ecological implications. Acta Oecol, 35: 422−428.
Pérez-Ramos IM, Gómez-Aparicio L, Villar R, García LV, Marañón T. 2010. Seedling growth and morphology of three oak species along field resource gradients and seed mass variation: a seedling age-dependent response. J Ecol, 21: 419−437.
Pio Corrêa M. 1984. Dictionary of useful plants of Brazil and cultivated exotics. Rio de Janeiro: Brazilian National Press, p. 747. (Portuguese)
Poorter L, Bongers F. 2006. Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology, 87: 1733−1743.
Rigamonte-Azevedo OC, Wadt PGS, Wadt LHO. 2004. Copaíba: ecology and production of oil resin. Rio Branco: Brazilian Enterprise for Agricultural Research (Embrapa), p. 28. (Portuguese with abstract in English) Sambuichi RHR. 2009. List of native trees of south Bahia. In: Sambuichi RHR; Mielke MS, Pereira CE. (eds), Our Trees: Conservation, Uses and Management of Native Trees of South Bahia. Ilhéus: Editus, p. 171−257. (Portuguese)
Santos Júnior N, Botelho SA, Davide AC. 2004. Study of germination and survival of three species in direct seeding system, aiming riparian forest restoration. Cerne, 10: 103−117. (Portuguese with abstract in English)
Schmidt LH. 2007. Tropical Forest Seed. Berlin: Springer, p. 409.
Schravendijk HW, van Andel OM. 1985. Interdependence of growth, water relations and abscisic acid level in Phaseolus vulgaris during waterlogging. Physiol Plantarum, 63: 215−220.
Veiga-Junior VF, Pinto AC. 2002. The Copaifera L. genus. Quim Nova, 25: 273−286.
Woods K, Elliot S. 2004. Direct seeding for forest restoration on abandoned agricultural land in Northern Thailand. J Trop For Sci, 16: 248−259.
ORIGINAL PAPER
.
DOI 10.1007/s11676-014-0537-z
The online version is available at http:// www.springerlink.com
Daniela Baldez Vidal · Eusinia Louzada Pereira Andrade
Department of Agricultural and Environmental Sciences, State University of Santa Cruz, BR 415, km 16, Ilhéus 45662-300, Bahia, Brazil.
Isis Leite Andrade · Marcelo Schramm Mielke ()
Department of Biological Sciences, State University of Santa Cruz, BR 415, km 16, Ilhéus 45662-300, Bahia, Brazil.
Email: msmielke@uesc.br
Corresponding editor: Zhu Hong
杂志排行
Journal of Forestry Research的其它文章
- Growth and yield of two grain crops on sites former covered with eucalypt plantations in Koga Watershed, northwestern Ethiopia
- Carbon stock in Korean larch plantations along a chronosequence in the Lesser Khingan Mountains, China
- Biomass accumulation and nutrient uptake of 16 riparian woody plant species in Northeast China
- Cloning and sequence analysis of nine novel MYB genes in Taxodiaceae plants
- Genetic and morphological variation in natural teak (Tectona grandis) populations of the Western Ghats in Southern India
- Improved salt tolerance of Populus davidiana × P. bolleana overexpressed LEA from Tamarix androssowii
