Enhancement of seed germination in Macaranga peltata for use in tropical forest restoration
2014-09-06CassieRodriguesBernardRodrigues
Cassie R. Rodrigues · Bernard F. Rodrigues
ORIGINAL PAPER
Enhancement of seed germination in Macaranga peltata for use in tropical forest restoration
Cassie R. Rodrigues · Bernard F. Rodrigues
Received: 2013-01-08; Accepted: 2013-06-18
© Northeast Forestry University and Springer-Verlag Berlin Heidelberg 2014
We used pre-sowing treatments viz., soaking seeds in concentrated sulphuric acid (CSA), gibberellic acid (GA), combined treatment of CSA + GA and mechanical scarification to overcome seed dormancy and enhance synchronous germination of Macaranga peltata seeds. We analysed percent seed germination data by logistic regression and confirmed that except in GA treatment, time and acid concentration together were crucial for enhancing germination. The combination treatment of CSA and GA resulted in higher percent germination (up to 74%) than either treatment used separately, but produced defective seedlings (26%). Mechanical scarification of seed coat had the greatest impact in enhancing germination (78%) and reducing imbibition time (6 days) against the control (0%). Germination studies and SEM analysis confirmed that seed germination in M. peltata was inhibited by seed coat dormancy.
dormancy, gibberellic acid (GA), mechanical scarification, restoration, seed coat
Introduction
Pioneer tree species form an integral part of a wasteland restoration process. They are key players for facilitating the establishment of late successional species under their canopy and accelerate the restoration of forest ecosystem services. Macaranga is a large genus of the old world tropics consisting of approximately 250 pioneer plant species with its centre of distribution in sub-tropical Asia and Pacific (Eck et al. 2001). Most of the species are restricted to tree fall gaps, stream banks and unstable slopes in the primary forest, but are often common followingdisturbance, frequently dominating secondary forest (Whitmore 1967and 1969). Macaranga peltata (Roxb.) Mull. Arg.is a tropical tree of the family Euphorbiacea. It is a pioneer species for stand initiation and yields a mixed canopy early in stand development (Ashton et al. 2001). M. peltata is widely studied as an early secondary species for restoration in India and Sri Lanka (http://en.wikipedia.org/wiki/Macaranga_peltata) and is regarded as a native recoloniser. It is a light-demanding tree restricted to large canopy openings and is a disturbance tolerant species (Chandrashekara and Ramakrishnan 1993; Goodale et al. 2012), hence is suitable for revegetating large forest gaps. It commonly occurs as an early succession tree, growing rapidly to a height of 11 m (Goodale et al. 2009). In response to greater sunlight penetration at disturbed sites, it exhibits a high shoot:root ratio thus facilitating efficient light capture and faster growth (Chandrashekara and Ramakrishnan 1993). It produces large amounts of leaf litter which rapidly decompose (Sundarapandian and Swamy 1999) and contributes to an increase in soil organic matter in nutrient deficient areas. Recruitment of viable seeds, germination, seedling establishment and growth are indicators of regeneration potential of a plant species. Macaranga peltata produces light weight seeds and has responds well to coppicing, thus readily invades nearby areas. Tropical forests of the Western Ghats in India have greater germinable seed banks of M. peltata outside the monsoon period, i.e., during October to December, however, staggered germination over a period of five months has been recorded (Chandrashekara and Ramakrishna 1993). In nature, seeds of M. peltata mature during April−May and are dispersed by birds just prior to the onset of monsoon. Murali (1997) noted that seeds of M. peltata were viable for a period of 15 months in field conditions and germination began 23 days after dispersal. The hard seed coat contains phenolic compounds and might confer protection against fungal and parasitic attacks, thereby reducing the rate of field deterioration (Haris 1987).
Although M. peltata is a promising candidate for revegetation and reclamation of wastelands in tropical Asia (Ashton et al. 2001), its asynchronous, slow germination reduces its utility in restoration, where large numbers of viable seedlings produced rapidly and on predetermined time frames are required. There-fore, the knowledge of seed germination and requirements for breaking dormancy are essential for restoration projects where there is often a limited supply of seeds (Marques and Draper 2012) and seedlings. Better understanding of germination traits of forest tree species is important for achieving revegetation objectives in forest management and ecological restoration.
Physical dormancy is common in most hard-seeded plants and is imposed by the seed coat. It is an ecological mechanism to allow seeds to germinate only when conditions are suitable for supporting seedling growth. The hard seed coat in many early secondary species might be a mechanism to prevent seed damage during dispersal rather than for building persistent soil seed banks (Chandrashekara and Ramakrishnan 1993). However, this is a limitation when prompt germination is required at high percentages. Since little information is available on the germination characteristics of M. peltata seeds and the technique/s for breaking dormancy, this study was aimed at evaluating the effectiveness of various seed treatments in increasing percent seed germination and at determining the causes of dormancy in M. peltata.
Materials and methods
Collection and storage of fruits
Fruits of M. peltata were collected in May from trees growing around Goa University campus. The seeds were separated from the outer oily black layer and sun dried to constant weight. Moisture content of fresh seeds was determined by oven drying (103°C for 17 h) and expressed as percentage of water on a fresh weight basis (ISTA 1996). Viability of fresh seeds was assessed using the tetrazolium test (Moore 1985). Embryo morphology was studied in a small sample of seeds after dissection. Dried seeds were stored in zip lock polythene bags at 27°C in the dark until commencement of the experiment (one week).
Experimental design for seed germination
A total of 21 pre-sowing seed treatments were tested in addition to the control and 50 seeds were used per treatment. The pre-sowing treatments were:
Sulphuric acid treatment
Seeds were scarified with commercial grade concentrated H2SO4(CSA) for five time intervals, viz., 5, 10, 15, 20 and 25 min.
GA treatment
Seeds were treated with three concentrations of GA, viz. 100, 200 and 300 μg·L-1for 2h.
Combined treatment (CSA + GA)
Following treatment with CSA for four time intervals (15, 20, 25, and 30 min), seeds were treated with three concentrations of GA (100, 200 and 300 μg·L-1) for 2 h.
Mechanical scarification
The hard sclerotic dark brown seed coat was mechanically removed (exposing the endosperm) by applying pressure using a micrometer screw gauge with care taken not to damage the embryo.
Following treatments, the seeds were rinsed with distilled water, surface sterilized using 0.1% mercury chloride for 2 min and placed in sterile Petri plates lined with moist filter papers. Plates were incubated at 27°C at 16 h of photoperiod and germination percentage was recorded daily. Seed imbibition time was recorded as the number of days from treatment to commencement of germination. Germination was defined as emergence of a radical at least 2 mm long (Mackay et al., 1995).
Exomorphic micro-characterisation of seeds
The seed coat of M. peltata was studied using Scanning Electron Microscopy (SEM). Treated and control seeds were washed thoroughly with distilled water and air dried for 24 h prior to SEM analysis. Seeds were mounted on clean dry aluminium stubs using double sided adhesive carbon tape. A coat of platinum was sprayed on the seeds using a JEOL 1600 auto fine coater device. Coated seeds were then examined and photographed using JEOL JSM–6360LV scanning electron microscope (NCAOR, Goa, India) which operated at an accelerating voltage of 12kV.
Statistical analysis
Seed germination was expressed as percentage of the total number of seeds sown after each treatment. Percentage germination data were analyzed using logistic regression to identify the treatments facilitating germination. The variables tested for their effect on germination included treatment time, concentration and treatment type. To test the precision of the model, the value of pseudo R2(Nagelkerke R2) was calculated. All statistical analyses were carried out using SPSS 17.0 (Chicago, IL, USA).
Results
M. peltata fruits are globose dehiscent capsules green in colour. The capsules split open when fruits mature and seeds are ready for dispersal. The seeds are dark brown in colour, rounded to slight oval in shape and exhibit epigeal germination (Fig. 1a). Seeds have an outer oily covering and a very hard seed coat surrounding a rigid endosperm, which is the major storage tissue (Fig. 1b and 1c). The embryo is spatulate and axile, composed of two symmetrical foliaceous cotyledons (axis and radical), having an average length of 2.0mm (Fig. 1d). Average diameter of seeds was (3.7±0.03) mm, and the seed moisture content (fresh wt. basis) at dispersal was (37.24±4.75)%. The tetrazolium test of fresh seeds recorded high viability upto 84%.Viable seeds stained pink while non-viable or defective seeds stained lightly or did not stain (Fig. 1e). SEM images of the untreated hard seed coat indicated absence of pores. Seed coats were rough and ridged, and a honeycomb network of deposits was observed onthe surface, with cells tightly packed, and anti-clinal walls slightly raised (Fig. 2a & 2b). The seed coat was approximately 607.5 µm thick and contained a palisade cell layer of macrosclereids (Fig. 1b) making it hard and rigid.
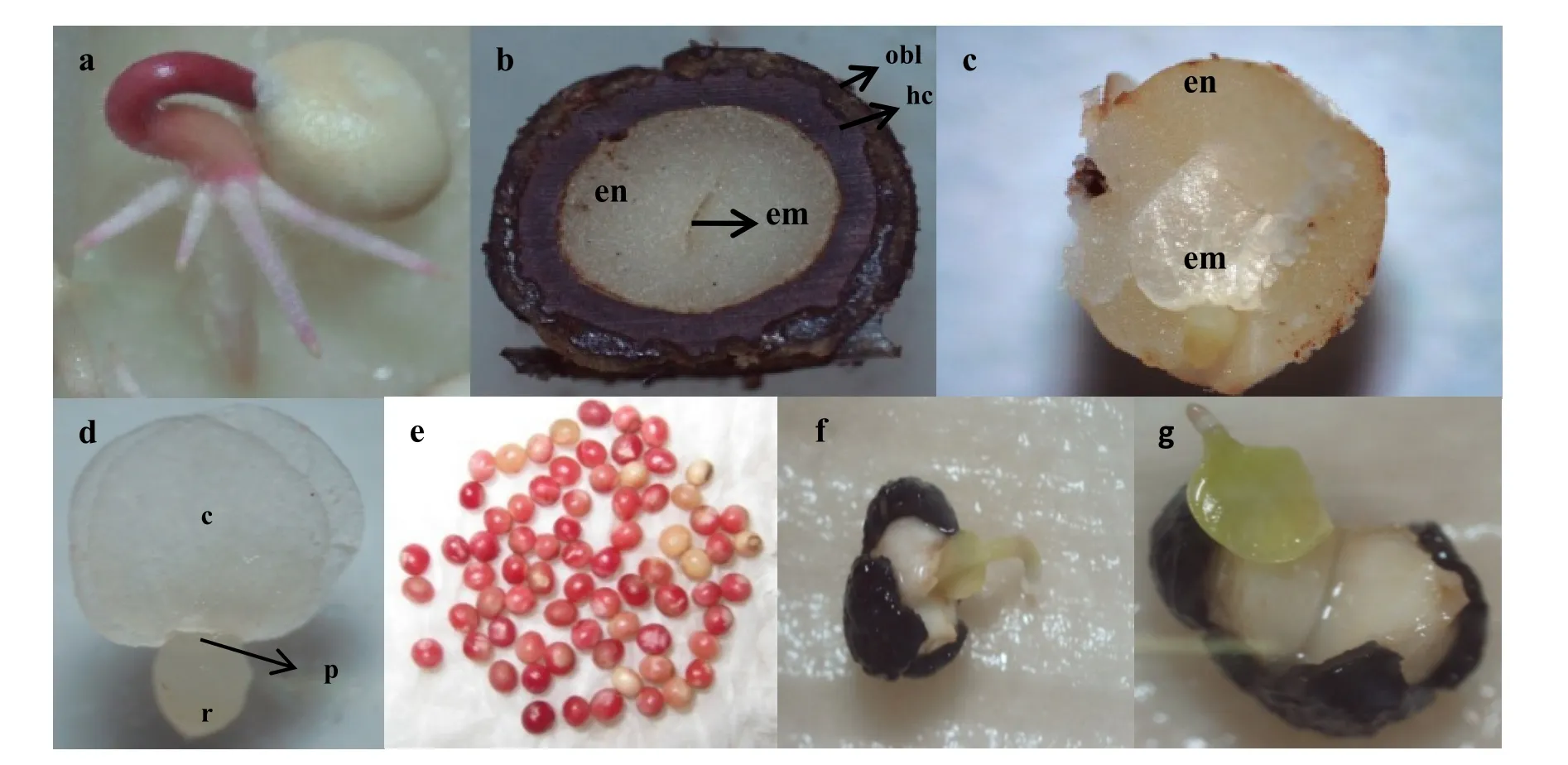
Fig. 1: M. peltata seed structure and germination. a: Normal epigeal seed germination; b: M. peltata seed layers (obl- oily black layer, hc- hard coat, emembryo, en- endosperm); c: Dissected seed with embryo surrounded by the rigid endosperm; d: Embryo (c-cotyledon, r- radicle, p- plumule); e: Viable and non-viable seeds treated with tetrazolium; f & g: Abnormal seedlings.

Fig: 2: Scanning electron micrographs of M. peltata seed. a: untreated seed; b: Seed coat ornamentation before acid treatment; c: Seed treated with conc. H2SO4; d: Closed view of sloughed seed coat; e: Distorted seed coat ornamentation of acid treated seed; f: Cracks in the palisade layer (arrows pointing to regions where cracks have developed).
Seed germination ranged from 0−78% in various treatments (Table 1), and no germination was observed in control seeds. Logistic regression analysis confirmed the existence of a significant relationship between treatments and germination (χ2= 428.303, P = 0.0001). Treatment with CSA caused leaching of phenolic compounds from the seed coat into the acid solution, however, at varying time durations it did not have a marked influence in increasing germination percentage. A maximum germination of 14.0% was obtained when treated for 15, 20 and 25 min, with germination commencing 24, 23 and 26 days after treatment, respectively. SEM images revealed that seeds treated with CSA for more than 15 minutes led to complete degeneration of the outer testa due to cracking and sloughing of the seed coat, thus facilitating imbibition and slightly initiating germination (Fig. 2c−2f). Treatment with GA alone at different concentrations did not promote germination (0%). A combination treatment of CSA and GA resulted in higher percent germination compared to either treatment used alone, however, it produced defective seedlings (>20%). In all treatments, 20%−30% seedlings were abnormal with restricted radical growth eventually resulting in seedling death (Fig. 1f and 1g).

Table 1: Effect of pre-sowing treatments on seed germination.
Increased treatment time reduced the probability of germination (χ2=1.675, B=−0.057), while increased treatment concentration significantly reduced the probability of germination (χ2=30.754, B=−0.578). Among the treatments used the combination treatment was most effective in increasing percent seed germination and was followed by mechanical scarification (Table 2). Mechanical scarification of the seed coat increased germination upto 78% compared to other treatments in a relatively shorter period (6 days) and the percentage of defective plantlets was also reduced (20%).
Variables B SE Wald χ2DF Sig. Exp (B)

Table 2: Logistic regression analysis predicting the effect of different parameters on seed germination probability
Discussion
The hard, impermeable seed coat surrounding the embryo and endosperm restricts seed germination in M. peltata. Strong impermeable seed coats protect the embryos during dormancy and maintain an environment that is conducive to quiescence (Bewley 1997). A honeycomb network of deposits on the seed coat as observed in scanning electron micrographs revealed the absence of pores, which made seeds impermeable to water. A similar network of deposits was reported for the hard-seeded variety of Glycine Max (L.) Merr. (Haris 1987), suggesting that this might be a characteristic feature of hard seeds. As observed in the present study, seeds treated with GA alone did not germinate, suggesting the impermeable nature of the seed coat. Although treatment with CSA alone was able to overcome this impermeability, it resulted in poor germination (14%). However, the combination of both treatments proved more efficient in enhancing seed germination. The seed coat was weakened using CSA, while GA treatment assisted in overcoming the endospermic barrier. GA is reported to overcome the mechanical restraint conferred by the seed-covering layers by weakening the tissues surrounding the radicle (Kucera et al. 2005).This increases the growth potential of the embryo to a point at which resistance of the seed coat is overcome and germination results. On the other hand, complete removal of the seed coat by mechanical scarification reduces the mechanical resistance to embryo growth to the extent the radicle can elongate. In such a situation, even though the embryo is at a relatively low growth potential, the seed eventually germinates. Similar observations were reported earlier for seeds of Suaeda aralocaspica (Wang et al. 2008). Seed germination treatments and the tetrazolium test confirmed that around 20% of the seeds produced by M. peltata plants were not viable. Also, in all treatments around 20%−30% of seedlings were abnormal, perhapsdue to the presence of underdeveloped embryos.
Seeds of M. peltata exhibited physical dormancy, but successfully germinated in an appreciably reduced time period after complete removal of the seed coat. This technique would be useful to restoration and reforestation practitioners as it is simple, cost effective and we recommend it for generating large numbers of propagules for revegetation of degraded lands.
Acknowledgements
We thank the Ministry of Minority Affairs - UGC, New Delhi, India for providing financial assistance (MANF). We also thank the National Centre for Antarctic Ocean Research (NCAOR), Goa for assisting with SEM imaging. Special thanks to Dr. Uromi Goodale, Xishuangbanna Tropical Botanical Gardens, Chinese Academy of Sciences, for critically reviewing the manuscript and for her valuable suggestions.
Ashton MS, Gunatilleke CVS, Singhakumara BMP, Gunatilleke IAUN. 2001. Restoration pathways for rain forest in southwest Sri Lanka: A review of concepts and models. Forest Ecology and Management, 154: 409−430.
Bewley JD. 1997. Breaking down the walls - a role for endo-ß-mannanase in release from seed dormancy. Trends in Plant Science, 2: 464−469.
Burgess PF. 1971. The effect of logging on Hill Dipterocarp Forests. Malaysian Nature Journal, 24: 231−237.
Chandrashekara UM, Ramakrishnan PS. 1993. Gap phase regeneration of tree species of differing successional status in a humid tropical forest of Kerala, India. Journal of Biosciences, 18: 279−290.
Eck G, Fiala B, Linsenmair KE, Hashim RB, Proksch P. 2001. Trade-off between chemical and biotic antiherbivore defense in the South East Asian plant genus Macaranga. Journal of Chemical Ecology, 27(10): 1979−1996.
Goodale UM, Berlyn GP, Gregoire TG, Ashton MS. 2009. Ecological significance of crown functional traits across size classes and disturbance environments in eight pioneer species in a Sri Lankan rainforest. Journal of Sustainable Forestry, 28: 22−47.
Goodale UM, Ashton MS, Berlyn GP, Gregoire TG, Singhakumara BMP, Tennakoon KU. 2012. Disturbance and tropical pioneer species: Patterns of association across life history stages. Forest Ecology and Management, 227: 54−66.
Haris WM. 1987. Comparative ultrastructure of developing seed coats of“hard-seeded” and “soft seeded” varieties of soybean, Glycine max (L.) Merr.. Botanical Gazette, 148: 324−331.
International Seed Testing Association (ISTA). 1996. Seed science and technology rules: International rules for seed testing. Seed Science and Technology, 24.
Kucera B, Cohn MA, Leubner-Metzger G. 2005. Plant hormone interactions during seed dormancy release and germination. Seed Science Research, 15: 281–307.
Mackay WA, Davis TD, Sankhla D. 1995. Influence of scarification and temperature treatments on seed germination of Lupinus havardii. Seed Science and Technology, 23: 815−821.
Marques I, Draper D. 2012. Seed germination and longevity of autumn-flowering andautumn-seed producing Mediterranean geophytes. Seed Science Research, 22: 299−309.
Moore RP. 1985. Handbook on Tetrazolium Testing. Zurich, Switzerland: International Seed Testing Association, p. 99.
Murali KS. 1997. Patterns of seed size, germination and seed viability of tropical tree species in southern India. Biotropica, 29: 271−279.
Sundarapandian SM, Swammy PS. 1999. Litter production and leaf-litter decomposition of selected tree species in tropical forests at Kodayar in the Western Ghats, India. Forest Ecology and Management, 123: 231−244.
Wang L, Huang Z, Baskin C, Baskin JM, Dong M. 2008. Germination of dimorphic seeds of the desert annual halophyte Suaeda aralocaspica (Chenopodiaceae), a C4 plant without Kranz anatomy. Annals of Botany, 102: 757–769.
Whitmore TC. 1967. Studies in Macaranga an easy genus of Malayan wayside trees. Malaysian Nature Journal, 20: 89−99.
Whitmore TC. 1969. Studies in Macaranga III. First thoughts on species evolution in Malayan Macaranga. Biological Journal of Linean Society, 1: 223−231.
DOI 10.1007/s11676-014-0536-0
Project funding: the Ministry of Minority Affairs - UGC, New Delhi, India for providing financial assistance (MANF)
The online version is available at http://www.springerlink.com
Cassie R. Rodrigues (), Bernard F. Rodrigues Department of Botany, Goa University, Goa 403206, India
Tel.: (+91) 08326519345; Fax: (+91) 832-2451184
E-mail: cassierodrigues@gmail.com
Corresponding editor: Yu Lei
杂志排行
Journal of Forestry Research的其它文章
- Growth and yield of two grain crops on sites former covered with eucalypt plantations in Koga Watershed, northwestern Ethiopia
- Carbon stock in Korean larch plantations along a chronosequence in the Lesser Khingan Mountains, China
- Biomass accumulation and nutrient uptake of 16 riparian woody plant species in Northeast China
- Cloning and sequence analysis of nine novel MYB genes in Taxodiaceae plants
- Genetic and morphological variation in natural teak (Tectona grandis) populations of the Western Ghats in Southern India
- Improved salt tolerance of Populus davidiana × P. bolleana overexpressed LEA from Tamarix androssowii
