Wildland fires and moist deciduous forests of Chhattisgarh, India: divergent component assessment
2014-09-06KitturSwamyBargaliManojKumarJhariya
B. H. Kittur · S. L. Swamy · S. S. Bargali · Manoj Kumar Jhariya
ORIGINAL PAPER
Wildland fires and moist deciduous forests of Chhattisgarh, India: divergent component assessment
B. H. Kittur · S. L. Swamy · S. S. Bargali · Manoj Kumar Jhariya
Received: 2012-12-09; Accepted: 2013-10-21
© Northeast Forestry University and Springer-Verlag Berlin Heidelberg 2014
We studied moist deciduous forests of Chhattisgarh, India (1) to assess the effect of four levels of historic wildland fire frequency (high, medium, low, and no-fire) on regeneration of seedlings in fire affected areas during pre and post-fire seasons, (2) to evaluate vegetation structure and diversity by layer in the four fire frequency zones, (3) to evaluate the impact of fire frequency on the structure of economically important tree species of the region, and (4) to quantify fuel loads by fire frequency level. We classified fire-affected areas into high, medium, low, and no-fire frequency classes based on government records. Tree species were unevenly distributed across fire frequency categories. Shrub density was maximum in zones of high fire frequency and minimum in lowfrequency and no-fire zones. Lower tree density after fires indicated that regeneration of seedlings was reduced by fire. The population structure in the high-frequency zone was comprised of seedlings of size class (A) and saplings of size class (B), represented by Diospyros melanoxylon, Dalbergia sissoo, Shorea robusta and Tectona grandis. Younger and older trees were more abundant for Tectona grandis and Dalbargia sissoo after fire, whereas intermediate-aged trees were more abundant prefire, indicating that the latter age-class was thinned by the catastrophic effect of fire. The major contributing components of fuel load included duff litter and small woody branches and twigs on the forest floor. Total fuel load on the forest floor ranged from 2.2 to 3.38 Mg/ha. The netchange in fuel load was positive in high- and medium-frequency fire zones and negative under low- and no-fire zones. Repeated fires, however, slowly reduced stand stability. An ecological approach is needed for fire management to restore the no-fire spatial and temporal structure of moist deciduous forests, their species composition and fuel loads. The management approach should incorporate participatory forest management. Use of controlled fire, fire lines and mapping of fire prone areas are fundamental principles of fire hazard reduction in these areas.
forest fire, diversity, fuel load, vegetation structure
Introduction
Forest fire is a driving factor in shaping forest vegetation and the landscape of the region (Schmerbeck and Hiremath 2007). It causes enormous losses to the forest ecosystem in terms of biodiversity and economic wealth of villagers. About 55% of India’s 67.5 million ha of forest cover is subjected to annual fires (Gubbi 2003). Most are surface fires deliberately started by villagers seeking to improve their socio-economic conditions. The Forest Survey of India reported that around 50% of the forest areas are fire prone. The influence of fire on vegetation varies with the type and nature of the fire. Different vegetation types have varying frequencies and intensities of fire. Interactions between climate, soil type and topography are all involved, as these features determine not only the vegetation type but also the likelihood of fire.
Shifts in species composition in natural forests typically occur slowly under normal conditions (Swaine et al. 1987). But fire can reduce structural and biological complexity of forests (Schindele et al. 1989; Swamy et al. 2010). Generally forest fires have negative impacts on plant diversity. Fire severity mainly depends on fuel loads of litter, dead and dying seedlings, herbs/shrubs and trees. The changes in quantity of litter on the forest floor can greatly influence the spread of fire. Other environmental factors such as atmospheric temperature, relative humidity, moisture content of litter, and wind speed and direction can also affect the severity of forest fire. Several studies have evaluated short-term impact of wild fire on vegetation. But relatively few studies (Co-vington and Moore 1994; Arno et al. 1996; Östlund et al. 1997; Hörnberg et al. 1999) have examined the long-term impact of wildland fire frequency on regeneration, vegetation structure, diversity and fuel load in moist deciduous forests. This study was undertaken (1) to assess the effect of wildland fire frequency on regeneration of seedlings in four categories of fire frequency (high, medium, low, and no-fire) during pre- and post-fire seasons; (2) to evaluate structure and diversity in different layers of vegetation in these four categories of fire frequency; (3) to describe the impact of fire on the structure of economically important tree species of the region; and (4) to quantify fuel loads by fire frequency category. Our goal was to provide information to silviculturists and ecologists to enhance understanding of fire ecology for scientific forest management.
Material and methods
Study site
The study was conducted in Achanakmar–Amarkantak biosphere reserve, Chhattisgarh, India. The reserve covered an area of 3836 sq. km (Fig. 1). The Amarkantak is one among the important natural heritage sites of Chhattisgarh. It is well known for its rich, complex and diverse flora and fauna. The forest area represents tropical moist and dry deciduous vegetation. Champion and Seth (1968) classified the forests as Northern Tropical Moist Deciduous and Southern Dry Mixed Deciduous forests. The predominant trees are Tectona grandis, Shorea robusta and various Terminalia sp. The study region experiences a typical monsoon climate, with three distinct seasons: summer from March - June, rainy from July - October and winter from November-February.
Experimental design
For the assessment of fire affected areas of Achanakmar-Amarkantak biosphere reserve, the coordinates of fire affected regions were provided by the National Remote Sensing Agency (NRSA) Hyderabad, India. Historical information on fire occurrence was compiled through local enquiry to quantify fire frequency on the fire-affected areas. Based on historic frequency of fire occurrence, we categorized fire-affected areas into four classes of fire frequency, viz. high, medium, low, and no-fire. We sampled vegetation in 20 quadrats in forest stands in each of these four classes before and after the fire season in 2009 and 2010 to evaluate regeneration, understorey and overstorey vegetation structure, diversity and composition.
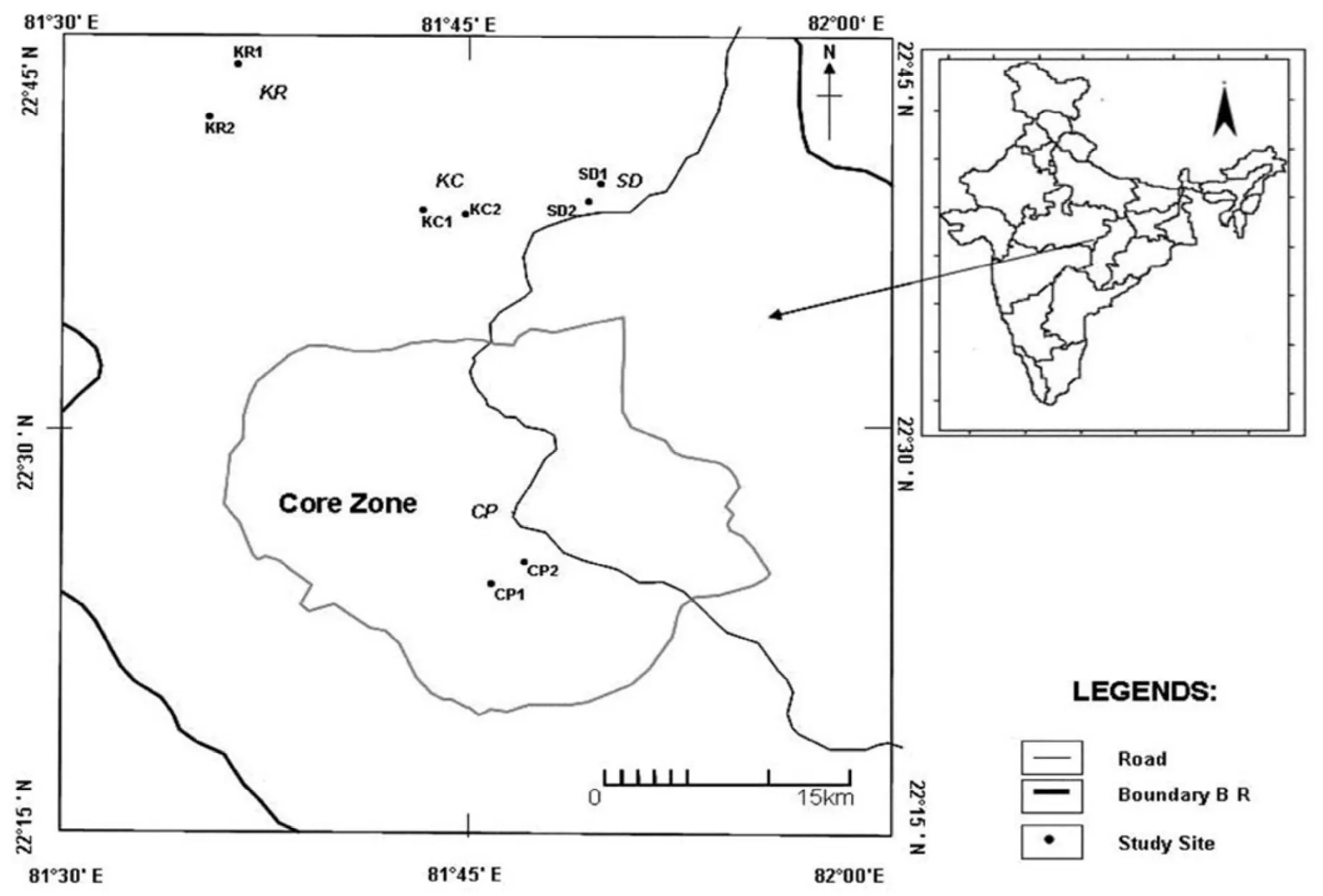
Fig. 1: Location map of the Achanakmar-Amarkantak Biosphere Reserve, Chhattisgarh, India.
Fire-affected areas were randomly located over the entire Achanakmar–Amarkantak biosphere reserve. We identified and counted all trees and saplings in 20 quadrats of 20 m × 20 m size in each fire frequency class. The girth at breast height (GBH) of all trees and saplings in each quadrat were measured and recorded. Trees >31.5 cm GBH were categorized as tree, those 10-31.5 cm GBH as saplings, and those <10 cm GBH as seedlings. In each quadrat five sub-quadrats of 5 m × 5 m size were randomly selected for measurement of seedlings and shrubs. Similarly, within each sub-quadrat, a quadrat of 1 m × 1 m was laid for measuring herbs and litter during the pre-fire season (March-April). The boundary demarcations were properly made for postfire sampling of the same plots and subplots.
Vegetation data were quantitatively analysed for frequency, density, abundance (Curtis and McIntosh 1950). The relative frequency, relative density and relative basal area values were calculated following Phillips (1959). Relative frequency, relative density, and relative basal area values were summed to yield an Importance Value Index (IVI) for each species.
Plant diversity analysis
Plant diversity in different fire frequency zones was quantified by the following diversity indices.
Shannon Index (Shannon & Weaver 1963) was used for species diversity:

where, Ni was the total number of species i and N was the total number of all the species. The factor 3.3219 was used to convert the index value to log2.
Concentration of dominance was measured by Simpson’s Index (Simpson 1949):

where, Ni and N were the same as explained above.
Equitability (e) was calculated following Pielou (1966):

where, H′ = Shannon index and S = the number of species. Species richness was calculated following Margalef (1958):

where, S = total number of species, N = basal area of all species (m2·ha-1).
Beta diversity is often been used to indicate habitat variation with regarded to species composition and was calculated (Whitaker 1977). where, Sc = total number of species in all sites and S = average species per site.

The population structures were developed based on different tree girth classes. The total number of individuals belonging to these girth classes was calculated for each species on each site following Saxena and Singh (1984), Tripathi et al. (1991) and Jhariya et al, (2012). In addition to seedling (A) and sapling (B) classes, three more size classes (based on GBH) i.e., 31.5−70.0 cm (C); 70.1−110.0 cm (D) and >110 cm (E) were arbitrarily established for each tree species. The total numbers of individuals belonging to these size classes were calculated.
For fuel load quantification, a sub-quadrat of 1 m × 1 m was laid in each fire frequency zone to sample during pre- and postfire seasons. The total weight of litter (duff) and other small woody components which may be easily burned was estimated. The weights were then converted to Mg/ha.
Results and discussion
Ecologically important changes
The density of trees across the fire affected zones ranged from 250 to 335 trees·ha-1(Table 1). The density of trees in all fire affected zones was lower than the 633−1203 trees·ha-1reported by Singh et al. (2006) in the same forest type. The lower tree density might be attributed to the effect of fire on trees in the region. Other factors reducing tree density might have been biotic and abiotic factors, the true character of the forests, depending on the degree of impact (Champion and Seth 1968). Tree basal area ranged from 12 to 20 m2·ha-1, it was highest in the nofire zone. IVI values ranged from 5.69 to 43.53. The composition of the tree layer in the high-frequency fire zone during the prefire season revealed that IVI was highest for Ougeinia oojeinensis and lowest for Terminalia tomentosa. On the basis of IVI, the Ougeinia oojeinensis-Anogeissus latifolia association was recognized as the predominant plant community, while Lagerstroemia parviflora, Shorea robusta were codominants and the Pterocarpus marsupium-Terminalia tomentosa plant community was suppressed. A/F ratio ranged from 0.02 to 0.35, indicating that most of the species showed contiguous distribution and some random distribution. Total individuals in the sapling layer of the high-frequency fire zone ranged from 10 to 13, at densities of 130 to 215 saplings ha-1(Table 2A). The density of saplings was highest in the low-frequency fire zone while fewer saplings were recorded in high, medium and no-fire zones. Saccopetalum tomentosum occurred at highest density and maximum frequency followed by Diospyros melanoxylon and L. parviflora. IVIs of saplings ranged from 8.44 to 63.31. The density of shrubs was maximum in the high-frequency fire zone and minimum in lowfrequency and no-fire zones (Table 2B). In the low-frequency fire zone during the pre-fire season, the densities of Holarrhena antidysenterica and Ventilago maderaspatana were 160 and 80 individuals/ha, respectively. A/F ratios ranged from 0.05 and 0.10, indicating that most of the species showed contiguous dis-tribution and few species showed random distribution. The higher density of shrubs in the high-frequency fire zone was possibly due to the fact that heat may stimulate the seed germination of the shrub species and resulted in sudden increase in population density (Rikhari and Bargali 1989).
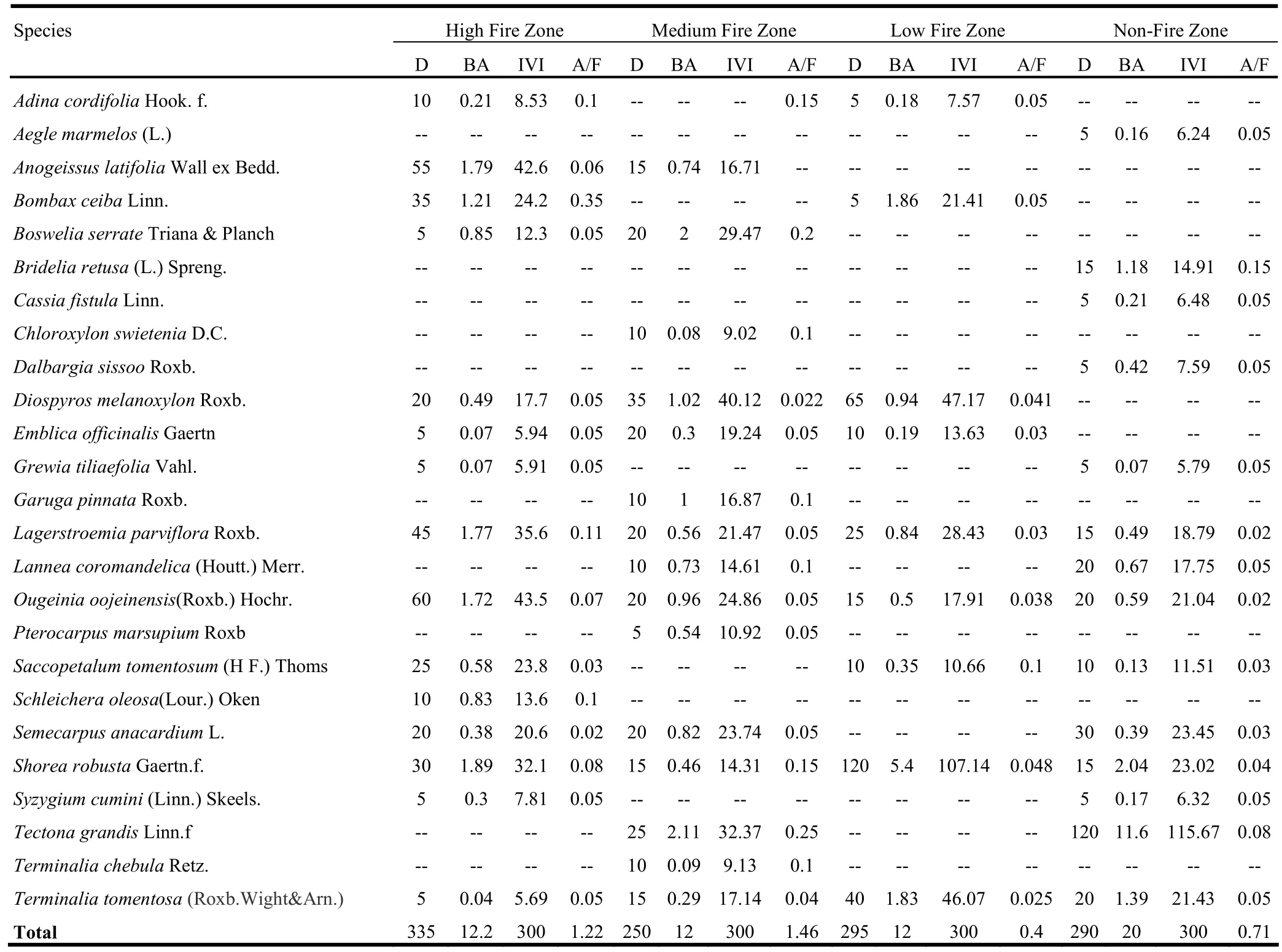
Table 1. Species structure and composition of the tree layer by fire-frequency zone during pre-fire season of Achanakmar- Amarkantak Biosphere Reserve.
Tree diversity was highest in the high-frequency fire zone, whereas the concentration of dominance had an inverse relationship with diversity (Table 3). Equitability ranged from 0.22 to 1.39 and beta diversity from 6.93 to 11.60. The diversity parameters of these forests were comparable with diversity indices reported for other tropical forests (Jha 1990). Seedling diversity ranged from 2.86 to 3.30, equitability from 1.06 to 1.25, species richness from 1.46 to 1.71, concentration of dominance from 0.13 to 0.20 and beta diversity from 5.41 to 6.57. These results supported the findings of Naidu and Sribasuki (1994). Perhaps young plants are more severely affected by fires than mature plants. Shrub diversity ranged from 0.50 to 1.91, equitability from 0.73 to 1.37, species richness from 0.15 to 0.46, concentration of dominance from 0.63 to 1.63 and beta diversity from 7 to 14. In the low-frequency fire zones, a decline in species richness was recorded after fire. This was perhaps caused primarily by the elimination of some early species which are over topped and shaded out by rapidly growing woody plants, especially resprouters (Miller 2000). The effect of declining species richness was also documented by Gill et al. (1999).
Many changes after disturbance are directly related to changes in vegetation characteristics, these features may be allied on forest type, forest fire, local weather and other anthropogenic factors. Due to disturbances such as forests fire and logging, the species composition has been altered, the species predominantly found in the dry deciduous forests such as Cassia fistula, Anogeissus latifolia and Xeromphis spinosa are now found in these vegetation types (Daniel et al. 2005; Hegde et al. 1998). The diversity parameters of herb layer showed that Shannon index in different fire zones varied from 2.21 to 2.57, equitability from 1.02 to 1.24, species richness from 0.34 to 0.67, concentration of dominance from 0.21 to 0.31 and beta diversity from 7.27 to 13.33.
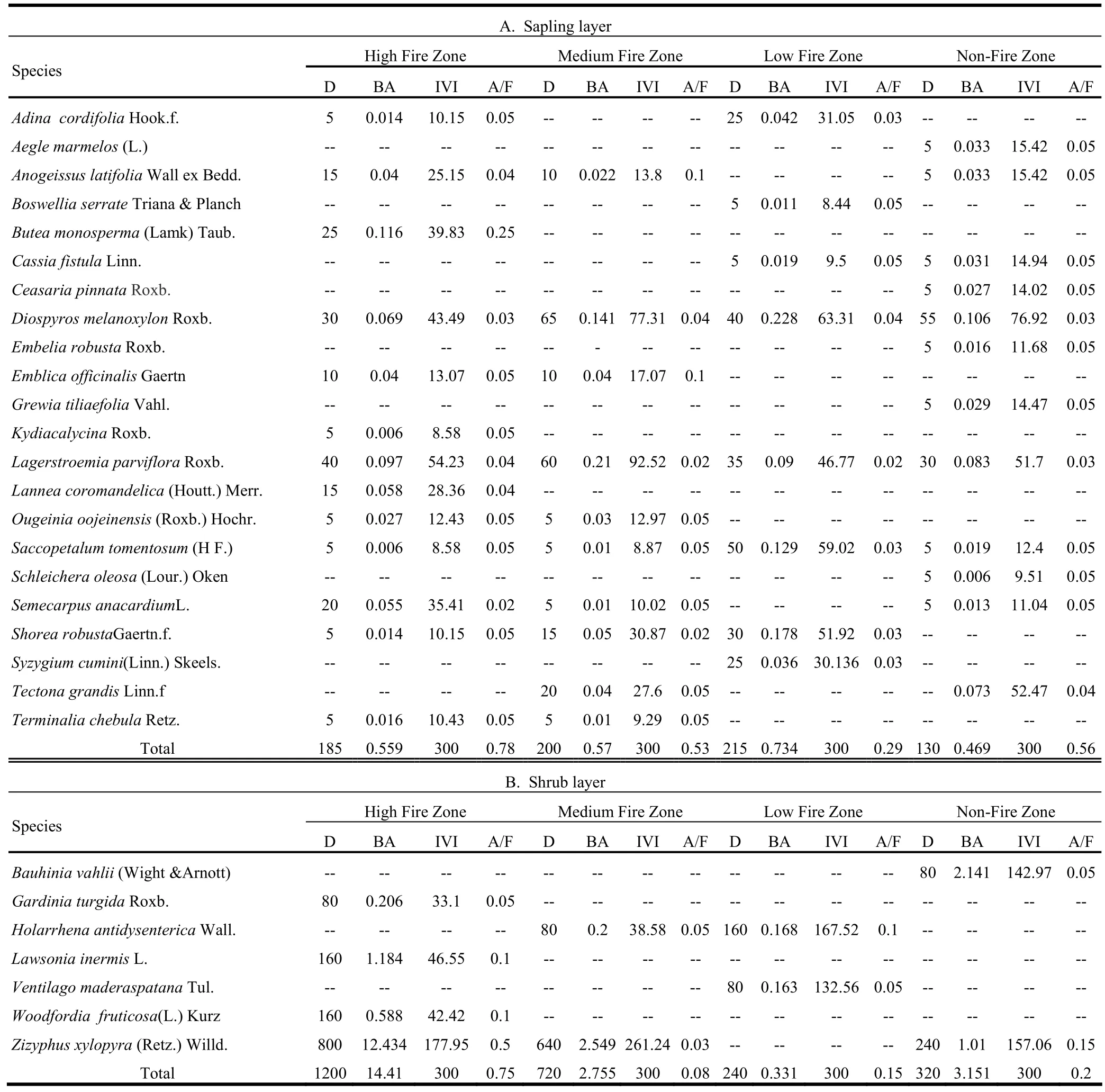
Table 2: Species structure and composition of saplings and shrubs by fire-frequency zone during the pre-fire season in Achanakmar- Amarkantak Biosphere Reserve
Wildfire and understorey recovery
Density during the pre-fire season ranged from 7200 to 12200 individuals·ha-1(Fig. 2). While after fire, the density was 5840 to 10000 individuals·ha-1. The reduced density of seedlings after fire indicated that regeneration of seedlings was presumably affected by fire irrespective of previous fire frequency. Clark (1990) and Malamud et al. (1998) revealed that the area affected by fire depends on the size and configuration of a forest patch; in general, frequent fires favor small patch sizes. During the prefire season, the density of seedlings was maximum in the medium-frequency fire zone and minimum in the no-fire zone. During the post-fire season, the low-frequency fire zone had the highest density of seedlings followed by the high-frequency, medium-frequency and no-fire zones, respectively.
The comparative reduction in understorey regeneration in the no-fire zone was perhaps due to un-decomposed and partially decomposed litter present on the forest floor that might have inhibited regeneration. Molofsky and Augspurger (1992) statedthat regeneration in forests under litter is species specific, the smaller seeded, shade intolerant species had fewer seedlings under leaf litter than on bare ground. In the post-fire season, seedling density increased considerably in the low-frequency fire zone. The increase in seedling density might have been due to the trees that were partially burnt by low-intensity fire and, in consequence, set more seed (Otto et al. 2010). Therefore, mild disturbance is in accordance with the intermediate disturbance hypothesis (Connell 1978). Mild fire enhances the productivity of ecosystems by releasing chemicals and nutrients locked up in the old herbage (Kodandapani 2001).
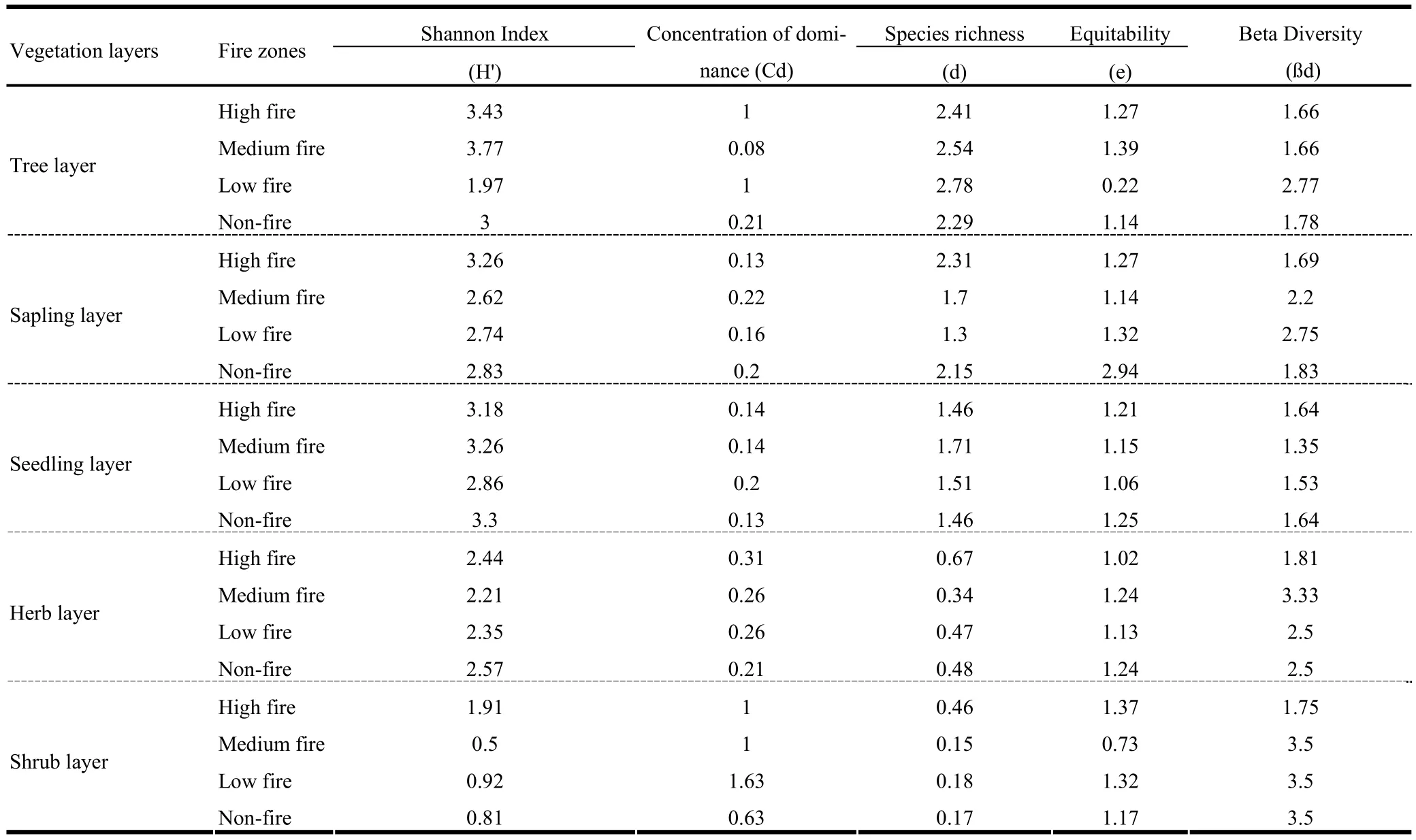
Table 3: Diversity by vegetation layer during post-fire season in Achanakmar- Amarkantak Biosphere Reserve.
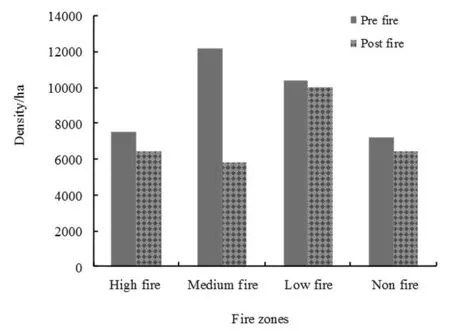
Fig. 2: Seedling density by fire-frequency zone during pre-fire and postfire seasons in Achanakmar-Amarkantak Biosphere Reserve.
Post fire population structure
Post-fire population structure data are presented in Fig. 3 and Fig. 4. In the high-frequency fire zone, seedling size class (A) and sapling size class (B) were represented by D. melanoxylon, D. sissoo, S. robusta and T. grandis. O. oojeinensis and A. latifolia were represented by intermediate size (B) or (C) classes. The younger and older trees were mostly Tectona grandis and Dalbargia sissoo and they disappeared after forest fire. These species are referred to as infrequent reproducers (Knight 1975).
In the low-frequency fire zone (Fig. 5) the seedling layer of all species was dominant, followed by saplings class (B) and small trees (C), exemplified by Lagerstroemia parviflora, Shorea robusta, Cassia fistula and Diospyros melanoxylon. Ougeinia oojeinesis, Buchanania lanzan and Semecarpus anacardium had more saplings (B) and younger trees (C) in the low-frequency fire zones. The no-fire zone was represented more by (Fig. 6) seedlings (A) except Terminalia tomentosa. The size classes (C), (D) and (E) were represented by fewer older trees, illustrated by Saccopatalum tomentosum, Schleichaera oleosa, Diospyros melanoxylon, Embelia robusta and Lagerstroemia parviflora. The sapling size represented by class (B) to older size class (E) increased proportionally (Shorea robusta and Terminalia tomentosa).
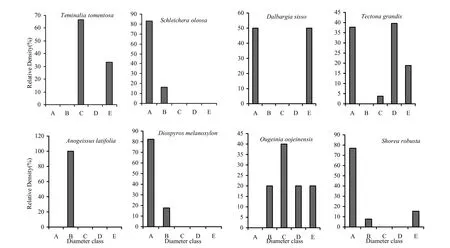
Fig. 3: Population structures of major tree species during post-fire season in the high-frequency fire zone. A= Seedlings, B= Saplings, C= Trees (31.5-70.0 cm GBH), D= Trees (70.1-110.0 cm GBH), E= Trees (> 110 cm GBH)
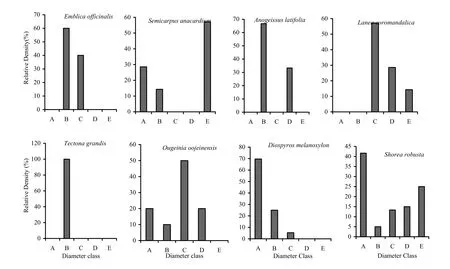
Fig. 4: Population structures of major tree species during post-fire season in medium-frequency fire zone. A= Seedlings, B= Saplings, C= Trees (31.5-70.0 cm GBH), D= Trees (70.1-110.0 cm GBH), E= Trees (> 110 cm GBH)
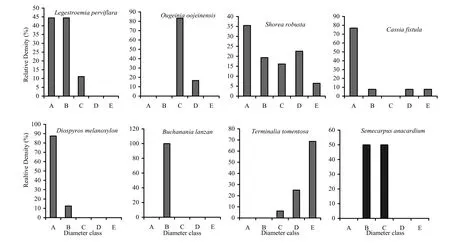
Fig. 5: Population structures of major tree species during post-fire season in low-frequency fire zone. A= Seedlings, B= Saplings, C= Trees (31.5-70.0 cm GBH), D= Trees (70.1-110.0 cm GBH), E= Trees (> 110 cm GBH)
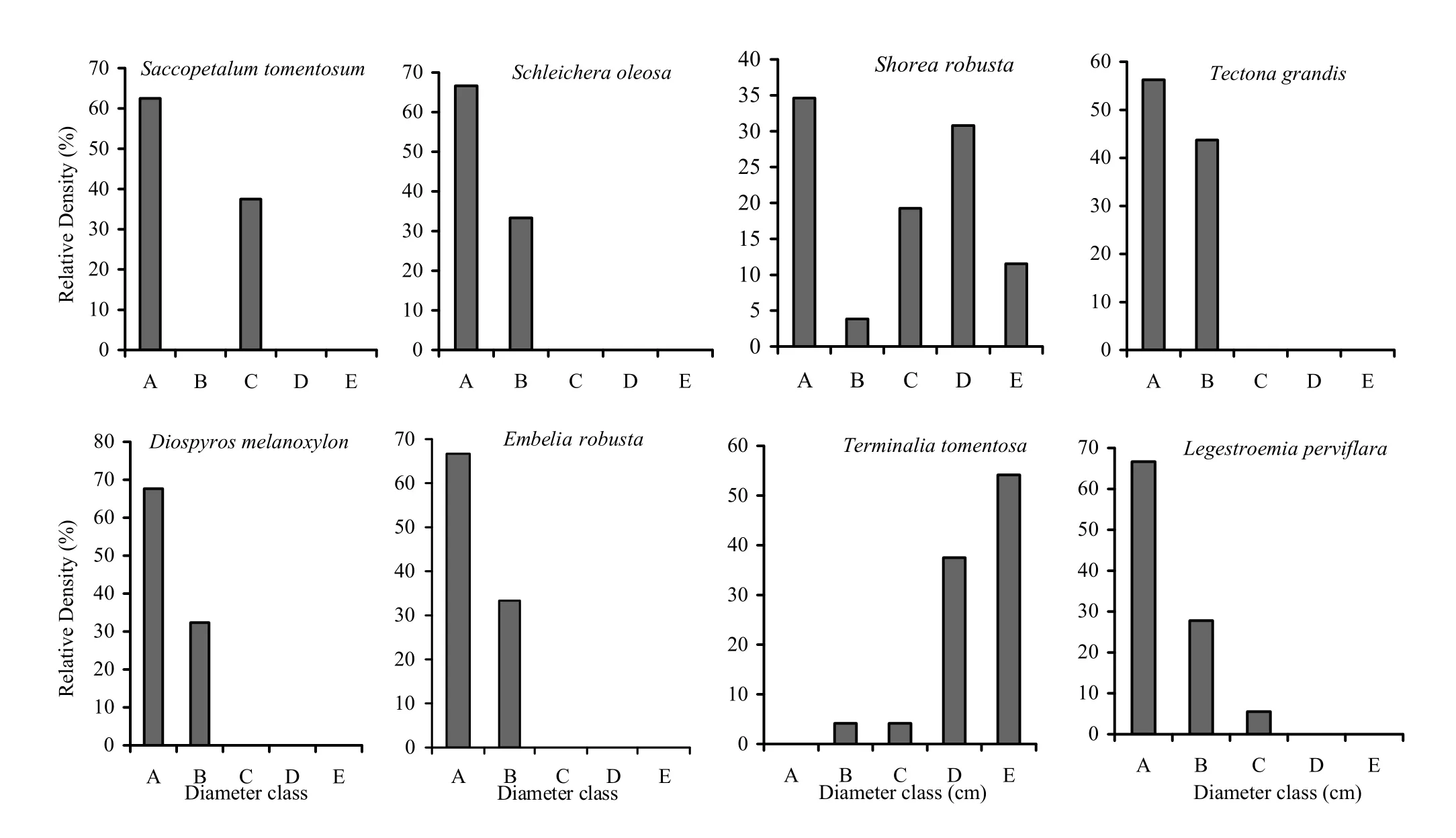
Fig. 6: Population structures of major tree species during post-fire season in no-fire zone. A= Seedlings, B= Saplings, C= Trees (31.5-70.0 cm GBH), D= Trees (70.1-110.0 cm GBH), E= Trees (> 110 cm GBH)
The ratio of saplings (B) and small trees (C) to older tree size (classes D and E) increased gradually for Shorea robusta while for Lannea coramandelica class (C) to class (E) the ratio decreased and this population is on the way to extinction if this tendency continues (Saxena and Singh 1984; Bargali et al.1987, 1989). Hence the younger species with short-duration repeated fires may stimulate the understorey annotation. Perhaps, it is important to note that the retrogressive succession (vegetation shift from taller trees with higher species richness to shorter woody communities of lower diversity) might lead to inferior forest condition. The hump in the middle size classes may indicate comparatively fast growth or less mortality in individuals once they successfully cross the sapling layer and attain the first tree size class (C), as exemplified by T. grandis, T. tomentosa and O. oojeinensis in the high-frequency fire zone and O. oojeinensis in the medium-frequency fire zone, S. anacardium and O. oojeinensis in the low-frequency fire zone, and, in the no-fire zone by S. robusta. West et al. (1981) reported that this pattern indicates heavy exploitation of older individuals and greater mortality among young individuals due to repeated forest fires.
Consequences of fuel load
The major contributing components of fuel load include the duff litter and small woody components on the forest floor. The duff litter ranged from 1.77 to 2.62 Mg/ha, while small woody components ranged from 0.43 to 0.76 Mg/ha (Fig. 7). Lower fuel loads were recorded in high- and medium-frequency fire zones during the pre-fire season. Similarly, in post-fire season the fuel load was increased considerably. Little or no burning of fuel accompanied by addition of litter possibly contributed higher litter cover during the post-fire season. Both duff litter and small woody components were most dense in the no-fire zone, whereas they were low in the high- and low-frequency zones. Wanthongchai et al. (2010) reported that pre-burning heaps of fine fuels increased with the length of the previous fire-free interval and this led to higher accumulation of fuel load. Total fuel load on the forest floor ranged from 2.2 to 3.38 Mg/ha. The fuel load during pre-fire season followed the order: No-fire > Medium->High- >Low-frequency zone.
The duff litter during post-fire season ranged from 0.26 to 3.25 Mg/ha. The high- and medium-frequency zones had little duff litter. There was 0.392 Mg/ha and 0.37 Mg/ha of litter lost due to burning, decomposition and other process during pre- and postfire seasons, respectively. Where as 0.11 Mg/ha and 0.83 Mg/ha of total load accumulated in the low-frequency and no-fire zones, respectively during post-fire season. The accumulated fuel load was comprised of senescent and dry leaves that were burned by low-intensity ground fires in medium- and low-frequency fire zones (visual observation). The zone unaffected by fire underwent further litter deposition and decomposition but usually the litter deposition was greater than decomposition due to the deciduous nature of the vegetation. The greater fuel accumulation in no-fire zones leads to greater fire frequency (Singh and Singh 1992). Small woody components ranged from 0.96 to 1.88 Mg/ha. Values were higher in high- and medium-frequency fire zones, and lower in the low-frequency fire zone. Total quantity of litter ranged from 1.8 to 4.21 Mg/ha. The total litter during post-fire season was in the order: no-fire > Low- > Medium- >High-frequency zone (Fig. 7).
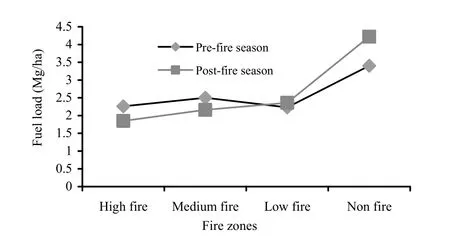
Fig. 7: Total litter (Mg/ha) by fire-frequency zone in pre- and post-fire season in Achanakmar-Amarkantak Biosphere Reserve
Management perspectives
The moist deciduous forests of Chhattisgarh, India are ecologically rich as compared to other tropical forests of the country in terms of structure, composition and diversity. However, due to the repeated effect of fire, stability was slowly decreasing. We suggest more ecological approach is warranted; one that would restore of moist deciduous forest. The approach can be through participatory forest management by forest department personnel and villagers. Fuel load management, including controlled fire, construction of fire lines and mapping of fire-prone areas, are fundamental principles of fire hazard reduction. An ecological approach to landscape management is needed and should be based on knowledge of natural vegetation structure and composition. Litter collection by tribal communities for mulching their crops would help to reduce forest fuel loads. Furthermore, ecologically driven management that would foster the restoration of vegetation is urgently needed for Achanakmar-Amarkantak biosphere reserve. Various combinations of precommercial and profitable thinning, mechanical fuel treatments and controlled burning could be used at a stand-level to reduce the fuel addition.
Acknowledgement
Sincere thanks are to the reviewers and editor for the closer look into the article and valuable suggestions made.
Arno SF. 1976.The historical role of fire on the Bitterroot National Forest. Research Patent INT-187, U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station, Missoula, MT, 29 pp.
Bargali SS, Rana BS, Rikhari HC, Singh RP. 1989. Population structure of Central Himalayan blue pine (Pinus wallichiana) forest. Environment and Ecology, 7: 431-436.
Bargali SS, Tewari JC, Rawat YS, Singh SP. 1987. Woody vegetation in high elevation blue-pine mixed oak forest of Kumaun Himalaya, India. In: Y. P. S. Pangty and S. C. Joshi (eds.), Western Himalaya: Environment, Problems and Development. Gyanodaya Parakashan, Nainital. pp. 121−155.
Champion HG, Seth SK. 1968. A Revised Survey of the Forest Types of India. New Delhi: Manager of Publications, Government of India, p.404.
Clark JS. 1990. Fire and climate change during the last 750 years in northwestern Minnesota. Ecological Monographs, 60: 135–159.
Connell JH. 1978. Diversity in tropical rain forests and coral reefs. Science, 199: 1302–1310.
Covington WW, Moore MM. 1994. Southwestern ponderosa forest structure, changes since Euro-American settlement. Journal of Forestry, 92: 39–47. Curtis JT, McIntosh RP. 1950. The interrelations of certain analytic and synthetic phytosociological characters. Ecology, 31: 434– 455.
Danielle van OF, Markus H, Patrick de O Jan, Rein de W. 2005. Effect of tree species on within-forest distribution of understorey. Annual Review of Ecology and Systematics, 18: 431–451.
Gill M, Woinarski J, York A. 1999. Australia’s biodiversity responses to fire. Biodiversity technical report No. 1, Environment Australia.
Gubbi S. 2003. Fire burning. Deccan Herald, daily news, Bangalore. India. Available at http://wildlifefirst.info/images/wordfiles/fire.doc.
Hegde V, Chandran MD, Gadgil M. 1998. Variation in bark thickness in a tropical forest community of Western Ghats in India. Functional Ecology, 12: 313–318.
Schmerbeck J, Hiremath A. 2007. Forest fires in India: Dealing with the issue – The project, India. In: J. Schmerbeck, A. Hiremath, C Ravichandran (eds), Forest fires in India -- Workshop proceedings. Madurai, India: Pillar Human Resource Development Centre, pp.5–8.
Hörnburg L, Östlund L, Zackrisson O, Bergman I. 1999. The genesis of two Piceacladina forests in northern Sweden. Journal of Ecology, 87: 800−814.
Jha CS. 1990. Land use and vegetation analysis of dry tropical forest region. Ph.D. Thesis, Banaras Hindu University, Varanasi, India.
Jhariya MK, Bargali SS, Swamy SL, Kittur B. 2012. Vegetational structure, diversity and fuel load in fire affected areas of tropical dry deciduous forests in Chhattisgarh. Vegetos, 25(1): 210–224.
Knight DH. 1975. A phytosocioligical analysis of species-rich-tropical forest on Barro Colorado Island; Panama. Ecological Monograph, 45: 259–284.
Malamud BD, Morein G, Turcotte DL. 1998. Forest fires: an example of selforganized critical behavior. Science, 281: 1840–1842.
Marglef DR. 1958. Information theory in ecology. General Systems Yearbook, 3: 36–71.
Miller M. 2000. Wildland fire in ecosystems, effects of fire on flora, Gen. Tech. Rep. Vol. 2. Ogden, UT, U.S. Department of Agriculture and Forest Service, Rocky Mountain Research Station, p.257.
Molofsky J, Augspurger CK. 1992. The effect of leaf litter on early seedling establishment in a tropical forests. Ecology, 73(1): 68–77.
Naidu CV, Srivasuki KP. 1994. Effect of forest fire on tree species on different areas of Aeshachalam Hills. Journal of Tropical Forestry, 10(3): 56–64.
Östlund L, Zackrisson O, Axelsson AL. 1997. The history and transformation of a Scandinavian boreal forest landscape since the 19thcentury. Canadian Journal of Forest Research, 27: 1198–1206.
Otto R, García-del-Rey E, Muñoz PG, Fernández-Palacios JM. The effect of fre severity on frst-year seedling establishment in a Pinus canariensis forest on Tenerife, Canary Islands. European Journal of Forest Research, 129(4): 499–508.
Phillips EA. 1959. Methods of Vegetation. New York, USA: Henry Holt and Claremont.
Pielou EC. 1966. The measurement of diversity in different types of biological collections. Journal of Theoretical Biology, 13: 131–144.
Rikhari HC, Bargali SS. 1989. Shrub layer composition in different forest ecosystems of Kumaun Himalaya. Vegetos, 2(2): 195–200.
Saha S. 2002. Anthropogenic fire regime in a deciduous forest of central India. Current Science, 82(9): 1144–1147.
Saxena AK, Singh JS. 1984. Tree population structure of central Himalayan forest associations and implications concerning their future composition. Vegetatio, 58(2): 61–69.
Schindele W, Thoma W, Panzer K.1989. The forest fire 1982/3 in East Kalimantan Part 1: The fire, the effects, the damage and technical solutions. In: FR-Report No. 5. Investigation of the steps needed to rehabilitate the areas of East Kalimantan seriously affected by fire. Jakarta: German Forest Inventory Service Ltd. for German Agency for Technical Cooperation (GTZ) and International Tropical Timber Organization (ITTO).
Shannon CE, Weaver W. 1963. The Mathematical Theory of Communication. Urbana, USA: University of Illinois Press.
Simpson EH. 1949. Measurment of diversity. Nature, 163: 688.
Singh JS, Singh VK. 1992. Phenology of seasonally dry tropical forest. Current Science, 63(11): 684– 689.
Singh L, Puri S, Bargali SS. 2006. Biodiversity and importance of some lesser known woody species of Moist Deciduous Forest of Achanakmar-Amarkantak Biosphere Reserve. The Botanica, 56: 97–103.
Swaine MD, Hall JB, Alexander CJ. 1987. Tree population dynamics at Kade, Ghana (1968-1982). Journal of Tropical Ecology, 3: 331–345.
Swamy SL, Dutt CBS, Murthy MSR, Mishra A, Bargali SS. 2010. Floristics and dry matter dynamics of Tropical Wet Evergreen Forests of Western Ghats, India. Current Science, 99(3): 353–364.
Tripathi BC, Rikhari HC, Bargali SS, Rawat YS. 1991. Species composition and regeneration in disturbed forest sites in the oak zone in and around Nainital. Proceedings of Indian National Science Academy, B57: 381–390.
Wanthongchai K, Goldammer JG, Bauhus J. 2010. Effects of fire frequency on prescribed fire behaviour and soil temperatures in dry dipterocarp forests. International Journal of Wildland Fire,20(1): 35–45.
West DC, ShugartJr HH, Ranney JW. 1981. Population structure of forest cover in a large area in the boreal Swedish forest. Forest Science, 27: 701–710.
Whittaker RH. 1972. Evolution and measurement of species diversity. Taxon, 21: 213–251.
DOI 10.1007/s11676-014-0471-0
Project funding: This research was financed by NRSA, Hyderabad, Forest Department of Chhattisgarh, India.
The online version is available at http://www.springerlink.com
B. H. Kittur
Department of Silviculture & Agroforestry, College of Forestry, Kerala Agriculture University, Vellanikara, Thrissure, Kerala, India.
S. L. Swamy, Manoj Kumar Jhariya
Department of Forestry, College of Agriculture, Indira Gandhi Krishi Vishwavidyalaya, Raipur (C.G.) 492006, India.
Department of Botany, DSB Campus, Kumaun University, Nainital –263001 (Uttarakhand) India. Email: surendrakiran@rediffmail.com
Corresponding editor: Hu Yanbo
杂志排行
Journal of Forestry Research的其它文章
- Growth and yield of two grain crops on sites former covered with eucalypt plantations in Koga Watershed, northwestern Ethiopia
- Carbon stock in Korean larch plantations along a chronosequence in the Lesser Khingan Mountains, China
- Biomass accumulation and nutrient uptake of 16 riparian woody plant species in Northeast China
- Cloning and sequence analysis of nine novel MYB genes in Taxodiaceae plants
- Genetic and morphological variation in natural teak (Tectona grandis) populations of the Western Ghats in Southern India
- Improved salt tolerance of Populus davidiana × P. bolleana overexpressed LEA from Tamarix androssowii
