Changes in foliar carbon isotope composition and seasonal stomatal conductance reveal adaptive traits in Mediterranean coppices affected by drought
2014-09-06GiovanniDiMatteoLuigiPeriniPaoloAtzoriPaoloDeAngelisTizianoMeiGiadaBertiniGianfrancoFabbioGiuseppeScarasciaMugnozza
Giovanni Di Matteo · Luigi Perini · Paolo Atzori · Paolo De Angelis ·Tiziano Mei Giada Bertini · Gianfranco Fabbio · Giuseppe Scarascia Mugnozza
ORIGINAL PAPER
Changes in foliar carbon isotope composition and seasonal stomatal conductance reveal adaptive traits in Mediterranean coppices affected by drought
Giovanni Di Matteo · Luigi Perini · Paolo Atzori · Paolo De Angelis ·Tiziano Mei Giada Bertini · Gianfranco Fabbio · Giuseppe Scarascia Mugnozza
Received: 2013-03-14 Accepted: 2014-03-30
© Northeast Forestry University and Springer-Verlag Berlin Heidelberg 2014
We estimated water-use efficiency and potential photosynthetic assimilation of Holm oak (Quercus ilex L.) on slopes of NW and SW aspects in a replicated field test examining the effects of intensifying drought in two Mediterranean coppice forests. We used standard techniques for quantifying gas exchange and carbon isotopes in leaves and analyzed total chlorophyll, carotenoids and nitrogen in leaves collected from Mediterranean forests managed under the coppice system. We postulated that responses to drought of coppiced trees would lead to differential responses in physiological traits and that these traits could be used by foresters to adapt to predicted warming and drying in the Mediterranean area. We observed physiological responses of the coppiced trees that suggested acclimation in photosynthetic potential and water-use efficiency: (1) a significant reduction in stomatal conductance (p<0.01) wasrecorded as the drought increased at the SW site; (2) foliar δ13C increased as drought increased at the SW site (p<0.01); (3) variations in levels of carotenoids and foliar nitrogen, and differences in foliar morphology were recorded, and were tentatively attributed to variation in photosynthetic assimilation between sites. These findings increase knowledge of the capacity for acclimation of managed forests in the Mediterranean region of Europe.
Mediterranean forest ecosystems, forest acclimation, stable isotopes, leaf gas exchanges, water-use efficiency, foliar traits, adaptive silviculture.
Introduction
Most Mediterranean forests are broadleaved forests managed for centuries under the coppice system. Historically, coppice forests have been subjected to intensive management on short rotations for firewood and charcoal. Most of these forests have been abandoned over the last 60 years because of the depopulation of rural areas, the change in socio-economical conditions, and the replacement of firewood by fossil fuels. These ecosystems survived intensive management because of their ability to adapt to different light regimes and drought conditions by maximizing their photosynthetic potential and water-use efficiency (WUE). The main role currently attributed to the unmanaged coppice forests is the restoration of more functional cover in terms of biodiversity, societal and ecosystem services, landscape quality and climate change mitigation.
Recent global warming caused a significant upward elevational shift of growth potential of forest ecosystems. This trend is predicted to continue in future but many forest species have limited capacity to migrate to higher elevations due to their sensitivity to late frosts (Lindner et al. 2010; Maxime and Hendrik 2011). Trees in particular are susceptible to climate change due to their longevity that extends from decades to centuries. Mediterranean evergreen species such as Quercus ilex are characterized by highleaf plasticity that may explain their wide distribution under varying ecological environments. This attribute might be advantageous in response to altered environmental conditions such as global climate change (Gratani et al. 2006). Climate projections indicate drying trends due to decreasing precipitation and increasing evaporation on most continents, and these are supported by current evidence in the Mediterranean region (Christensen et al. 2007).
The coppice system is still widespread in European Mediterranean countries and covers about 8.5 million hectares (Morandini 1998). A typical “coppice with standards” forest consists mainly of shoots in the growth form of “simple coppice” with fewer scattered mature standard trees. Standard trees are the long-lived trees originating mainly from seed and untouched by coppicing or thinning for a few cycles. Shoots are the younger trees regenerating from root stocks resprouting after each coppicing. Although of different ages, standard trees and shoots live together in the same stand.
Coppices are adapted to a wide range of environments and soil water conditions because of their deep rooting and stomatal control of gas exchange. Coppices tolerate negative water potentials by osmotic adjustment and other morphological and physiological adaptations (Damesin et al. 1998; Tognetti et al. 2007). Coppices are also characterized by trees of varying ages that can capture different water sources, thereby avoiding water limitation (Valentini et al. 1992). The repeated cuts (coppicing) create an‘artificial disturbance regime’ similar to the periodical natural opening of large canopy gaps. Since the abandonment of coppicing on a portion of the managed forest area over the last decades, new forest structures have been created through the conversion of former coppice into high-forest physiognomies from which one shoot grows from each stump. Resulting structures are similar to single-story young stands and scattered standard trees.
The carbon isotope ratio in organic material is a useful tool for identifying medium- and long-term effects of environmental factors on gas exchange in plants. Changes in stomatal conductance (gs) and/or photosynthetic capacity (A) are reflected in changes in the pi/pa ratio (intercellular and atmospheric partial pressures of CO2), affecting the carbon isotope composition of the plant (δ13C) (Farquhar et al. 1982 and references therein). Changes in foliar δ13C along environmental gradients have been reported for a wide range of forest ecosystems (Körner et al. 1991; Hultine and Marshall 2000; Sah and Brume 2003; De Lillis et al. 2004; Shi et al. 2006, Klein et al. 2013). Correlations have also been reported along elevational gradients between δ13C and abiotic factors such as soil moisture (Beerling et al. 1996; Sun et al. 1996), air temperature (Panek and Waring 1995), atmospheric CO2concentration (Gale 1972; Ehleringer and Cerling 1995) and barometric pressure (Marshall and Zhang 1994). The variation in δ13C along environmental gradients has been correlated to morphometric and physiological features that vary with drought and elevation, such as leaf thickness (Vitousek et al. 1990; Cordell et al. 1998; Kogami et al. 2001), foliar nitrogen content (Evans 1989; Friend et al. 1989; Morecroft and Woodward 1996; Cordell et al. 1999; Kogami et al. 2001; Hikosaka et al. 2002; Shi et al. 2006) and stomatal density (Körner et al. 1988; Hultine and Marshall 2000; Paridari et al. 2013).
We conducted a replicated field trial in two Mediterranean coppice forests of southern Italy to: (1) characterize the interacting effects of aspect and intensifying drought on foliar δ13C; (2) quantify temporal variation in gs. Our hypothesis was that intensifying drought leads to physiological responses of coppiced trees at both sites. We tested six parameters to represent tree physiology (carbon isotope composition, stomatal conductance) and tree photosynthetic potential (total chlorophyll, carotenoids, nitrogen and leaf morphology). Observations were made over three years (i.e., 2004, 2005 and 2006) with focus on June-September, when plant physiological activities ranged between summer minima and summer maxima.
Materials and Methods
Study site, climate and experimental design
The study was conducted in a mature 9,500 ha Holm oak (Quercus ilex L.), Arbutus unedo L. and Phillyrea latifolia L. coppice forest at Pula in the southern Sulcis region of Sardinia, Italy (39° 03' N, 8° 50' E) between September 2004 and September 2006. Q. ilex is a deep-rooted tree widely distributed in the sub-humid areas of the Mediterranean basin at 0−1400 m elevation. A. unedo is an evergreen laurophyllous shrub or tree, occurring mainly on siliceous soils. Both Q. ilex and A. unedo are species of the Mediterranean sclerophyllous vegetation characterized by deep root systems for summer drought survival (Cubera and Moreno 2007; David et al. 2007). Our two experimental sites were located on opposing sides of the same valley. Mean annual air temperature was 15.2 °C, and mean temperature of the warmest months was 21.0 °C. Mean annual precipitation was 976 mm, mostly occurring in winter and spring. Due to the evidence that during the study period the meteorological conditions were drier than their long-term average (Table 1), we assumed an intensifying of drought conditions at both sites.
Soil profiles were characterized as Eutric Cambisol or Mediterranean brown earths of xerophytic and mesophytic forests, whereas volcanic porphyritic rocks constituted the main geological substrate (Palaeozoic) (Amorini et al. 1996).
At one site, a set of experimental plots faced northwest (NW) and at the other site plots faced southwest (SW). Rainfall varied across the valley with higher total precipitation at the NW site (1,179 mm) than at the SW site (773 mm). Relative humidity over the drought season (May to August) was higher at the NW site (72.3%) than at the SW site (42.5%) (Fabbio et al. 1996; ARPA Sardinia). Dry summer periods are on average very long, from May to September, and the lowest mean monthly rainfall is recorded in July. The SW site was affected by one additional month of dry summer than the NW site.
Both coppice forests were historically intensively coppiced for firewood and charcoal production and then progressively abandoned over the last 60-70 years. Hence Q. ilex occurred both as a standard tree (hereafter “uncoppiced tree”) and as a shoot (here-after “coppiced trees”). A. unedo occurred only as a coppiced tree. In 2005, trees at both sites had reached average ages of 30-40 yrs for coppiced trees and 70 or more for uncoppiced trees. During the pre-sampling 2004 inventory, trees had mean diameter at breast height (DBH) of 7.1 cm and density of about 7,560 stems ha-1at the NW site, compared to mean DBH of 5.5 cm and density of 8,286 stems·ha-1at the SW site. Total basal area (BA) of trees varied between sites from 44.1 m2·ha-1at the NW site to about 30 m2·ha-1at the SW site (Table 2).
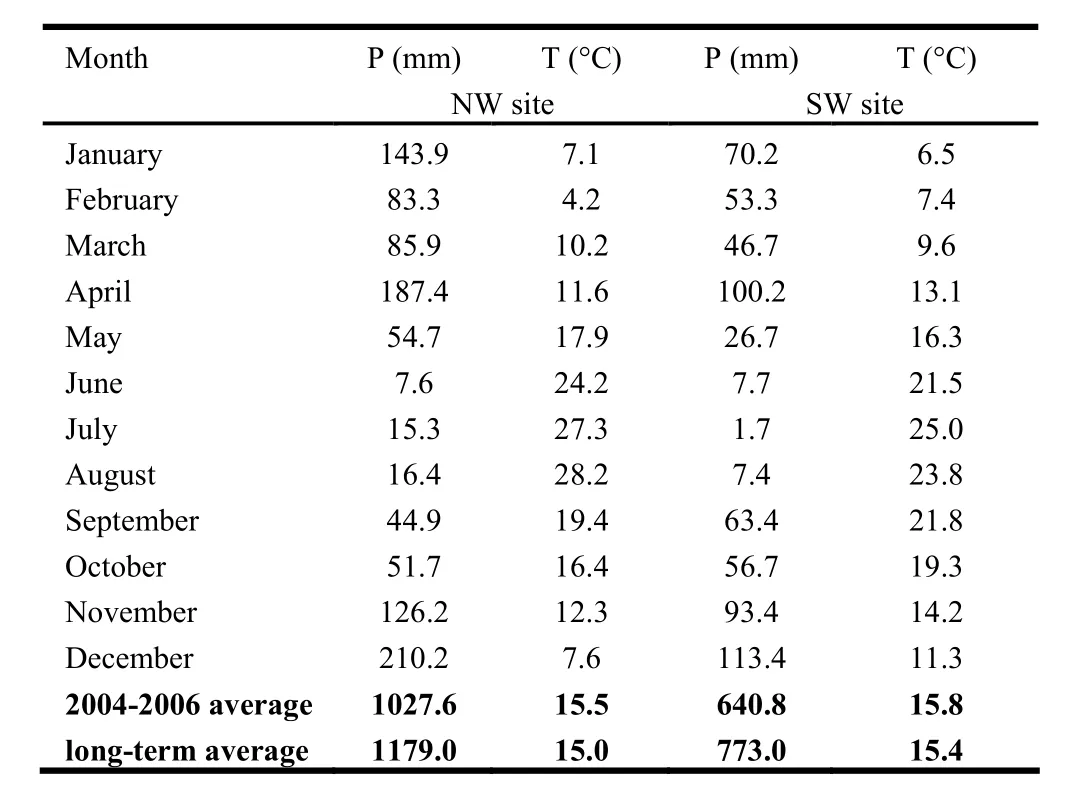
Table 1: Mean monthly precipitation (P) and mean monthly air temperature (T) recorded during the study period (i.e., 2004, 2005 and 2006) at both sites compared with their long-term averages. Precipitation and mean air temperature were either recorded at two climate stations located approximately few km distance from the experimental sites or collected directly from regional meteorological services (i.e., ARPA Sardinia website: http://www.sar.sardegna.it/pubblicazioni/riepiloghimensili/mensili.asp).
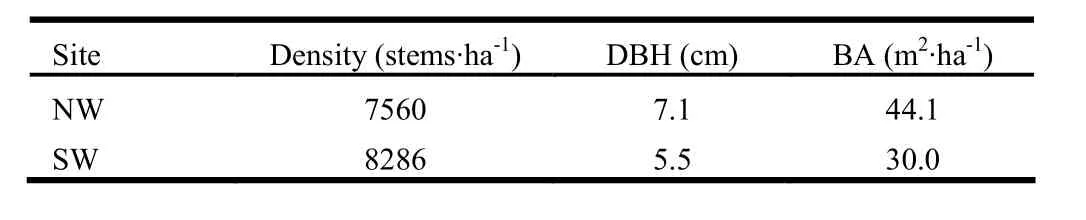
Table 2. Forest mensurational parameters for the experimental sites. DBH = mean diameter at breast height; BA= basal area.
Sampling procedures
A 1,000 m2plot was established at each experimental site and 15 trees were sampled randomly on the basis of three growth forms: Q. ilex uncoppiced trees (mean tree height: 12.2 m), Q. ilex coppiced trees (mean tree height: 8.6 m) and A. unedo coppiced trees (mean tree height: 5.3 m). Altogether, 45 trees were sampled at each site (15 trees of each of 3 growth forms).
Fully expanded sun-exposed leaves from the middle-upper part of the crown were selected to measure seasonal gs, foliar δ13C, total chlorophyll, carotenoids, foliar nitrogen and foliar morphology.
Total chlorophyll and carotenoids analyses
In order to quantify total chlorophyll (a + b) and carotenoid concentrations on a leaf-area basis, fifteen sunlit leaves per growth form were sampled at each site (n = 15 leaves × 15 trees × 3 growth forms). Immediately after collection, samples were sealed in cryovials and stored in a dark environment under liquid nitrogen and transported to the lab. Total chlorophyll and carotenoid extraction was performed using 95% ethanol. Absorbance measurements were performed with a spectrophotometer (Lambda 3B, Perkin Elmer, Norwalk, USA) at wavelengths of 664.2 nm and 648.6 nm. Total chlorophyll and carotenoid contents were calculated following Lichtenthaler (1987).
Foliar carbon isotope composition analyses (δ13C)
We ground the foliar samples using a Retsch mill (Mixer Mills, MM 200, Haan, Germany). The resulting powder was placed in tin capsules and then weighed (approximately 1.5 mg) using a microbalance (Mettler AT 21 Comparator, Mettler Toledo AG, Greifensee, Switzerland). Foliar carbon isotope ratios were determined by combustion of the samples in an Elemental Analyzer (Carlo Erba, model 1108EA, Milan, Italy) coupled to an isotopic ratio mass spectrometer (Finnigan MAT, model S delta, Bremen, Germany). Analysis precision was greater than ± 0.11‰. The results were expressed as delta (δ13C) in ‰ relative to the Pee Dee Belemnite (PDB) standard where: [δ13C= (Rsample/Rstandard–1) × 1000], and Rsampleand Rstandardrepresent the13C/12C molar ratios of the sample and the standard, respectively.
Stomatal conductance measurements
We measured stomatal conductance at both sites during spring and summer (between June and September). Measurements were made at three times during the day (08:00, 12:00 and 16:00 hrs) on sunlit leaves formed in the previous growing season along the upper-middle portion of the crown using a steady-state Porometer LI-COR, LI-1600 (Li-Cor Inc, Lincoln, NE, USA). Stomatal conductance measurements were carried out under constant PAR values between 1,000 and 1,200 μE·m-2·s-1and under leaf temperatures in the range of 28−30°C.
Foliar nitrogen analyses
The same foliar powder obtained for carbon isotope analyses was used for nitrogen elemental analyses. Powder was placed in tin capsules and then weighed (approximately 2.5 mg) using a microbalance (Mettler AT 21 Comparator, Mettler Toledo AG, Greifensee, Switzerland). Foliar nitrogen concentration was determined by combustion of the samples in an Elemental Analyzer (Carlo Erba, model 1108EA, Milan, Italy). Data were processed using Carlo Erba software and reported as the dry weight percentage for each sample.
Foliar morphological analyses
The measured leaf morphology variables were: leaf area, leaf length and specific leaf area (SLA) (the ratio of surface area to dry weight). Leaf area was measured using a Leaf Area Meter (LI-3100, Li-Cor Inc, Lincoln, NE, USA). In order to determinethe foliar dry weight, samples were dried at 70 °C to constant dry weight.
Statistics
All measured and calculated variables were compared between sites, species and growth forms, and analysed by analysis of variance (ANOVA) using PRISM software version 4.01 (Graph-Pad Software, Inc. San Diego, CA, USA). Measurements of individual trees were used as observations, whereas site, species, growth forms and their interactions were defined as factors. Because stomatal conductance was measured in four months (i.e., June, July, August and September) by species, growth form and site, a four-way ANOVA was performed with time as the fourth factor. Means were compared using Tukey’s HSD test, reported as letters indicating significant differences in Fig. 1.
Results
Total chlorophyll, carotenoids and nitrogen
Total chlorophyll and carotenoid contents differed significantly by growth form (p <0.01) and species (p <0.05), and carotenoids differed significantly between sites (p <0.01). We found significant interactions Si × Gf in carotenoids (p <0.05) and Si × Sp for both total chlorophyll and carotenoids (p <0.05) (Table 3). Significant total chlorophyll differences (p <0.01) were found between uncoppiced (87.5 μg cm-2) and coppiced trees (82.8 and 84.1 μg cm-2, for Q. ilex and A. unedo, respectively). Mean foliar nitrogen values were similar for uncoppiced (1.1%) and coppiced trees (1.0%).
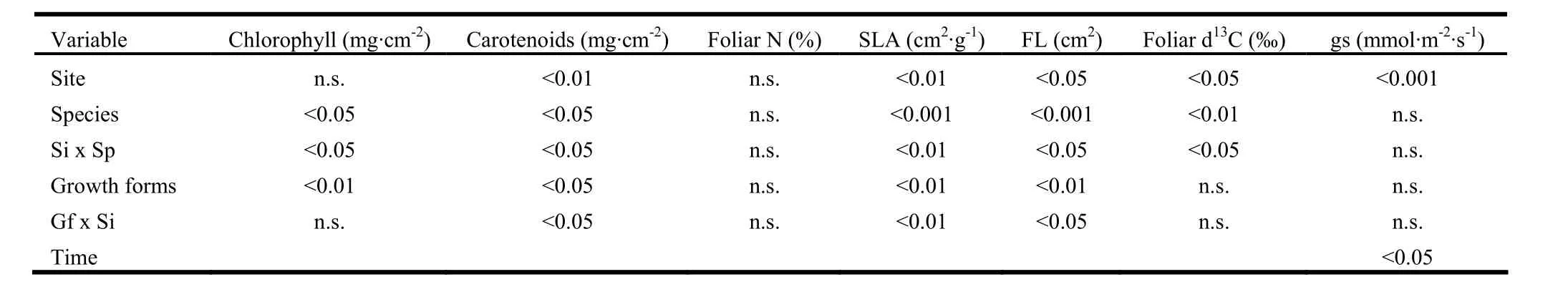
Table 3: P values (probability > F, an effect is significant if P <0.05, in bold) from ANOVA for the effects of site, species, growth forms, time and their interactions on main response parameter.
Specific Leaf Area and Leaf Length
Specific leaf area (SLA) and leaf length (FL) differed between sites (p <0.01 and p <0.05, respectively). FL and SLA were significantly lower in uncoppiced than in coppiced trees (p<0.01). Both FL and SLA showed significant Si × Gf and Si × Sp interactions, indicating high variability between sites (Table 3).
Foliar carbon isotope composition
Foliar carbon isotope ratios were higher at the SW site (p <0.05) (Table 3). δ13C values differed by species with higher mean values recorded for coppiced trees of A. unedo (-25.1‰) compared to Q. ilex (-26.3‰) (p <0.01).
Stomatal conductance
Stomatal conductance was higher in June and September than in July and August (Figures 1a and 1b) at both sites. July and August were characterized by lower (p <0.05) gs values for all three growth forms at both sites. Stomatal conductance values were markedly lower at the SW site during summer when they were consistently <100 mmol·m-2·s-1. Conversely, summer was characterized by higher gs values at the NW site, always >100 mmol·m-2·s-1with a maximum value of 164.9 mmol·m-2·s-1recorded in July on coppiced Q. ilex trees.
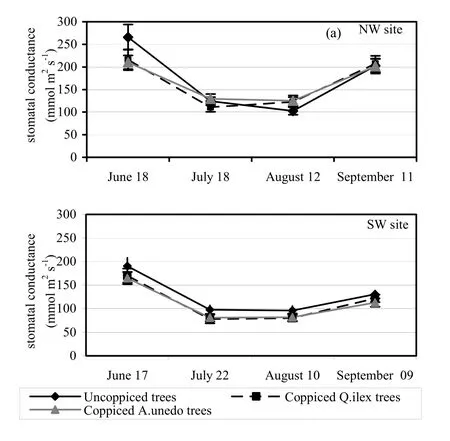
Fig. 1: Spring and summer pattern of stomatal conductance (June to September) for the experimental sites. The reported values refer to the average daily figures, the same trees having been measured at 8.00 a.m., again at noon and again at 4.00 p.m. Bars indicate the standard error of the mean. Different letters denote significant differences between growth forms (sample size = 45, p <0.05, Tukey’s HSD test).
Discussion
Physiological differences between sites
The hypothesis that intensifying drought caused physiological changes to Mediterranean coppice species and growth forms was supported by the data. Changes in the six selected parameters between sites and growth forms indicated two lines of adjustment (Fig. 2): (1) a trait indicated acclimation by difference in values between sites and growth forms, e.g., large differences by site in carotenoids, foliar δ13C, gs and SLA, and small differences by growth form in total chlorophyll, foliar nitrogen (coppiced trees), and foliar δ13C (both uncoppiced and coppiced trees); (2) a trait indicated marked acclimation by differences in value between sites and between growth forms, e.g., gs, foliar δ13C, SLA and leaf length.
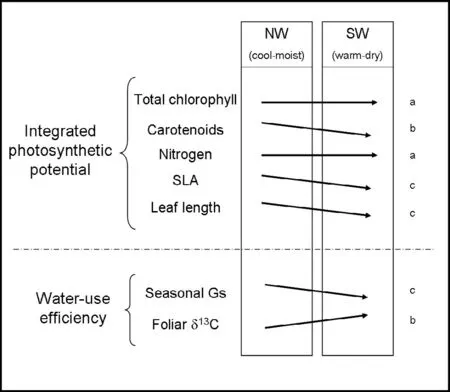
Fig. 2: Physiological parameters describing the photosynthetic potential and water-use efficiency of growth forms for the experimental sites. The direction of the arrows indicates the increase or decrease of the parameters measured between NW and SW aspect. a) Similar trait: refers to small changes between sites; b) Acclimation trait: refers to the larger site differences and small growth forms differences; c) Marked acclimation trait: refers to the differences between sites and among growth forms.
Growth-form traits showed acclimation by differences in total chlorophyll, carotenoids and foliar morphology. This was probably because (1) they had different potential photosynthetic responses at both sites, and (2) the coppiced trees were subject to greater shading than the uncoppiced trees.
Garzia-Plazaola et al. (1997) and Faria et al. (1998) reported that carotenoid concentration provide an index for dissipation of excessive thermal energy in response to drought, e.g., when carbon assimilation is limited by reduced stomatal conductance the observed variability in carotenoid would indicate a physiological adjustment, suggesting that photo-protection processes were the primary physiological responses to differences between sites and drought intensity or duration. Faria et al. (1998) and Oliveira & Penuelas (2000) reported significantly lower total chlorophyll in Mediterranean forest stands populated mainly by A. unedo, Q. ilex and Q. suber than we recorded here. In Mediterranean forest ecosystems, lower chlorophyll contents could be a strategy to avoid capture of excessive light at colder sites and at those with greater sunlight intensity (Núñez-Olivera et al. 1994) or could be an adaptive response against photo-inhibition as chlorophyll reduces the light harvesting capacity of the leaf (Kyparissis et al. 1995).
Foliar δ13C and stomatal conductance responded to drought at the site- and species-level
Foliar δ13C varied by site and species. Foliar δ13C was greater at the drier SW site and lower in Q. ilex than in A. unedo. At the site-level, this result was due to the extra month of summer drought that affected the SW site. Air temperature and rainfall data showed an intensifying drought at both sites with respect to long-term averages, an extended drought period in spring and summer with less rainfall at the SW site (May to September) than at the NW site (June to September). This was supported by comparison of the three years mean spring rainfall (April to June) during the study period at both sites: the SW site recorded 134.6 mm, 46.1% less than the 249.7 mm at the NW site. Foliar δ13C values at the NW site were lower in uncoppiced than in coppiced trees. At the SW site, all growth forms showed higher net δ13C values than at the NW site (i.e., +2.45‰ for uncoppiced trees, +0.58 and +1.20‰ for coppiced trees). Since δ13C is directly related to intrinsic water-use efficiency (iWUE= A/gs), δ13C variations can be the result of changes in gs or A. For instance, an increase in δ13C, interpreted as a reduction in pi (intercellular partial pressures of CO2inside the leaf) in the Farquhar model (1982), can be the result of either (1) decreased gs (at constant A); or (2) increased A (at constant gs). In this study A was not measured directly, therefore we did not calculate iWUE, but seasonal gs measurements were carried out under constant PAR values (i.e., 1,000 and 1,200 μE·m-2·s-1), hence we can assume that the lower gs values at the SW site explained the foliar δ13C increases (greater decrease in gs over A). At the same time, the higher gs values at the NW site explained the lower foliar δ13C values. With regard to the slight differences in foliar δ13C by growth form, trees naturally grow more slowly with increasing age, thus decreasing their carbon gain (Piper and Fajardo 2011). We assumed that the photosynthetic rate decreased with increasing age, therefore the lower A values probably explained the lower foliar δ13C values in the uncoppiced trees at the NW site. However all growth forms at the SW site had higher foliar δ13C values, rendering unlikely the hypothesis that an increase in δ13C was due to increasing A.
Conclusions
Our comparatively dry SW site had higher foliar δ13C, lower stomatal conductance, lower carotenoid content, and similarfoliar nitrogen concentration and total chlorophyll. Similar results were observed by Van de Water et al. (2002) comparing foliar δ13C values at dry and wet sites. The lower rainfall at the SW site was associated with lower gs and higher foliar δ13C values, supporting the evidence that spring rainfall can markedly influence water-use efficiency in Mediterranean environments. Within the general frame of references, the paper’s findings provide further knowledge of acclimation capacity of Mediterranean forest ecosystems.
Acknowledgements
We thank Matilde Tamantini (DIBAF, University of Viterbo) for her help with lab analyses, and Ermenegildo Magnani (Stable Isotopes Laboratory, Agrital Ricerche, Maccarese, Rome) for his technical support with carbon isotopic analyses. Many thanks to the staff of Regional Forest Service of Is Cannoneris forests for their kind hospitality and support during sampling activities. This study was supported by the Italian Ministry for Agricultural and Forest Policy, under the programme Ri. Selv. Italia (Research unit 3.2.1, DIBAF, University of Viterbo). The authors thank the editors and the anonymous reviewers for their many valuable comments.
Amorini E, Bruschini S, Cutini A, Fabbio G, Manetti MC. 1996. Silvicultural treatment of Holm oak (Quercus ilex L.) coppices in Southern Sardinia: thinning and related effects on stand structure and canopy cover. Annals of the Forestry Centre of the Agricultural Research Council (CRA-SEL), 27: 167−175.
Beerling DJ, Heath J, Woodward FI, Mansfield TA. 1996. Interaction in trees: observations and mechanism. New Phytologist, 134: 235−242.
Christensen JH, Hewitson B, Busuioc A, Chen A, Gao X, Held I, Jones R, Kolli RK, Kwon WT, Laprise R, et al. 2007. Regional climate projections. In: Solomon S, Quin D, Manning M, Chen Z, Marquis M, Averyt KB, Tingor M, Miller HL (eds), Climate Change 2007: The Physical Science Basis. Contributions of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambride, United Kingdom/New York, NY: Cambridge University Press.
Ciancio O, Corona P, La Monaca A, Portoghesi L, Travaglini D. 2006. Conversion of clearcut beech coppices into high forests with continuous cover: A case study in central Italy. Forest Ecology and Management, 22: 235−240.
Cordell S, Goldstein G, Mueller-Dombois D, Webb D, Vitousek PM. 1998. Physiological and morphological variation in Metrosideros polymorpha, a dominant Hawaiian tree species, along an altitudinal gradient: role of phenotypic plasticity. Oecologia, 113: 188−196.
Cordell S, Goldstein G, Meinzer FC, Handley L. 1999. Allocation of nitrogen and carbon in leaves of Metrosideros polymorpha regulates carboxylation capacity and δ13C along altitudinal gradient. Functional Ecology, 13: 811−818.
Cubera E, Moreno G. 2007. Effect of single Quercus ilex trees upon spatial and seasonal changes in soil water content in dehesas of central western Spain. Annals of Forest Science, 64: 355−364.
Damesin C, Rambal S, Joffre R. 1998. Seasonal and annual changes in leaf δ13C in two co-occurring Mediterranean oaks: relations to leaf growth and drought progression. Functional Ecology, 12: 778−785.
David TS, Henriques MO, Kurz-Besson C, Nunes J, Valente F, Vaz M, Pereira JS, Siegwolf R, Chaves MM, Gazarini LC, et al. 2007. Water-use strategies in two co-occurring Mediterranean evergreen oaks: surviving the summer drought. Tree Physiology, 27: 793−803.
De Lillis M, Matteucci G, Valentini R. 2004. Carbon assimilation, nitrogen, and photochemical efficiency of different Himalayan tree species along an altitudinal gradient. Photosynthetica, 42: 597−605.
Di Matteo G, De Angelis P, Scarascia Mugnozza G. 2005. Application of isotope discrimination techniques to evaluate the functional response of Mediterranean coppices to high-forest conversion cut. Forest@, 2: 367−377.
Ehleringer JR, Cerling TE. 1995. Atmospheric CO2and the ratio of intercellular to ambient CO2concentrations in plants. Tree Physiology, 15: 105−111.
Evans JR. 1989. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia, 78: 9−19.
Fabbio G, Cutini A, Mascia V. 1996. Silvicultural treatment of holm oak coppices (Quercus ilex L.) in Southern Sardinia: effects of canopy and crop thinning on microclimate. Annals of the Forestry Centre of the Agricultural Research Council (CRA-SEL), 27: 55−63.
Faria T, Silverio D, Breia E, Cabral R, Abadia A, Abadia J, Pereira JS, Chaves MM. 1998. Differences in the response of carbon assimilation to summer stress (water deficits, high light and temperature) in four Mediterranean tree species. Physiologia Plantarum, 102: 419−429.
Farquhar GD, O’Leary MH, Berry JA. 1982. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Australian Journal of Plant Physiology, 9: 121−137.
Field C, Mooney HA. 1986. The photosynthesis-nitrogen relationship in wild plants: In: Givinish TJ (eds), On the Economy of Plants Form and Function. Cambridge: Cambridge University Press, pp. 25−55.
Friend AD, Woodward FI, Switsur VR. 1989. Field measurements of photosynthesis, stomatal conductance, leaf nitrogen and δ13C along altitudinal gradients in Scotland. Functional Ecology, 3: 117−122.
Gale J. 1972. The availability of carbon dioxide for photosynthesis at high altitudes: theoretical considerations. Ecology, 53: 494−497.
Gale J. 2004. Plants and altitude – revisited. Annals of Botany, 94: 199.
Garzia-Plazaola JI, Faria T, Abadia J, Abadia A, Chaves MM, Pereira JS. 1997. Seasonal changes in xanthophylls composition and photosynthesis in cork oak (Quercus suber L.) leaves under Mediterranean climate. Journal of Experimental Botany, 48: 1667−1674.
Gratani L, Covone F, Larcher W. 2006. Leaf plasticity in response to light of three evergreen species of the Mediterranean maquis. Trees, 20: 549−558.
Hikosaka K, Nagamatsu D, Ishii HS, Hirose T. 2002. Photosynthesis-nitrogen relationships in species at different altitudes on Mount Kinablau, Malaysia. Ecological Research, 17: 305−313.
Hultine KR, Marshall JD. 2000. Altitude trends in conifer leaf morphology and stable carbon isotope composition. Oecologia, 123: 32−40.
Klein T, Di Matteo G, Rotemberg E, Cohen S, Yakir D. 2013. Differential ecophysiological response of a major Mediterranean pine species across a climatic gradient. Tree Physiology, 33: 26−36.
Kogami H, Hanba YT, Kibe T, Terashima I, Masuzawa T. 2001. CO2transfer conductance, leaf structure and carbon isotope composition of Poly-gonum cuspidatum leaves from low and high altitudes. Plant, Cell and Environment, 24: 529−538.
Körner C, Farquhar GD, Roksandic Z. 1988. A global survey of carbon isotope discrimination in plants from high altitude. Oecologia, 74: 623−632.
Körner C, Farquhar GD, Wong SC. 1991. Carbon isotope discrimination by follows latitudinal and altitudinal trends. Oecologia, 88: 30−40.
Kyparissis A, Petropoulou Y, Manetas Y. 1995. Summer survival of leaves in a soft-leaved shrub (Phlomis fruticosa L., Labiatae) under Mediterranean field conditions: avoidance of photoinhibitory damage through decreased chlorophyll contents. Journal of Experimental Botany, 46: 1825−1831.
Lichtenthaler HK. 1987. Chlorophylls and carotenoids: pigments of photosynthetic apparatus biomembranes. Methods in Enzymology, 148: 349−382.
Lindner M, Maroschek M, Netherer S, Kremer A, Barbati A, Garcia-Gonzalo J, Seidl R, Delzon S, Corona P, Kolstro M, Lexer MJ, Marchetti M. 2010. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. Forest Ecology and Management, 259(4): 698–709.
Marshall JD, Zhang J. 1994. Carbon isotope discrimination and water use efficiency of native plants of the north-central Rockies. Ecology, 75: 1887−1895.
Maxime C, Hendrik D. 2011. Effects of climate on diameter growth of cooccurring Fagus sylvatica and Abies alba along an altitudinal gradient. Trees, 25: 265−276.
Morandini R. (Coordinator). 1998. 1996: Special issue on Improvement of Mediterranean coppices. MEDCOP project. Annals of the Forestry Centre of the Agricultural Research Council (CRA-SEL), 27: 224 pp.
Morecroft MD, Woodward FI, Marrs RH. 1992. Altitudinal trends in leaf nutrient contents, leaf size and δ13C of Alchemilla alpina. Functional Ecology, 6: 730−740.
Morecroft MD, Woodward FI. 1996. Experiments on the causes of altitudinal differences in leaf nutrient contents, size, and δ13C of Alchemilla alpina. New Phytologist, 134: 471−479.
Núñez-Olivera E, Martínez-Abaigar J, Escudero JC. 1994. Chlorophyll content of a Mediterranean shrub (Cistus ladanifer L.) over a latitude and altitude gradient in the Iberian Peninsula. Photosynthetica, 30: 133–142.
Oliveira G, Penuelas J. 2000. Comparative photochemical and phenomorphological responses to winter stress of an evergreen (Quercus ilex L.) and a semi-deciduous (Cistus albidus L.) Mediterranean woody species. Acta Oecologica, 21: 97−107.
Panek JA, Waring RH 1995. Carbon isotope variation in Douglas-fir foliage: improving the δ13C-climate relationship. Tree Physiology, 15: 657−663.
Paridari IC, Jalali SG, Sonboli A, Zarafshar M, Bruschi P. 2013. Leaf macroand micro-morphological altitudinal variability of Carpinus betulus in the Hyrcanian forest (Iran). Journal of Forestry Research, 24: 301−307.
Piper FI, Fajardo A. 2011. No evidence of carbon limitation with tree age and height in Nothofagus pumilio under Mediterranean and temperate climate conditions. Annals of Botany, 108: 907−917.doi: 10.1093/aob/mcr195
Sah SP, Brume R. 2003. Altitudinal gradients of natural abundance of stable isotopes of nitrogen and carbon in the needles and soil of a pine forest in Nepal. Journal of Forest Science, 49: 19−26.
Sakata T, Yokoi Y. 2002. Analysis of the O2dependency in leaf-level photosynthesis of two Reynoutria japonica populations growing at different altitudes. Plant, Cell and Environment, 25: 65−74.
Shi Z, Shirong L, Xingliang L, Centritto M. 2006. Altitudinal variation in photosynthetic capacity, diffusional conductance and δ13C of butterfly bush (Buddleja davidii) plants growing at high elevations. Physiologia Plantarum, 128: 722−731.
Sun ZJ, Livingston NJ, Guy RD, Ethier GJ. 1996. Stable carbon isotopes as indicators of increased water use efficiency and productivity in white spruce (Picea glauca (Moench) Voss)) seedlings. Plant, Cell and Environment, 19: 887-894.
Terashima I, Masuzawa T, Ohba H, Yokoi Y. 1995. Is photosynthesis suppressed at higher elevation because of low CO2pressure? Ecology, 76: 2663−2668.
Tognetti R, Cherubini P, Marchi S, Raschi A. 2007. Leaf traits and tree rings suggest different water-use and carbon assimilation strategies by twooccurring Quercus species in a Mediterranean mixed-forest stand in Tuscany, Italy. Tree Physiology, 27: 1741−1751.
Valentini R, Scarascia Mugnozza G, Ehleringer JR. 1992. Hydrogen and carbon isotope ratios of selected species of a Mediterranean macchia ecosystem. Functional Ecology, 6: 627−631.
Van De Water PK, Leavitt SW, Betancourt JL. 2002. Leaf δ13C variability with elevation, slope aspect, and precipitation in the southwest United States. Oecologia, 132: 332−343.
Vitousek PM, Field CB, Matson PA. 1990. Variation in foliar δ13C in Hawaiian Metrosideros polymorpha: a case of internal resistance? Oecologia, 84: 362−370.
DOI 10.1007/s11676-014-0532-4
Project funding: This study was supported by the Italian Ministry for Agricultural and Forest Policy, under the programme Ri. Selv. Italia (Research unit 3.2.1, DIBAF, University of Viterbo).
The online version is available at http://www.Link.springer.com
Giovanni Di Matteo (), Luigi Perini
Consiglio per la Ricerca e la sperimentazione in Agricoltura, Research Unit for Climatology and Meteorology applied to Agriculture, CRACMA, I-00186 Rome, Italy.
Tel.: 0039-06-69531228. E-mail: giovanni.dimatteo@entecra.it
Paolo Atzori, Paolo De Angelis, Giuseppe Scarascia Mugnozza University of Viterbo, Department for Innovation in Biological, Agrofood and Forest systems, DIBAF, I-01100, Viterbo, Italy.
Tiziano Mei
Ente Foreste della Sardegna, Servizio Territoriale di Cagliari, Viale Luigi Merello 86, I-09123 Cagliari, Italy.
Giada Bertini, Gianfranco Fabbio Consiglio per la Ricerca e la sperimentazione in Agricoltura, Forestry Research Centre, CRA-SEL, I-52100, Arezzo, Italy.
Corresponding editor: Chai Ruihai
杂志排行
Journal of Forestry Research的其它文章
- Growth and yield of two grain crops on sites former covered with eucalypt plantations in Koga Watershed, northwestern Ethiopia
- Carbon stock in Korean larch plantations along a chronosequence in the Lesser Khingan Mountains, China
- Biomass accumulation and nutrient uptake of 16 riparian woody plant species in Northeast China
- Cloning and sequence analysis of nine novel MYB genes in Taxodiaceae plants
- Genetic and morphological variation in natural teak (Tectona grandis) populations of the Western Ghats in Southern India
- Improved salt tolerance of Populus davidiana × P. bolleana overexpressed LEA from Tamarix androssowii
