纳米金/碳催化剂的合成及其在多巴胺电化学检测中的应用
2014-09-02杨敬贺李亚敏
杨敬贺,杨 朵,李亚敏
(1.河南大学 化学化工学院,河南 开封 475004; 2.北京大学 化学与分子工程学院,北京 100871; 3.河南师范大学 新联学院(理学院),河南 新乡 453007)
纳米金/碳催化剂的合成及其在多巴胺电化学检测中的应用
杨敬贺1, 2*,杨 朵3,李亚敏1
(1.河南大学 化学化工学院,河南 开封 475004; 2.北京大学 化学与分子工程学院,北京 100871; 3.河南师范大学 新联学院(理学院),河南 新乡 453007)
建立了一种合成碳负载型金纳米颗粒(AuC)的新方法. 将酵母菌、葡萄糖及HAuCl4溶液混合后置于恒温摇床中,在310 K下振荡数天得到酵母菌吸附的金前体盐(AuY);将AuY在氮气气氛中1 273 K下煅烧得到AuC. 采用扫描电镜和透射电镜观察了AuC的形貌; 将AuC修饰在玻碳电极上并用于多巴胺的电化学检测. 结果表明, AuY培养3 d后,其酵母菌颗粒粒径分布不均匀, 这主要是由于大量葡萄糖的吸附和包裹以及酵母孢子的存在所致; 而AuY培养6 d后得到的金颗粒粒径分布均匀,粒径约为10 nm. 此外, AuC在多巴胺电化学检测中具有非常好的响应,且检出限较低, 其电化学过程为混合动力学控制过程.
纳米金/碳催化剂;酵母吸附;碳化法;合成;多巴胺;电化学检测;应用
As we all know, metal nanostructures have been widely applied in many different fields such as catalysis, electronics, adsorption, life sciences, medicine and aerospace owing to their unique properties compared with bulk metals[1]. Many traditional physical and chemical methods have been explored to synthesize nanostructures. However, these methods are energy intensive or not environmentally benign, which makes it imperative to develop cleaner and non-toxic technologies for synthesizing nanostructures[1]. Recently, the use of microbial biomass in preparing nanoparticles (denoted as NPs) has emerged as one of the research highlights in nanotechnology[2]. For example, actinomycetes, bacteria, fungi, and even bacteria have been used to prepare metal NPs such as Au[3-4], Pd[5-6]and Ag[7-9]. It has been demonstrated that enzymes of live microorganisms can catalyze the reduction from metal ions to NPs[10]. However, the main drawback of enzyme-catalyzed reduction is that as-synthesized NPs are usually formed in the cell periplasm, even if some NPs exist on the cell surface, and the biological substrate has poor stability and conductivity. These would limit the application scope of the NPs, especially in catalysis and electrochemistry. Carbon material is a class of carrier with excellent stability and conductivity. More importantly, in the course of the synthesis of supported metal nanostructures, especially NPs, the dispersion of metal salt precursor on the support is very important. Volumetric impregnation is a widely used method. However, this method is time-consuming and unsuitable for the synthesis of large quantities of materials.
We anticipate that microbial biomass can be combined with calcining to prepare carbon supported metal NPs. Namely, microbial biomass can be adopted to prepare supported NPs from the dispersion of expected metal salt precursor, and then the carbon supported metal NPs can be prepared by calcination the microbial biomass precursor containing metal. Herein, we report a facile method for the synthesis of carbon-supported gold NPs (AuC) by 2 h calcination of yeast-biosorption-Au species (AuY) at 1 273 K in N2. The electrochemical performance of as-prepared AuC in the electro-oxidation and determination of dopamine (denoted as DA) is also reported.
1 Experimental
1.1 Reagents
Nafion (5% ethanol solution, mass fraction)was purchased from Alfa Aesar and diluted to 0.1% (mass fraction) with doubly distilled water in use. Chloroauric acid (HAuCl4·3H2O), yeast extract powder, glucose and other agents were purchased from Sinopharm Chemical Reagent Co., Ltd. All stock solutions were prepared with deionized water (resistance not less than 18.2 MΩ·cm).
1.2 Synthesis of various target products
10 g of yeast extract powder, 10 g of glucose and 10 mL of chloroauric acid solution (10 g·L-1) were dissolved in 300 mL of deionized water in an Erlenmeyer flask. Then the flask was put into a shaker and shaked at 310 K for 3 d and 6 d, respectively. AuY species were separated by filtration and dried in an air oven at 310 K overnight. AuY species obtained after 3 d of reaction and 6 d of reaction are denoted as AuY-3 and AuY-6, respectively.
AuC was obtained by calcining AuY in a tubular furnace under nitrogen atmosphere. The temperature rose at the rate of 10 K·min-1until 1 273 K and then held there for 2 h. AuC samples obtained from AuY-3 and AuY-6 are marked as AuC-3 and AuC-6, respectively.
1.3 Characterization
The morphologies of various products were observed with a scanning electron microscope (SEM, XL30S-FEG, 5 kV) and a transmission electron microscope (TEM, FEI Tecnai G2 T20). To-be-tested products were seperately dissolved in ethanol under ultrasonic irradiation, and then a drop of resultant solutions was put onto a Cu grid providing samples for TEM analysis.
1.4 Preparation of modified glassy carbon electrode (GCE)
The glassy carbonelectrodes (GCEs, 3 mm diameter, Tianjin Aida, Inc.) were polished withα-Al2O3powder (40 nm), rinsed twice by deionized water and ethanol, and then dried at room temperature. 1 mg of as-ynthesized catalyst was dispersed in 1 mL of 0.1% nafion solution. Then the mixture was dropped on a pre-treated GCE to fabricate a modified GCE. Finally the modified GCEs were dried under infrared light.
1.5 Electrochemical measurements
Electrochemical experiments were performed at 298 K with a CHI660D electrochemical workstation (CHI, Shanghai) equipped with a three-electrode setup. GCE or modified GCEs acted as working electrode; saturated calomel electrode (SCE) was used as reference electrode, and platinum wire as the counter electrode.
2 Results and discussion
AuC hybrid was developed by a facile two-step method (Fig. 1A). Firstly, yeast as the organism precursor vector for dispersing metal was mixed with glucose and HAuCl4aqueous solution for 3 d and 6 d at 310 K in a shaker, generating AuY-3 and AuY-6, respectively. Then AuY-3 and AuY-6 were calcined at 1 273 K under N2atmosphere affording AuC-3 and AuC-6, respectively. At the beginning, HAuCl4would be slowly adsorbed onto yeast in the mixture. Some HAuCl4would be reduced by the catalysis of enzymes of yeast besides the direct reduction by glucose[1]. The yeast would grow and divide over time. The absorbed gold and HAuCl4would be dispersed with the growth of yeast and the glucose was slowly consumed. As a result, AuY-3 is obtained (Fig.1B). The size of yeast in AuY-3 is not uniform because of the absorption and package of plenty of glucose and the presence of many yeast spores. After 6 d, most of the glucose is consumed and the yeast is able to fully grow and multiplicate. Thus, the number and density of yeast are larger than that of AuY-3 (Fig.1C). The dispersion of gold would be also higher than that of AuY-3. When AuY is calcined at high temperature under a nitrogen atmosphere, the yeast is carburized and gold is completely reduced; and gold NPs have no significant accumulation because of the protection of yeast (Fig.2A). Though AuC-3 and AuC-6 exhibit the same amount of gold, the gold NPs of AuY-6 have better dispersion than those of AuY-3. In other words, the size of gold NPs of AuC-6 is smaller than that of AuC-3, and the number of gold NPs of AuC-6 is greater than that of AuC-3 (Fig. 2B and 2C). As shown in Fig. 2D, the size of gold NPs of AuC-6 is about 10 nm. We suppose that such differences in the number and size of gold NPs should influence the electro-catalytic activity of relevant carbon-supported gold catalyst, which is to be discussed in the forthcoming section.

Fig.1 Scheme of preparation of AuC-3 and AuC-6 (A) as well as SEM images of AuY-3 (B) and AuY-6 (C)

Fig.2 TEM images of AuC-3 (A) and AuC-6 (B, C and D)
Fig.3A shows the steady-state cyclic voltammograms (CVs) illustrating electro-oxidation of DA. It can be seen that the anodic peak current of DA at the bare/GCE shows a small anodic peak with a peak potential of 0.35 V, whereas two times larger anodic peak current and a marked reduction of the anodic peak potential about 80 mV compared to unmodified bare/GCE are observed for AuC-6/GCE. AuC-6/GCE also presents well-defined and quasi-reversible redox peaks corresponding to the electrochemical reaction of DA, which well corresponds to its good electro-catalytic activity. These phenomena are clear evidences of catalytic effect of the chemically modified electrode towards DA oxidation. In terms of the anodic peak current and anodic peak potential, AuC-6/GCE exhibits better electrochemical performance (Fig.3B). The effect of AuC could have a relationship with the size and dispersion of gold NPs on AuC. CVs of DA over AuC-6/GCE are depicted in Fig. 3C with the scan rate ranging from 20 to 300 mV/s. The relation between redox peak currents and the scan rates implies a hybrid kinetic mechanism. At lower scan rates from 20 to 130 mV·s-1, the electrochemical redox behavior of DA over the AuC-6/GCE surface is a diffusion-controlled process, while at higher scan rates (> 160 mV/s) it is a surface absorption-controlled process with the current peak being proportional to the scan rate. This means that the reaction occurs not only at reactive sites within the adsorbed assembly but also at the outer surface of the electrode[11-14]. In other words, Au and C constitute a stable composite and AuC-6 is a good and sensitive sensor for DA. Square wave voltammogram (SWV) of DA was carried out with AuC-6/GCE (Fig.3D). The SWV amperometric currents show a prefect linear relationship with the concentration of DA in the range of 5-50 μmol·L-1and 60-100 μ·L-1, with the detection limit being 1.5 μmol·L-1at a signal-to-noise ratio of 3. This means that the AuC-6/GCE electrode exibits high effectiveness in accurate determination of DA.
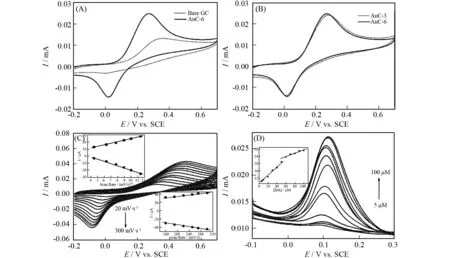
Fig.3 CVs (A and B) in the presence of phosphate buffer (PBS) solution (0.2 mol·L-1) containing 1.0×10-3 mol·L-1 DA at pH=7.4 and 50 mV·s-1; CVs on AuC-6/GCE in a 0.2 mol·L-1 PBS solution containing 1.0×10-3 mol·L-1 DA at pH = 7.4 and different sweep rates (C). Linear relationship between peak current versus the square root of the scan rate (scan rate < 130 mV·s-1) and the scan rate (scan rate > 160 mV·s-1) (C, inset); Square wave voltammogram (SWV) for AuC-6/GCE in 0.2 mol·L-1 PBS solution containing the DA (concentrations from 5 to 100 μmol·L-1 at pH =7.4) (D); Linear calibration curve for the determination of DA (D, inset)
3 Conclusions
Carbon-supported gold NPs hybrids were prepared by calcination precursor AuY obtained through mixing and shaking of yeast, glucose and HAuCl4solution at a pre-set temperature for different durations. The feasibility of as-prepared AuC catalyst in the electrochemical detection of dopamine was investigated. It has been found that the present method is significant for the synthesis of carbon-supported metal NPs. As-prepared AuC catalyst exhibits excellent activity at low peak potential, showing potential in the investigation and diagnosis of dopamine-related diseases.
[1]LIN Liqin, WU Weiwei, HUANG Jiale, et al. Catalytic gold NPs immobilized on yeast: From biosorption to bioreduction [J]. Chem Eng J, 2013, 225: 857-864.
[2]KATZ E, WILLNER I. Integrated nanoparticle-biomolecule hybrid systems: Synthesis, properties, and applications [J]. Angew Chem Int Ed, 2004, 43 (45): 6042-6108.
[3]HE Shiying, GUO Zhirui, ZHANG Yu, et al. Biosynthesis of gold NPs using the bacteria Rhodopseudomonas capsulata [J]. Mater Lett, 2007, 61 (18): 3984-3987.
[4]AGNIHOTRI M, JOSHI S, KUMAR A R, et al. Biosynthesis of gold NPs by the tropical marine yeast Yarrowia lipolytica NCIM 3589 [J]. Mater Lett, 2009, 63 (15): 1231-1234.
[5]SRIVASTAVA S K, CONSTANTI M. Room temperature biogenic synthesis of multiple NPs (Ag, Pd, Fe, Rh, Ni, Ru, Pt, Co, and Li by Pseudomonas aeruginosa SM1 [J]. J Nanopart Res, 2012, 14 (4): 831-840.
[6]YONG P, ROWSON N A, FARR J P G, et al. Bioreduction and biocrystallization of palladium by Desulfovibrio desulfuricans NCIMB 8307 [J]. Biotechnol Bioeng, 2002, 80 (4): 369-379.
[7]YANG Dapeng, CHEN Shouhui, HUANG Peng, et al. Bacteria-template synthesized silver microspheres with hollow and porous structures as excellent SERS substrate [J]. Green Chem, 2010, 12 (11): 2038-2042.
[8]JUIBARI M M, ABBASALIZADEH S, JOUZANI G S, et al.Intensified biosynthesis of silver NPs using a native extremophilic Ureibacillus thermosphaericus strain [J]. Mater Lett, 2011, 65 (6): 1014-1017.
[9]SNEHA K, SATHISHKUMAR M, MAO J, et al. Corynebacterium glutamicum-mediated crystallization of silver ions through sorption and reduction processes [J]. Chem Eng J, 2010, 162 (3): 989-996.
[10]SEN K, SINHA P, LAHIRI S. Time dependent formation of gold NPs in yeast cells: A comparative study [J]. Biochem Eng, J 2011, 55 (1): 1-6.
[11]HOU S F, KASNER M L, SU S J, et al. Highly sensitive and selective dopamine biosensor fabricated with silanized graphene [J]. J Phys Chem C, 2010, 114 (35): 14915-14921.
[12]RASSAEI L, SILLANPM, MARKEN F. Modified carbon nanoparticle-chitosan film electrodes: Physisorption versus chemisorption [J]. Electrochim Acta, 2008, 53 (19): 5732-5738.
[13]SHAHROKHIAN S, ZARE-MEHRJARDI H R. Application of thionine-nafion supported on multi-walled carbon nanotube for preparation of a modified electrode in simultaneous voltammetric detection of dopamine and ascorbic acid [J]. Electrochim Acta, 2007, 52 (22): 6310-6317.
[14]HAN H S, LEE H K, YOU J M, et al. Electrochemical biosensor for simultaneous determination of dopamine and serotonin based on electrochemically reduced GO-porphyrin [J]. Sensor Actuat B-Chem, 2014, 190: 886-895.
[责任编辑:毛立群]
date:2014-04-25.
Henan University Research Foundation (2013YBZR007).
Biography:YANG Jinghe (1986-), male, lecturer, majoring in energy and environment catalysis.*
, E-mail: jhyang@henu.edu.cn.
