K3H3[As2W21O69(H2O)]·19H2O的合成及晶体结构
2014-09-02陈利娟
张 芳, 马 杏, 张 静, 陈利娟
(河南大学 化学化工学院,河南省多酸化学重点实验室, 河南 开封 475004)
K3H3[As2W21O69(H2O)]·19H2O的合成及晶体结构
张 芳, 马 杏, 张 静, 陈利娟*
(河南大学 化学化工学院,河南省多酸化学重点实验室, 河南 开封 475004)
利用二缺位K14[As2W19O67]·nH2O前驱体在酸性条件下的结构转变得到了饱和的砷钨酸盐K3H3[As2W21O69(H2O)]·19H2O (1), 借助傅立叶变换红外光谱和X射线单晶衍射对其结构进行了表征. 结果表明, 化合物1的多阴离子骨架由两个相同的三缺位[α-AsW9O33]9-片段通过三个W=O基团连接而成. 值得提及的是, 在化合物1的阴离子骨架中心空腔中存在一个游离的水分子.
多金属氧酸盐;砷钨氧酸盐;合成;晶体结构
As is known to all,polyoxotungstates (POTs) are a unique class of polynuclear metal-oxygen anionic clusters that exhibit a diverse compositional range and significant structural versatility, and they are usually composed of the early transition metal centered WO6polyhedra linked by shared corners, edges or faces, sometimes with additional heteroatoms (such as P, Si, As, etc) incorporated within the clusters[1-2]. Up to now, Keggin and Wells-Dawson polyoxoanions are probably the most famous POTs. Due to the advantages of their specific molecular composition, charge density, redox potentials, solubility characteristics and widespread applications in catalysis, medicine, magnetism and material science, the synthesis and characterization of novel arsenotungstates (ATs) are alluring[3-4]. In the past several decades, great efforts have been devoted to this field and some achievements have been made. For example, in 1986, a tetra-ZnIIsandwiched Keggin AT [Zn4(H2O)2(AsW9O34)]10-was first reported by EVANS et al[5]. With the expansion of research activity, in 2001, KORTZ et al discovered a gigantic chair-configuration AT cluster [As6W65O217(H2O)7]26-[6]. In 2010, WANG and co-workers prepared a dimeric 3d-4f heterometallic AT La[As2IIIW20CuO67(H2O)3]3-and a novel aggregate [As4IIIW19O69]12-[7-8]. In 2011, RITCHIE et al addressed a family of lanthanide substituted POTs with aminoacid components (HDABCO)8H5Li8[Ln4As5W40O144(H2O)10(Gly)2]·25H2O (Ln = Gd, Tb, Dy, Ho, Y, HDABCO = monoprotonated 1,4-diazabicyclooctane, Gly = glycine)[9]. To date, we also have made some progresses in the research of ATs. For instance, we synthesized a 65·8 CdSO4-like three-dimensional (denoted as 3D) framework [Cu(en)2]3[α-AsW11NaO39]·2H2O (en = ethylenediamine) in 2009[10], and we prepared a S-shaped multi-iron substituted AT [enH2]2[(α-H2AsVW6O26)Fe3(H2O)(B-α-H4AsVW9O34)]2Fe2·8H2O and quantitatively analyzed its magnetic behavior in 2011[11]. In 2012, we discovered organic-inorganic hybrids assembled by ATs and CuII-LnIII/IVheterometals Na3[Cu(en)2(H2O)][Cu(en)2]1.5[H3Ln(α-AsW11O39)2]·xH2O (Ln = PrIII,x= 5; Ln = NdIII,x= 4.5; Ln = SmIII,x= 5.5; Ln = EuIII,x= 4; Ln = TbIII,x= 4) and investigated their photodegradation towards rhodamine-B[12]. It is proved that [As2W19O67(H2O)]14-has versatile isomerization behavior and facile polymerization ability in an aqueous solution[11]. With the aim at discovering novel Ln substituted AT aggregates, recently we have launched our exploration on the reaction of [As2W19O67(H2O)]14-with lanthanide cations. In the present research, however, it is a pity that we failed to incorporate lanthanide cations into the outcome but only obtained a plenary POT, K3H3[As2W21O69(H2O)]·19H2O (1) (CSD: 427248). Here, we report its synthesis and structure characterization by Fourier transform infrared spectrometry (FT-IR) and single-crystal X-ray diffraction (XRD).
1 Experimental
1.1 Physical measurements
K14[As2W19O67]·nH2O was synthesized according to reference [6]and confirmed by FT-IR. Other reagents were obtained from commercial resources and used without further purification. FT-IR spectrum was obtained from a solid sample pelleted with KBr on Nicolet 170 SXFT-IR spectrometer in the range of 4 000-400 cm-1.
1.2 Synthesis of 1
0.282 g (0.053 mmol) K14[As2W19O67(H2O)](2) was suspended in 10 mL water at room temperature and stirred for 2 h (named as A solution). 0.077 g (0.176 mmol) Nd(NO3)3·6H2O was dissolved in 5 mL of water and its pH was adjusted to 0.66 by the addition of 0.3 mL (4 mol·L-1) HCl (named as B solution). B solution was rapidly poured into A solution under stirring. 1 h later, the pH value of the mixed solution changed to 1.27, and the solution was heated in water bath (80 ℃) for 1 h and then filtered after cooling. The yellow filtrate was slowly evaporated at room temperature, and yellow acicular crystals suitable for XRD analysis were obtained after filtering and drying in air for several days. Anal. calc. (%) for K3H3[As2W21O69(H2O)]·19H2O (1): K, 2.10; As, 2.68; W, 69.00. Found (%): K, 2.33; As, 2.40; W, 69.25. Unexpectedly, though Nd(NO3)3·6H2O was used as one of the raw materials in the reaction, no Nd3+ion was found in1. The specific role of Nd(NO3)3·6H2O in the reaction is not well understood at this stage.
1.3 X-ray crystallography
Intensity data of1was collected with a Bruker APEX-II CCD diffractometer at 296(2) K with graphite-monochromated Mo Kαradiation (λ= 0.071 073 nm). A total of 22 398 reflections was collected in the scanning range, and 2 635 independent reflections were included (Rint= 0.060 9). Cell constants and an orientation matrix for data collection were obtained from least-squares refinements of the setting angles in the range of 2.41° ≤θ≤ 24.99°. The structure was solved by direct methods and was refined by full-matrix least-squares methods with the SHELXTL-97 program package[13]. All intensity data was corrected for routine Lorentz polarization as well as for empirical absorption. Those hydrogen atoms attached to lattice water molecules were not located. Notably, the W4 atom is disordered over two sites (W4 and W4′). The crystallographic data and structural refinements for1are listed in Table 1. Selected bond lengths of1are presented in Table 2.

Table 1 Crystallographic data and structural refinements of 1
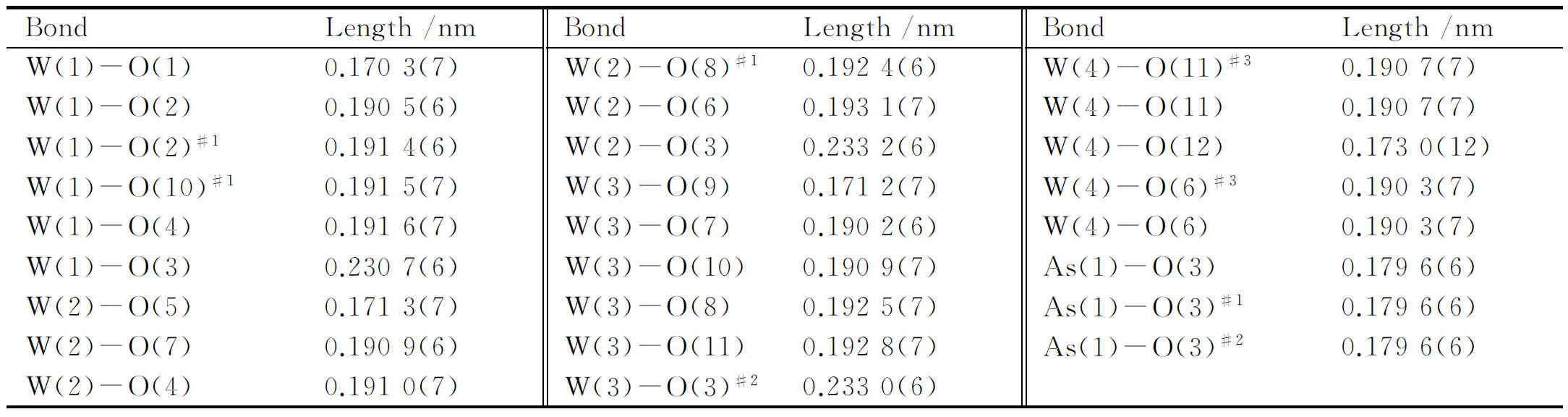
Table 2 Selected bond lengths of 1
2 Results and discussion
2.1 Crystal structure
Single-crystal XRD analysis shows that1crystallizes in the space groupP-31cand its molecule consists of a sandwich-type heteropolyanion [As2W21O69(H2O)]6-, three K+ions, three protons and nineteen lattice water molecules. The plenary polyoxoanion [As2W21O69(H2O)]6-is an interesting sandwich-like cluster, and it is constructed from two well-known trivacant Keggin [α-AsW9O33]9-fragments in eclipsed fashion joined together by three W=O groups. Interestingly, a dissociated water molecule is enclosed in the central cavity of the heteropolyanion of1. Viewing Fig.1a, we can have a good understanding of this amusing characteristic. Namely, the sandwich-type heteropolyanion [As2W21O69(H2O)]6-in1has theD3hsymmetry and theC3axis passes through two As atoms and a free water molecule in the central cavity. Two [α-AsW9O33]9-subunits are symmetrically situated at both sides of the plane constituted by three W=O groups. Each [α-AsW9O33]9-subunit consists of three corner-shared W3O13triads with a centered AsO3group (Fig.1b). Each AsIIIatom in [α-AsW9O33]9-subunit owns an unshared pair of electrons and exhibits a tri-coordinate environment defined by threeμ4-oxygen atoms from three W3O13triads with the As-O distances of 0.179 6(6) nm. The W centers in [α-AsW9O33]9-subunit display the octahedral coordination geometries. In each W3O13triad, three WO6octahedra are fused together in the edge-sharing mode with W-O distances of 0.170 3(7)-0.233 2(6) nm. Besides, it should be pointed out that three W atoms and three K atoms are bridged through twelve oxygen atoms forming a triangle whose three W atoms stand on the three vertexes while three K atoms sit on the midpoints of three edges, and the free water molecule is on the center of the triangle (Fig.1c). The edge length of the triangle is 0.817 5(1) nm. The crystallographically unique W4 atom employs the square-pyramidal geometry defined by fourμ3-oxygen atoms from two [α-AsW9O33]9-subunits standing on the bottom plane of square-pyramid while a terminal oxygen atom occupying the axial position. The K1 cation is coordinated by four terminal oxygen atoms and two bridging oxygen atoms from two [α-AsW9O33]9-subunits in one [As2W21O69(H2O)]6-unit, two terminal oxygen atoms from the other two [As2W21O69(H2O)]6-units and two water molecules [K-O: 0.279 7(14)-0.308 0(7) nm]. The precursor [As2W19O67(H2O)]14-(Fig.1d) can be viewed as a divacant derivative by removing two W=O groups away from the sandwich belt of the plenary polyoxoanion [As2W21O69(H2O)]6-, hence, the symmetry of the [As2W21O69(H2O)]6-polyoxoanion is higher than that of the [As2W19O67(H2O)]14-polyoxoanion.

Fig.1 (a) Ball-and-stick representation of the polyoxoanion in 1, (b) Ball-and-stick representation of the [α-AsW9O33]9-subunit, (c) Combination of three W atoms and three K atoms forming a triangle, and (d) Ball-and-stick representation of the polyoxoanion in 2 (symmetry code A: -y, 1+x-y, z, B: -1-x+y, -x, z, C: -1-x+y, y, 0.5-z, D: x, 1+x-y, 0.5-z, E: -y, -x, 0.5-z, F: -x, -x+y, -0.5+z, G: -x, 1-y, 1-z, H: -1+y, x, -0.5+z, I: x-y, x, 1-z, J: -1+y, -x+y, 1-z, K: x-y, 1-y, -0.5+z)
Notably, each [As2W21O69(H2O)]6-polyoxoanion is connected with six [As2W21O69(H2O)]6-polyoxoanions by way of nine K+cations giving rise to a 3D framework structure. Along the crystallographicalcaxis, six [As2W21O69(H2O)]6-polyoxoanions surround a hexagonal channel (Fig.2). The lattice water molecules are filled with the hexagonal channels.
2.2 FT-IR spectrum
FT-IR spectra of1and2are recorded between 4 000-400 cm-1with KBr pellets (Fig.3), and they display characteristic vibration patterns derived from the arsenotungstate frameworks in the low-wave number region[1,14]. IR spectra of1and2are similar. As to1, four characteristic vibration bands at 981, 894, 770 and 719 cm-1can be assigned toν(W-Ot),ν(As-Oa),ν(W-Ob) andν(W-Oc), respectively. In addition, the stretching vibration peak and bending vibration absorption peak of water molecules are observed at 3 444 and 1 628 cm-1, respectively. For2, four characteristic vibration absorption bands ofν(W-Ot),ν(As-Oa),ν(W-Ob) andν(W-Oc) appear at 974, 889, 715 and 631 cm-1, respectively. In comparison with those of1, all the vibration absorption bands of2have different red-shifts, because there are more K+cations in the periphery of the polyoxoanion of2than in the periphery of the polyoxoanion of1, and thus the electrostatic interactions between K+cations and polyoxoanion impair the W-O and As-O stretching vibrations. The stretching vibration peak and bending vibration absorption peak of water molecules are observed at 3 425 and 1 626 cm-1, respectively. Besides, the IR spectrum of2shows more splitting peaks than that of1in the low-wave number region of 1 000-400 cm-1, which suggests that the symmetry of the polyoxoanion in1is higher than that in2. These results are in good agreement with relevant structural analyses (Figs.1a and 1d).

Fig.2 The 3D framework showing hexagonal channels viewed along crystallographical c axis
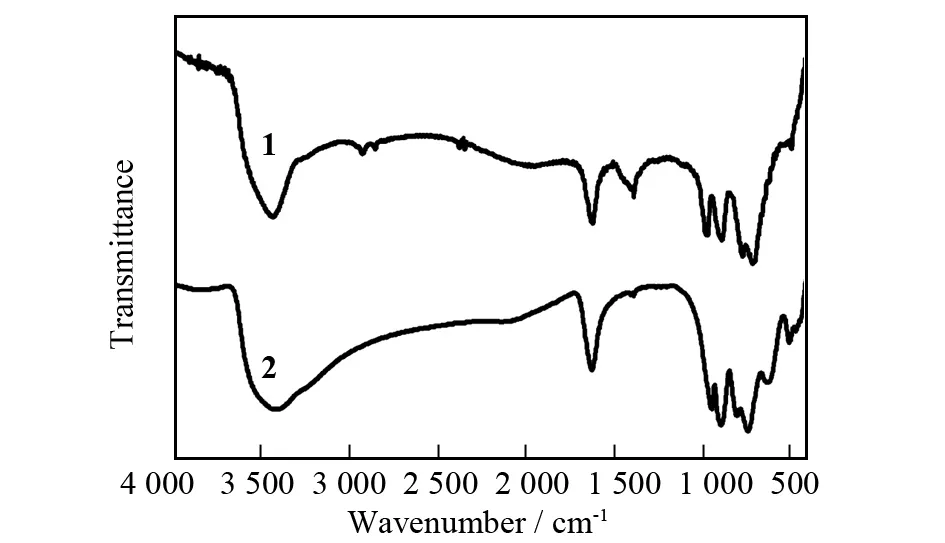
Fig.3 FT-IR spectra of 1 and 2
[1]SHI D Y, SHANG S S, ZHAO J W, et al. Three novel 2D organic-inorganic hybrid CuII-LnIIIheterometallic arsenotungstates [J]. Synth Met, 2012, 162: 1030-1036.
[2]RITCHIE C, REBER C, BOSKOVIC C,et al. Sensitization of lanthanoid luminescence by organic and inorganic ligands in lanthanoid-organic-polyoxometalates [J]. Inorg Chem, 2012, 51: 1142-1151.
[3]HUSSAIN F, KORTZ U, CLARK R J. The bis-phenyltin-substituted, lone-pair-containing tungstoarsenate [(C6H5Sn)2As2W19O67(H2O)]8-[J]. Inorg Chem, 2004, 43: 3237-3242.
[4]CHEN L J, ZHAO J W, MA P T, et al. An organic-inorganic hybrid nickel-substituted arsenotungstate consisting of three types of polyoxotungstate units [J]. Inorg Chem Commun, 2010, 13: 50-53.
[5]EVANS H T, TOURNÉ C M, TOURNÉ G F, et al. X-Ray crystallographic and tungsten-183 nuclear magnetic resonance structural studies of the [M4(H2O)2(XW9O34)2]10-heteropolyanions (M = CoIIor ZnII, X = P or As) [J]. J Chem Soc, Dalton Trans, 1986: 2699-2705.
[6]KORTZ U, SAVELIEFF M G, BASSIL B S, et al. A large, novel polyoxotungstate: [As6IIIW65O217(H2O)7]26-[J]. Angew Chem Int Ed, 2001, 40: 3384-3386.
[7]BI L H, WANG E B, PENG J, et al. Crystal structure and replacement reaction of coordinated water molecules of the heteropoly compounds of sandwich-type tungstoarsenates [J]. Inorg Chem, 2000, 39: 671-679.
[8]BI L H, HUANG R D, WANG E B,et al. Rational syntheses, characterization, crystal structure, and replacement reactions of coordinated water molecules of [As2W18M4(H2O)2O68]10-(M = Cd, Co, Cu, Fe, Mn, Ni or Zn) [J]. J Chem Soc, Dalton Trans, 2001(2): 121-129.
[9]RITCHIE C, SPELDRICH M, SORACE L, et al. Utilizing the adaptive polyoxometalate [As2W19O67(H2O)]14-to support a polynuclear lanthanoid-based single-molecule magnet [J]. Inorg Chem, 2011, 50: 7004-7014.
[10]ZHAO J W, HAN Q X, NIU J Y,et al. A CdSO4-like 3-D framework constructed from monosodium substituted Keggin arsenotungstates and copper(II)-ethylenediamine complexes [J]. Inorg Chem Commun, 2009, 12: 707-710.
[11]ZHAO J W, HAN Q X, SHI D Y, et al. Synthesis, structure and magnetism of a S-shaped multi-iron substituted arsenotungstate containing a trivacant Keggin [B-α-AsVW9O34]9-and a hexavacant Keggin [α-AsVW6O26]11-fragments [J]. J Solid State Chem, 2011, 184: 2756-2761.
[12]ZHAO J W,SHI D Y, CHEN L J, et al. Four types of 1D or 2D organic-inorganic hybrids assembled by arsenotungstates and CuII-LnIII/IVheterometals [J]. CrystEngComm, 2012, 14: 3108-3119.
[13]SHELDRICK G M. SHELXTL-97. Program for crystal structure solution [CP]. University of Göttingen, Germany, 1997.
[14]ZHAO J W, SHI D Y, CHEN L J, et al. Two 1-D multi-nickel substituted arsenotungstate aggregates [J]. CrystEngComm, 2011, 13: 3462-3469.
date:2014-01-21.
Supported by the Natural Science Foundation of China (21101055, 21301049, U1304208), the Natural Science Foundation of Henan Province (122300410106), the Foundation of Education Department of Henan Province (2010B150006) and the Students Innovative Pilot Plan of Henan University (2012, 2013).
Biography:ZHANG Fang (1989-), female, master, majoring in molecule-based functional materials.*
, E-mail: ljchen@henu.edu.cn.
