Size-controlled Pd Nanoparticles Supported on α-Al2O3as Heterogeneous Catalyst for Selective Hydrogenation of Acetylene*
2014-07-18ZHANGHuoli张火利YANGYuanyi杨元一DAIWei戴伟LUShuliang鲁树亮YUHaibo于海波andJIYuanyuan吉媛媛DepartmentofChemicalEngineeringBeijingUniversityofChemicalTechnologyBeijing0009ChinaSinopecBeijingResearchInstituteofChemicalIndustryBeijing0003
ZHANG Huoli (张火利), YANG Yuanyi (杨元一), DAI Wei (戴伟)**, LU Shuliang (鲁树亮) YU Haibo (于海波)and JI Yuanyuan (吉媛媛)Department of Chemical Engineering, Beijing University of Chemical Technology, Beijing 0009, ChinaSinopec Beijing Research Institute of Chemical Industry, Beijing 0003, China
Size-controlled Pd Nanoparticles Supported on α-Al2O3as Heterogeneous Catalyst for Selective Hydrogenation of Acetylene*
ZHANG Huoli (张火利)1, YANG Yuanyi (杨元一)1,2, DAI Wei (戴伟)2,**, LU Shuliang (鲁树亮)2, YU Haibo (于海波)2and JI Yuanyuan (吉媛媛)21Department of Chemical Engineering, Beijing University of Chemical Technology, Beijing 100029, China2Sinopec Beijing Research Institute of Chemical Industry, Beijing 100013, China
Size-controlled Pd nanoparticles (PdNPs) were synthesized in aqueous solution, using sodium carboxymethyl cellulose as the stabilizer. Size-controlled PdNPs were supported on α-Al2O3by the incipient wetness impregnation method. The PdNPs on α-Al2O3support were in a narrow particle size distribution in the range of 1-6 nm. A series of PdNPs/α-Al2O3catalysts were used for the selective hydrogenation of acetylene in ethylene-rich stream. The results show that PdNPs/α-Al2O3catalyst with 0.03% (by mass) Pd loading is a very effective and stable catalyst. With promoter Ag added, ethylene selectivity is increased from 41.0% to 63.8% at 100 °C. Comparing with conventional Pd-Ag/α-Al2O3catalyst, PdNPs-Ag/α-Al2O3catalyst has better catalytic performance in acetylene hydrogenation and shows good prospects for industrial application.
Pd nanoparticles, sodium carboxymethyl cellulose, acetylene hydrogenation
1 INTRODUCTION
Pd/α-Al2O3catalyst is one of the most important hydrogenation catalysts in the modern petrochemical industry. It is commonly used in fixed-bed reactors to remove acetylene (0.5%-2% by volume) in ethylene-rich stream. Industrially, it is generally required that the acetylene content should be less than 5×10−6in the ethylene product [1, 2]. Generally, Pd particles on α-Al2O3support participate in the reaction as active sites for the selective hydrogenation of acetylene in ethylene-rich stream. Catalytic performance of Pd/α-Al2O3catalyst strongly depends on the size and dispersion of Pd particles. However, for conventional Pd/α-Al2O3catalysts, the solution of Pd(II) ions or Pd particles will move over the α-Al2O3substrate surface to collide and form the aggregation of Pd particles during drying and calcining process [3]. Therefore, the conventional Pd/α-Al2O3catalysts are usually prepared using Ag as a promoter to block large Pd ensembles [4, 5]. Large active sites are undesirable for the selective hydrogenation of acetylene [6-8].
Transition-metal nanoparticles have attracted much attention due to their significant catalytic properties [9, 10]. Linear and dendritic polymers have been successfully used for the synthesis of Pd nanoparticles (PdNPs) in solutions [11-15]. It is highly significant to develop a supported catalyst with size-controlled PdNPs. Sodium carboxymethyl cellulose (CMC) is a semi-natural, renewable, inexpensive, nontoxic and linear polymer matrix in aqueous media, with which Pt nanoparticles [16] and Ag nanoparticles [17] have been synthesized. We have reported our first step in the development of an aqueous-phase catalytic process for the selective hydrogenation of acetylene using water soluble monodisperse Pd nanoparticles as catalyst [18]. In this work, size-controlled PdNPs are synthesized by the linear polymer of CMC in water and supported on α-Al2O3to develop a nanostructured heterogeneous catalyst for the selective hydrogenation of acetylene.
2 EXPERIMENTAL
2.1 Preparation of size-controlled PdNPs stabilized by CMC
The aqueous solution of Pd(NO3)2(50 mg·ml−1) was obtained from Sinopec Beijing Research Institute of Chemical Industry. Sodium carboxymethyl cellulose (Mw=90000) was supplied by Sigma-Aldrich. In a typical synthesis, a 150 ml of 1 mg·ml−1solution of Pd(NO3)2was added to 350 ml of 0.06% (by mass) aqueous solution of soluble CMC. The pH value was adjusted to about 7 by dropwise adding an aqueous solution of sodium hydroxide (NaOH, 0.5 mol·L−1) at stirring speed of 300 r·min−1. The mixture in a stainless steel autoclave was reduced to obtain size-controlled PdNPs by molecular hydrogen at 40 °C for at least 30 min at atmospheric pressure.
2.2 Preparation of catalysts
α-Al2O3was obtained from Sinopec Beijing Research Institute of Chemical Industry (surface area= 28 m2·g−1). Loaded catalysts with 0.01%, 0.02%, 0.03%, and 0.04% (by mass) Pd separately on α-Al2O3were prepared by the incipient wetness impregnation technique using the aqueous solution of PdNPs as mentioned in Section 2.1. PdNPs/α-Al2O3catalysts were obtained by drying at 120 °C for 8 h and calciningin air at 450 °C for 8 h to remove CMC and re-expose palladium active sites.
Ag-promoted PdNPs/α-Al2O3catalyst was also prepared by the incipient wetness impregnation method with a solution of AgNO3(Aldrich). PdNPs-Ag/α-Al2O3catalyst [0.03% (by mass) Pd and 0.07% (by mass) Ag] was obtained by drying at 120 °C for 8 h and calcining in air at 450 °C for 8 h. For comparison, conventional Pd-Ag/α-Al2O3catalyst [0.03% (by mass) Pd and 0.07% (by mass) Ag] was prepared by the same technique using an aqueous solution with desired amount of Pd(NO3)2and AgNO3(Aldrich), subsequently dried at 120 °C for 8 h and calcined in air at 450 °C for 8 h.
2.3 Catalyst characterization
The size and morphology of PdNPs in aqueous solution and PdNPs on α-Al2O3were examined by transmission electron microscopy (TEM). TEM images were recorded by a JEOL JEM-3010 (200 kV). The sample for TEM characterization was prepared by placing a drop of the solution onto a carbon-coated copper grid, followed by drying in vacuum at room temperature. The particle size distribution was determined by counting 100 particles from TEM image. The actual contents of Pd and Ag on α-Al2O3were analyzed by inductively coupled plasma mass spectrometry (ICP-MS 7500CX, Agilent). The ICP analysis was performed by taking 1.0 g sample into a teflon crucible, adding 20 ml HNO3(BV-III) and staying one night, followed by ultrasonic treatment for 3 h, being diluted 50-folds with 2% HNO3and analyzed with a Agilent 7500CX ICP-MS. The specific surface area of α-Al2O3was determined using the Brunauer-Emmett-Teller (BET) method by a ASAP 2020C instrument (Micromeritics Instruments, USA). CO chemisorption on catalysts was measured with in-situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) using a Brucker Vertex 70 spectrometer. For CO chemisorption measurement, the catalyst was grinded to powder and 30 mg powdered catalyst sample was prereduced in in-situ infrared cell at 300 °C for 2 h by 10% H2in Ar, then 10 ml·min−11% CO in Ar flowed through the sample at 40 °C for 60 min. After CO adsorption, the gas was switched to Ar purging at 80 °C. The Fourier transform infrared spectroscopy (FTIR) spectra of sample were measured every 2 min.
2.4 Catalytic test
The selective hydrogenation of acetylene in ethylene-rich stream was performed in an 8 mm (i.d.) stainless steel tube microreactor with 1 ml catalyst. Before reaction, the catalyst was reduced by H2at 180 °C for 2 h. A feed gas containing 0.45% (by mol) acetylene in ethylene was used for the reaction. Total gas hourly space velocity was 10000 h−1. The mole ratio H2/C2H2in ethylene-rich stream was 1.5 and the products were analyzed on line by gas chromatography (Agilent 7890) with flame ionization detector (FID) and thermal conductivity detector (TCD). Acetylene conversion and ethylene selectivity are defined as
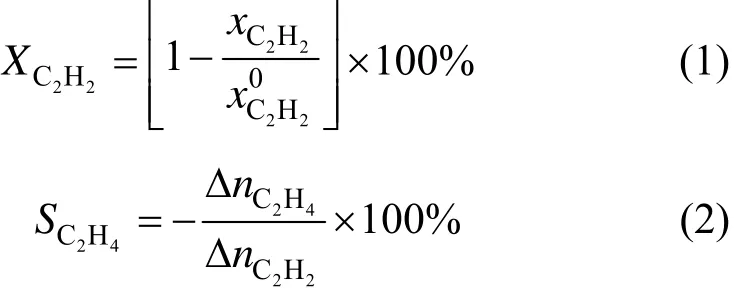
3 RESULTS AND DISCUSSION
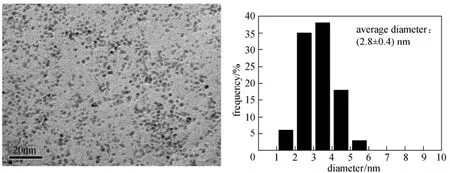
Figure 1 TEM image and size distribution of PdNPs stabilized by CMC in aqueous solution [Pd 0.03% (by mass), mCMC/mPd=2]
3.1 Catalysts characterization
TEM image of PdNPs stabilized by CMC is shown in Fig. 1. Size-controlled PdNPs are in an average diameter of 2.8±0.4 nm and a narrow particle size distribution in the range of 1-6 nm. The size and morphology of PdNPs on α-Al2O3are shown in Fig. 2. PdNPs/α-Al2O3catalysts form very small and uniform Pd particles on α-Al2O3. The diameter of PdNPs is in the range of 1 nm to 6 nm, which is the same with that of PdNPs stabilized by CMC in aqueous solution. Moreover, PdNPs on α-Al2O3are isolated nanoparticles, indicating isolated Pd active sites. The shape and morphology of supported metal nanoparticles are usually influenced by the metal-support interaction [19]. Thus CMC promotes the formation of isolated anddispersed PdNPs on α-Al2O3. For PdNPs/α-Al2O3catalysts, CMC inhibits PdNPs to move and prevents them from aggregation on α-Al2O3substrate surface during drying and calcining process. The inhibition mechanism is that CMC molecules could anchor to Pd particle surfaces and the association of CMC molecules with Pd nanoparticles via both the COO and
OH functional groups facilitates the encapsulation of Pd nanoparticles with a protective layer of CMC molecules [16], providing steric stabilization of Pd nanoparticles on α-Al2O3support. Therefore, it is desirable to obtain uniform and isolated active sites on α-Al2O3using size-controlled PdNPs.
Table 1 gives the palladium contents of PdNPs/α-Al2O3catalysts determined by inductively coupled plasma mass spectrometry. The Pd loadings on α-Al2O3support are in accordance with the design values.

Figure 2 TEM images of PdNPs/α-Al2O3catalysts (a) Pd 0.01% (by mass); (b) Pd 0.02% (by mass); (c) Pd 0.03%(by mass); (d) Pd 0.04% (by mass)

Figure 3 Acetylene conversion (a) and ethylene selectivity (b) with PdNPs/α-Al2O3catalysts
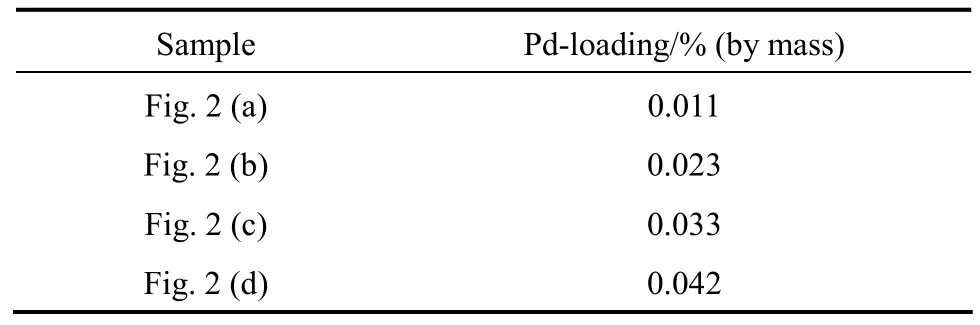
Table 1 The palladium contents of PdNPs/α-Al2O3catalysts determined by ICP
3.2 Catalytic performance of PdNPs/α-Al2O3catalysts
The catalytic performance of PdNPs/α-Al2O3catalysts is shown in Fig. 3. The catalytic activity of catalysts follows the order of Pd mass content (%) inthis way: 0.03, 0.04, 0.02, and 0.01. PdNPs/α-Al2O3catalyst with 0.03% Pd mass loading exhibits the highest catalytic activity among the four catalysts, while it presents the lowest ethylene selectivity. As the temperature increases, acetylene conversion increases and ethylene selectivity decreases. The main reason is that the selective hydrogenation of acetylene is a typical consecutive reaction with ethylene as an intermediate. Therefore, the ethylene selectivity decreases as acetylene conversion increases [3]. PdNPs/α-Al2O3catalysts have the same conversion-selectivity relationship for selective hydrogenation of acetylene as conventional Pd/α-Al2O3catalysts.
The reason for PdNPs/α-Al2O3catalyst with 0.03% (by mass) Pd to present higher hydrogenation activity is that the solution supported on α-Al2O3generates much larger Pd particles on the catalyst with 0.04% (by mass) Pd, as shown in Fig. 4. The formation of large Pd particles on α-Al2O3decreases the Pd active sites over the catalyst. Therefore, PdNPs/α-Al2O3catalyst with 0.04% (by mass) Pd has a lower activity than that with 0.03% (by mass) Pd. In order to test the stability, PdNPs/α-Al2O3catalyst with 0.03% (by mass) Pd was used in stream for 60 h at 100 °C. The result is shown in Fig. 5. It is found that PdNPs/α-Al2O3catalyst with 0.03% (by mass) Pd presents a high efficiency and long term stability without any deactivation in ethylene-rich stream.

Figure 4 Pd particles in the range of 5-10 nm on PdNPs/α-Al2O3catalysts

Figure 6 Comparison of catalytic performance of PdNPs/α-Al2O3catalyst and conventional Pd-Ag/α-Al2O3catalyst at 100 °C■ PdNPs/Al2O3; ● Pd-Ag/Al2O3

Figure 5 Stability of PdNPs/α-Al2O3catalyst with 0.03% (by mass) Pd at 100 °C
3.3 Comparison of PdNPs/α-Al2O3catalyst and conventional Pd-Ag/α-Al2O3catalyst
The catalytic performance of PdNPs/α-Al2O3catalyst with 0.03% (by mass) Pd is compared with that of conventional Pd-Ag/α-Al2O3catalyst [0.03% Pd (by mass) and 0.07% (by mass) Ag]. Table 2 shows the actual contents of Pd and Ag on α-Al2O3determined by ICP. For the catalysts in stream for 24 h at 100 °C, Fig. 6 shows that acetylene conversion with PdNPs/α-Al2O3catalyst is higher than that with conventional Pd-Ag/α-Al2O3catalyst, but ethylene selectivity with PdNPs/α-Al2O3catalyst is lower. It is likely that Ag as a promoter not only promotes the geometric blocking of large Pd ensembles but also enhances a synergetic effect on Pd-Ag/α-Al2O3catalyst [5, 20].
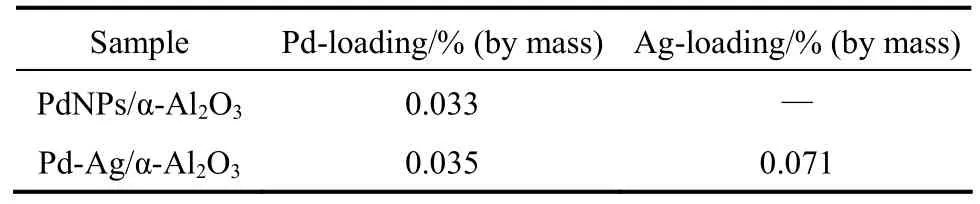
Table 2 The actual contents of Pd and Ag on α-Al2O3(determined by ICP)
3.4 Comparison of PdNPs-Ag/α-Al2O3catalyst and conventional Pd-Ag/α-Al2O3catalyst
In order to improve the catalytic performance, Agis introduced to PdNPs/α-Al2O3catalyst as promoter. The catalytic performance of PdNPs-Ag/α-Al2O3catalyst is also compared with conventional Pd-Ag/α-Al2O3catalyst. The actual contents of Pd and Ag on α-Al2O3are shown in Table 3.
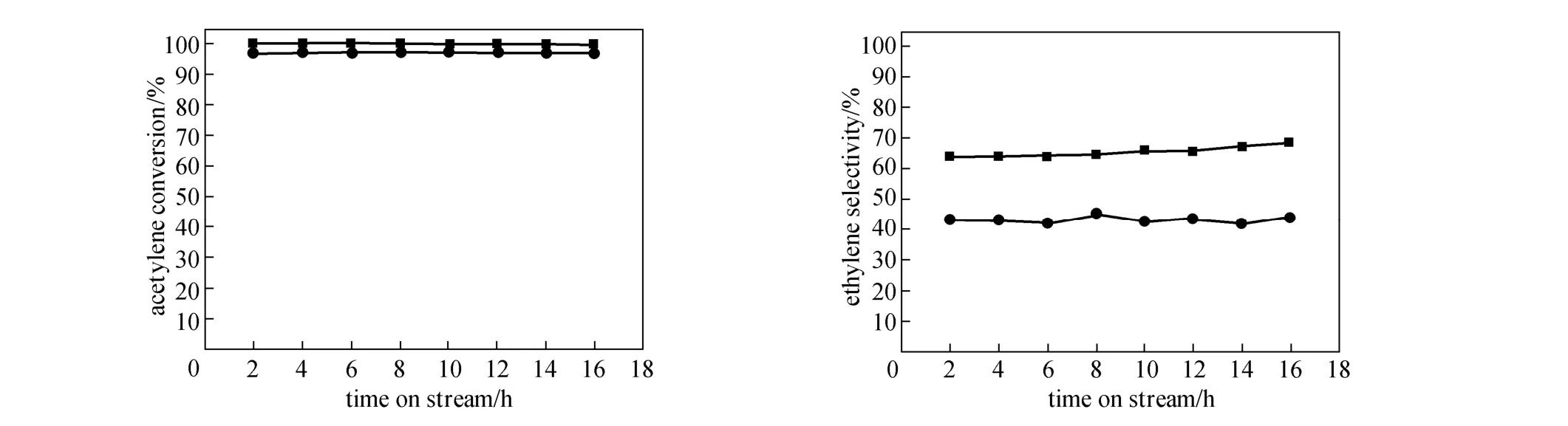
Figure 7 Comparison of catalytic performance of PdNPs-Ag/α-Al2O3catalyst and conventional Pd-Ag/α-Al2O3catalyst at 100 °C■ PdNPs/Al2O3; ● Pd-Ag/Al2O3
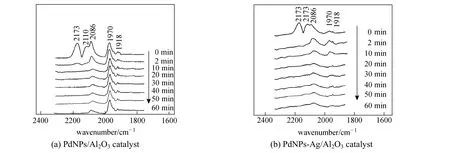
Figure 8 In situ DRIFTS spectra of Ar purging CO adsorbed on catalysts
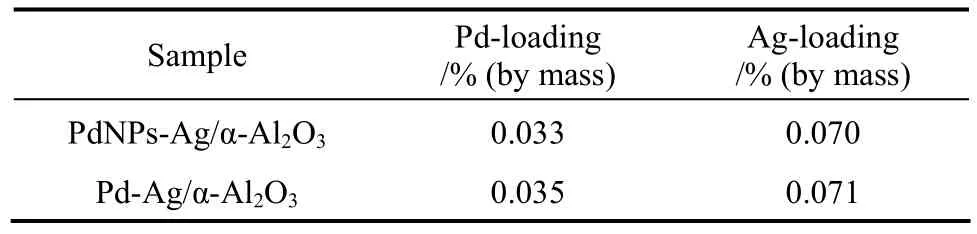
Table 3 The actual contents of Pd and Ag on α-Al2O3(determined by ICP)
Figure 7 shows the results with PdNPs-Ag/α-Al2O3catalyst and conventional Pd-Ag/α-Al2O3catalyst used in stream for 16 h at 100 °C separately. The catalytic performance of PdNPs-Ag/α-Al2O3catalyst is better. Moreover, ethylene selectivity of PdNPs-Ag/α-Al2O3catalyst is improved remarkably, compared with PdNPs/α-Al2O3catalyst. Ethylene selectivity is increased from 40.1% to 63.8%.
For the effect of Ag on Pd active sites, Fig. 8 shows the in-situ DRIFTS spectra of the two catalysts after CO adsorption. The peaks at 2173 cm−1and 2110 cm−1are adsorption bands owing to gas phase CO, which exist in the 0 min spectrum and disappear in the 2 min spectrum by N2purging. Meanwhile, two types of CO adsorption are observed in the spectra: linear adsorption (COl) at 2086 cm−1, bridge adsorption (COb) at 1970 cm−1and 1918 cm−1, which are attributed to CO adsorbed on Pd(100) plane and Pd(111) plane, respectively [21, 22]. There are two major differences between PdNPs-Ag/α-Al2O3catalyst and PdNPs/α-Al2O3catalyst. Firstly, the CO species adsorbed on Pd are mainly COlfor PdNPs-Ag/α-Al2O3catalyst, while those on Pd are COland CObfor PdNPs/α-Al2O3catalyst in the 0 min spectrum. Secondly, the relative absorption intensity of COb/COl is obviously different. In the 2 min spectrum, the peak height ratio of COb/COlis 0.47 for PdNPs-Ag/α-Al2O3catalyst and 1.75 for PdNPs/α-Al2O3catalyst. It has been demonstrated that Ag as a promoter effectively provides electronic and geometric effects on Pd surface for PdNPs-Ag/α-Al2O3catalyst [5]. Therefore, ethylene selectivity of PdNPs-Ag/α-Al2O3catalyst is higher than that of PdNPs/α-Al2O3catalyst.
In a word, PdNPs/α-Al2O3catalyst with 0.03% mass loading is a very effective catalyst owing to isolated active sites. With promoter Ag added, ethylene selectivity is improved and the catalytic performance of PdNPs-Ag/α-Al2O3catalyst is better than conventional Pd-Ag/α-Al2O3catalyst.
4 CONCLUSIONS
CMC was used as a suitable and good stabilizer to synthesize size-controlled PdNPs in aqueous solution. Low-loaded PdNPs/α-Al2O3catalysts were prepared by the incipient wetness impregnation technique using the aqueous solution of PdNPs. PdNPs/α-Al2O3catalysts form very small and uniform Pd particles on α-Al2O3. PdNPs/α-Al2O3catalyst with 0.03% Pd mass loading is a very effective and stable catalyst for the selective hydrogenation of acetylene in ethylene-rich stream. PdNPs-Ag/α-Al2O3catalyst exhibits better catalytic performance than conventional Pd-Ag/α-Al2O3catalyst, which is a kind of industrial candidate catalyst.
REFERENCES
1 Borodziński, A., Bond, G.C., “Selective hydrogenation of ethyne in ethene-rich streams on palladium catalysts. Part 1. Effect of changes to the catalyst during reaction”, Catal. Rev., 48, 91-144 (2006).
2 Molnár, Á., Sárkány, A., Varga, M., “Recirculation hydrogenation of carbon-carbon multiple bonds: Chemo-, regio- and stereo-selectivity”, J. Mol. Catal. A: Chem., 173, 185-221 (2001).
3 Kang, J.H., Shin, E.W., Kim, W.J., Park, J.D., Moon, S.H., “Selective hydrogenation of acetylene on TiO2-added Pd catalysts”, J. Catal., 208, 310-320 (2002).
4 Khan, N.A., Shaikhutdinov, S., Freund, H.J., “Acetylene and ethylene hydrogenation on alumina supported Pd-Ag model catalysts”, Catal. Lett., 108, 159-164 (2006).
5 Lee, J.H., Kim, S.K., Ahn, I.Y., Kim, W.J., Moon, S.H., “Performance of Pd-Ag/Al2O3catalysts prepared by the selective deposition of Ag onto Pd in acetylene hydrogenation”, Catal. Commun., 12, 1251-1254 (2011).
6 Borodziński, A., Cybulski, A., “The kinetic model of hydrogenation of acetylene-ethylene mixtures over palladium surface covered by carbonaceous deposits”, Appl. Catal. A: Gen., 198, 51-66 (2000).
7 Mei, D., Neurock, M., Smith, C.M., “Hydrogenation of acetyleneethylene mixtures over Pd and Pd-Ag alloys: First-principles-based kinetic monte carlo simulations”, J. Catal., 268, 181-195 (2009).
8 Komhom, S., Mekasuwandumrong, O., Praserthdam, P., Panpranot, J., “Improvement of Pd/Al2O3catalyst performance in selective acetylene hydrogenation using mixed phases Al2O3support ”, Catal. Commun., 10, 86-91 (2008).
9 Bronstein, L.M., Shifrina, Z.B., “Dendrimers as encapsulating, stabilizing, or directing agents for inorganic nanoparticles”, Chem. Rev., 111, 5301-5344 (2011).
10 Roucoux, A., Schulz, J., Patin, H., “Reduced transition metal colloids: A novel family of reusable catalysts?”, Chem. Rev., 102, 3757-3778 (2011).
11 Li, Y., Boone, E., EI-Sayed, M.A., “Size effects of PVP-Pd nanoparticles on the catalytic Suzuki reactions in aqueous solution”, Langmuir, 18, 4921-4925 (2002).
12 Esuni, K., Isono, R., Yoshimura, T., “Preparation of PAMAM- and PPI-metal (silver, platinum and palladium) nanocomposites and their catalytic activities for reduction of 4-Nitrophenol”, Langmuir, 20, 237-243 (2004).
13 Nemamcha, A., Rehspringer, J., Khatmi, K., “Synthesis of palladium nanoparticles by sonochemical reduction of palladium(II) nitrate in aqueous solution”, J. Phys. Chem. B, 110, 383-387 (2006).
14 Gallon, B.J., Kojima, R.W., Kaner, R.B., Diaconescu, P.L., “Palladium nanoparticles supported on polyaniline nanofibers as a semi-heterogeneous catalyst in water”, Angew. Chem. Int. Ed., 46, 7251-7254 (2007).
15 Evangelisti, C., Panziera, N., D’Alessio, A., Bertinetti, L., Botavina, M., “New monodispersed palladium nanoparticles stabilized by poly-(N-vinyl-2-pyrrolidone): Preparation, structural study and catalytic properties”, J. Catal., 272, 246-252 (2010).
16 Lu, J.C., Sutton, J., Roberts, C.B., “Synthesis and extraction of monodisperse sodium carboxymethylcellulose-stabilized platinum nanoparticles for the self-assembly of ordered arrays”, J. Phys. Chem. C, 111, 11566-11576 (2007).
17 Chen, J., Wang, J., Zhang, X., Jin, Y.L., “Microwave-assisted green synthesis of silver nanoparticles by carboxymethyl cellulose sodium and silver nitrate”, Mater. Chem. Phys., 108, 421-424 (2008).
18 Zhang, H.L., Yang, Y.Y., Dai, W., Yang, D., Lu, S.L., Ji, Y.Y., “An aqueous-phase catalytic process for the selective hydrogenation of acetylene with monodisperse water soluble palladium nanoparticles as catalyst”, Catal. Sci. Technol., 2, 1319-1323 (2012).
19 Liu, J., “Advanced electron microscopy of metal-support interactions in supported metal catalysts”, Chem. Cat. Chem., 3, 934-948 (2011).
20 Zhang, Q.W., Li, J., Liu, X.X., Zhu, Q.M., “Synergetic effect of Pd and Ag dispersed on Al2O3in the selective hydrogenation of acetylene”, Appl. Catal. A: Gen., 197, 221-228 (2000).
21 Zhang, Q., Dai, W., Mu, W., Yu, H.B., “Pd-Ag/Al2O3and Pd/Al2O3catalysts for selective hydrogenation of ethyne with in-situ DRIFTS”, CIESC Journal, 62, 71-77 (2011).
22 Palazov, A., Kadinov, G., Bonev, C.H., Shopov, D., “Infrared spectroscopic study of the interaction between carbon monoxide and hydrogen on supported palladium”, J. Catal., 74, 44-54 (1982)
2012-10-29, accepted 2013-02-28.
* Supported by SINOPEC Beijing Research Institute of Chemical Industry (01-09ZS0440, 11-08ZS0442).
** To whom correspondence should be addressed. E-mail: daiw.bjhy@sinopec.com
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Effect of Adsorbent Diameter on the Performance of Adsorption Refrigeration*
- Filtering Surface Water with a Polyurethane-based Hollow Fiber Membrane: Effects of Operating Pressure on Membrane Fouling*
- A Facile Route for Synthesis of LiFePO4/C Cathode Material with Nano-sized Primary Particles*
- High-Thermal Conductive Coating Used on Metal Heat Exchanger*
- Kinetics of Forward Extraction of Boric Acid from Salt Lake Brine by 2-Ethyl-1,3-hexanediol in Toluene Using Single Drop Technique*
- Soft Sensor Model Derived from Wiener Model Structure: Modeling and Identification*
