酸催化及竞争吸附对CeY分子筛吸附脱硫性能的影响
2014-06-23秦玉才高雄厚段林海范跃超于文广张海涛宋丽娟
秦玉才 高雄厚 段林海 范跃超于文广 张海涛 宋丽娟,*
(1中国石油大学(华东)化学化工学院,山东青岛266426;2辽宁石油化工大学,辽宁省石油化工催化科学与技术重点实验室,辽宁抚顺113001;3中国石油天然气股份有限公司,石油化工研究院,北京100007)
1 Introduction
Selectively adsorption desulfurization(SADS),based on the selectively adsorption capability for organosulfur compounds of zeolite adsorbents,is considered to be a promising approach for producing clean fuels.1Many attempts have been made to develop these adsorbents for desulfurization of liquid fuels.So far,a variety of adsorbents such as metal oxides,2-5active carbon,6-8mesoporous materials9-12and zeolites13-24have been studied for deep desulfurization.Among them,ion-exchanged zeolites are more promising and less expensive adsorbents being utilized for SADS.
Yang and coworkers13,15-19reported a series ofπ-complexation sorbents for selective adsorption desulfurization.Transitionmetal ion(Cu+,Ag+,Ni2+,Zn2+)exchanged Faujasite(FAU)zeolites were used to selectively remove organosulfur molecules from commercial fuels depending on theπ-complexation interaction.These interactions of sulfur compound molecules with transition-metal ions are stronger than those of aromatic and olefinic compounds.Theseπ-complexation sorbents,therefore,are effective for sulfur removal from transportation fuels.However,competitive adsorption of aromatics and olefines seems an inevitable drawback,which affects the selective adsorption of sulfur compounds by theπ-complexation interaction,because of the coexistence of large amounts of these compounds in liquid fuels.
Then,one kind of sulfur-metal(S-M)interaction sorbents was developed for selective adsorption desulfurization.Song and co-workers14reported that the Ce-exchanged Y(CeY)zeolite adsorbents present higher adsorption selectivity for sulfur compounds than for aromatics ascribed to the direct S-M interaction between the sulfur compound and the metal cation in the zeolite.Lin and co-worker24also demonstrated that CeY zeolites performed the best adsorption capacity for thiophenic sulfur among various Y zeolites.Herein,CeY zeolites are considered as one kind of ideal adsorbents for SADS from transportation fuels.However,the sulfur removal capability of CeY zeolites is influenced by the aromatic and olefinic compounds.Our previous work21,25,26focused on the effects of olefines on adsorptive deep desulfurization of gasoline over CeY zeolites,and confirmed that the adsorption capability for thiophene decreases significantly as the concentration of 1-octene increases.Tian and coworkers27explored the effects of toluene on thiophene adsorption over CeY zeolites,and convinced that the thiophene removal over CeY zeolites decreases with the increase of toluene concentration in model gasoline,however,the decline tendency on CeY zeolites is much smoother than that of NaY zeolites.It can be concluded that both olefines and aromatics can depress the capability of CeY zeolites for the selective adsorption of sulfur compounds.
Moreover,although many reports have demonstrated the suitability of CeY zeolites as adsorbents for the desulfurization of liquid fuel,no attention has been given to the effect of the acidic properties of rare earth cation exchanged zeolites which have been very well studied as acid catalysts in petrochemical processes.28Catalytic reaction of thiophene have been found due to Brönsted acidic site on zeolite even under mild condition,29-31but the influence of this reaction on the adsorption performance of sulfur compounds has rarely been reported.It is necessary therefore to study whether the catalytic reaction promoting or impeding the capability of adsorbents for SADS.
In the present work,the acid properties are measured by NH3temperature-programmed desorption(NH3-TPD)method andin-situpyridine-Fourier transform infrared(Py-FTIR)technique.The desulfurization capability and the effects of olefines or aromatics on thiophene removal over CeY zeolites are studied by fixed-bed adsorption/breakthrough experiments at room temperature(RT)with model gasoline.Thein-situFTIR technique is utilized to measure adsorption,co-adsorption or/and catalytic reaction of thiophene,benzene,and cyclohexene in CeY zeolites.
2 Experimental
2.1 Sorbent preparation
NaY zeolite(the molar ratio of Si and Al is 2.55),in powder form,was used as the starting material.The CeY zeolites were prepared by liquid phase ion exchange(LPIE)technique.In order to obtain the sorbents with higher Ce ion loading,the ionexchanged experiments were repeated.
2.2 Sorbent characterization
X-ray powder diffraction(XRD)patterns were collected in a 2θrange of 5.00°-59.98°using a D/MAXRB XRD instrument(Rigaku,Japan)equipped with CuKαradiation.The transmission electron microscopy(TEM)images were taken with a JEOL-2200 EX microscope operating at 400 kV(Jeol,Japan).Chemical compositions of the ion-exchanged zeolites were determined by inductively coupled plasma(ICP)elemental analysis using a high-resolution magnetic sector ICP-mass spectroscopy(MS)spectrometer(Thermo Elemental,USA).The BETsurface area and total pore volume were measured using a Micromeritics ASAP 2020 surface area and porosity analyzer(Micromeritics,USA).The surface acidity was monitored by thein-situPy-FTIR technique,with a Perkin-Elmer Spectrum TM GX spectrometer(Perkin-Elmer,USA)coupled to a conventional high vacuum system.NH3-TPD experiments were performed on a Micromeritics Auto Chem II Chemisorption Analyzer(Micromeritics,USA)with a thermal conductivity detector.The samples were treated in He gas flow at 600°C for 1 h.
2.3 Adsorption desulfurization experiments
Model gasolines were used to evaluate the desulfurization performance of the sorbents,with the model compounds,such as thiophene,benzene,1-octene,1-hexene,cyclohexene,and 1,5-hexadiene,dissolved inn-nonane according to certain proportions.The various model gasolines are listed in Table 1.All thiophene,benzene,1-octene,1-hexene,cyclohexene,1,5-hexadiene,andn-nonane were purchased from J&K Ltd.and were used without further purification.
All breakthrough experiments were performed in a vertical custom made quartz adsorber equipped on a setup consisted of a low-flow liquid pump,feed tanks,and a heating element.The quartz adsorber has a 500-mm length with an internal diameter of 6.0 mm to accommodate 1 g of sorbents.Initially,the sorbents(in powder form)were loaded inside the adsorber,and heatedin-situusing dry gases to eliminate the moisture.All the fuel samples collected during the breakthrough experiments were analyzed using a WK-2D microcoulommeter(Jiangfen Electroanalytical instrument Co.,Ltd.,China).The concentration of sulfur at 1 μg · g-1in outlet is defined as the breakthrough point.
2.4 In-situ FTIR study on the interaction between adsorbent and adsorbate
FTIR spectra of adsorbed thiophene,cyclohexene,benzene,and co-adsorbed thiophene and benzene,thiophene and cyclohexene on NaY and CeY zeolites were recorded using a Perkin-Elmer Spectrum TM GX spectrometer.Anin-situIR cell coupled to a conventional high vacuum system was utilized allowing the sample wafers can be heated under vacuum or exposed to vapor-phase probe molecules.The sorbents were pressed into thin wafers(12-15 mg·cm-2)and activated at 400 °C under vacuum(10-3Pa)for 4 h.Then,the wafers were exposed to the vapors of thiophene,benzene,cyclohexene,and the mixed vapors of thiophene and benzene,or thiophene and cyclohexene,respectively,at room temperature.The spectra were recorded between 4000 and 400 cm-1with 4 cm-1spectral resolution and 64 scans after desorbed at room temperature under vacuum.
3 Results and discussion
The results of XRD patterns of CeY zeolites have been reported in our previous study,25which reveal that the CeY zeolites retain the original FAU zeolite structure,and have no diffractions due to CeO2structure emerging.The TEM images(not shown in this paper)show that the samples maintain a perfect aperture and topology structure of the FAU zeolite,and no CeO2cluster species arise in the aperture or over the surface of the zeolite crystal.26The structure and composition data of the NaY and the CeY zeolites are presented in Table 2,showing that the Ce ion exchange degree of the CeY zeolites achieves 94%.The decrease of pore volume and specific surface area of CeY zeolites is ascribed to reduce of the crystallinity of the zeolites during the process of liquid cerium ion-exchanged.
Fig.1 shows the breakthrough curves of thiophene in a series of model gasolines with CeY sorbents.The treated volumes of model fuel at breakthrough point are about 10.5,7.5,and 2.0 cm3corresponding to MG-1,MG-2,and MG-3 to MG-6,respectively.These results display that olefines and aromatics in model gasolines depress the capacity of thiophene removal of CeY sorbents.Besides,the effect of olefines is more significant than aromatics.The different slopes of the breakthrough curves of MG-3 to MG-6 indicate that the effect of different olefines is unequal.It is not reasonable to explain the effect of different olefines and aromatic hydrocarbons only by the mode of interaction between adsorption sites and adsorbate molecules.
Fig.2 presents the photographs of NaY and CeY zeolite powders in model gasolines.The NaY zeolite powders remain white color(the NaY zeolites′color)after the samples are exposed to the four kinds of model gasolines.But for the CeY zeolites,the color of the samples becomes deep yellow,black,brown,and gray inn-nonane,MG-1,MG-2,and MG-5 instead of light yellow(the CeY zeolites′color).The phenomenon of“coke”(black color)is observed on the CeY zeolites in the MG-1(see Fig.2b′),indicating that some other processes should also take place during the selective adsorptive desulfurization process on the adsorbents.The gas chromatography(Clarus500)with sulfur chemiluminescence detection(SIEVERS 355)(GCSCD)profiles display that more than one signal was detected in the compounds trapped on the CeY zeolites(Fig.3c),indicat-ing that new macromolecular sulfur compounds generate in the adsorbents exposed to the MG-1.It can be speculated that the Brönsted acids on the adsorbents could lead to protonatic catalytic reaction of thiophene molecules,and then thiophene oligomers generate by electrophilic addition reaction.32However,the color of the samples only became brown and gray due to competitive adsorption of olefinic or aromatic molecules which cover the Brönsted acid sites.
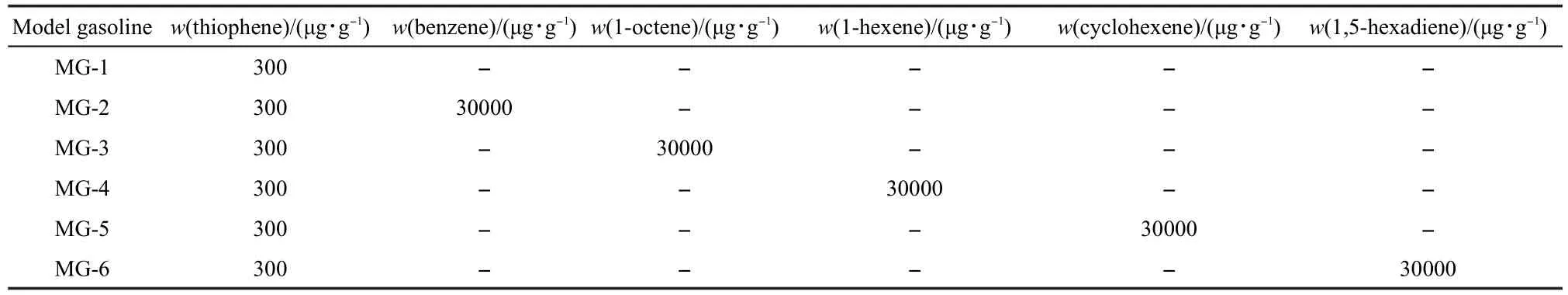
Table 1 Composition of model gasoline

Table 2 Structure and composition data for NaY and CeY zeolites
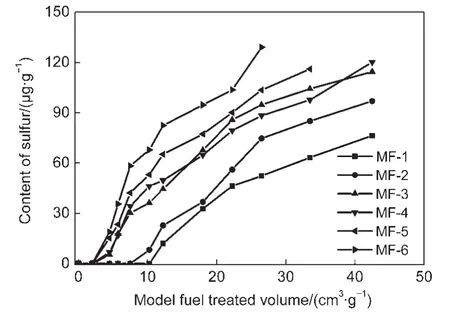
Fig.1 Breakthrough curves of thiophene in a fixed-bed adsorber with CeY adsorbents,with a model gasoline containing 300 μg·g-1 S of thiophene or mixed with 30000 μg·g-1benzene,1-octene,1-hexene,cyclohexene,and 1,5-hexadiene in n-octane respectively,at room temperature
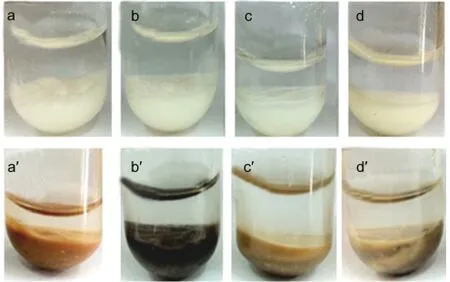
Fig.2 Photographs of NaY(a,b,c,d)and CeY(a',b',c',d')in model gasoline
The NH3-TPD spectra of NaY and CeY samples are depicted in Fig.4.The results show that strong peaks in the range of 100-300°C are all present in the NH3-TPD spectra of NaY and CeY,indicating that numerous weak acid sites exist in the two samples.The peaks in the range of 300-450°C and 500-600°C in the NH3-TPD curve of the CeY can be assigned to the medium strong acid sites and the strong acid sites,respectively.These results indicate that the plentiful medium strong and strong acid sites arise in the prepared process of CeY zeolites by LPIE method.
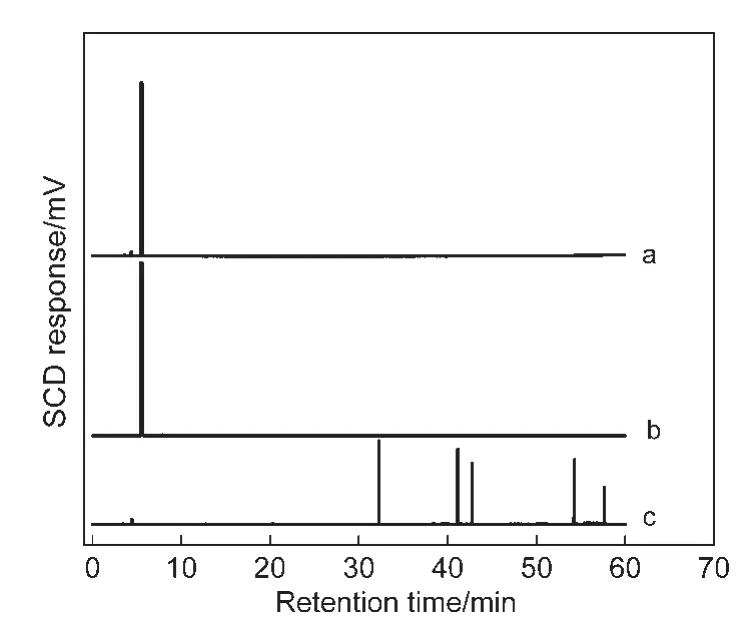
Fig.3 GC-SCD profiles of MG-1(a)and compounds trapped on the NaY(b)and CeY(c)zeolites exposed to the MG-1
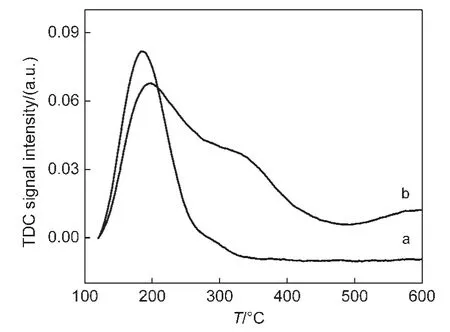
Fig.4 NH3-TPD spectra of NaY(a)and CeY(b)samples
Fig.5 shows the FTIR spectra of OH groups and pyridine adsorbed in NaY and CeY zeolites.Only one band at 3695 cm-1assigned to hydroxyls(Al―OH)in defect sites is observed in NaY zeolite(Fig.5A,left).33However,there are bands of the external Si―OH at 3740 cm-1,Si―OH―Al at 3639,3580 cm-1,and Ce―OH at 3527 cm-1in the FTIR spectra of CeY zeolites(Fig.5B,left).34-36Numerous Lewis acidic sites attributed to Na+identified by IR band of 1440 cm-1are present in NaY zeolite(Fig.5A,right).Plentiful Brönsted acid sites generate in the preparation process of CeY zeolites by LPIE method,according to IR band of 1543 cm-1(Fig.5B,right).
The FTIR spectra of thiophene,benzene,and cyclohexene adsorbed in the NaY and CeY zeolites at RT are shown in Fig.6.The bands at 3110 cm-1(C―H stretching vibrations)and at 1396 cm-lwere detected in the spectra of thiophene adsorbed in NaY(see Fig.6A(b)).The band at 1396 cm-1was assigned to the perturbed fundamental ring stretching vibration(ν5),which was assigned to be 14 cm-lshifted to the lower wavenumber compared with the band of thiophene adsorbed in SiO2.37Thus,all bands observed in Fig.6A(b)are attributed to modes of vibration of thiophene molecules strongly interacting with the Na+cations.The appearance of the new infrared bands at 1449 and 1439 cm-1in the IR spectra of thiophene adsorbed in theCeY zeolites(see Fig.6B(b))are assigned to the deformation vibrations of CH2groups close to a sulfur atom(*CH2―S―)or close to a double bond(*CH2―(CH=CH2)).38-40Besides,the broadening of the band in the range of 2990-2890 cm-1is attributed to the generation of saturated C―H species.These results indicate that protonization reactions of thiophene occurred as thiophene molecules adsorbed in the CeY zeolites.The band at 1504 cm-l(ring stretching fundamentalν14)related to the thiophene oligomers implies that oligomerizations of thiophene molecules take place in the CeY zeolites.The FTIR spectra of adsorbed thiophene in the NaY and CeY zeolites are different,indicating that the adsorption mechanism of thiophene on the two samples must be not the same.
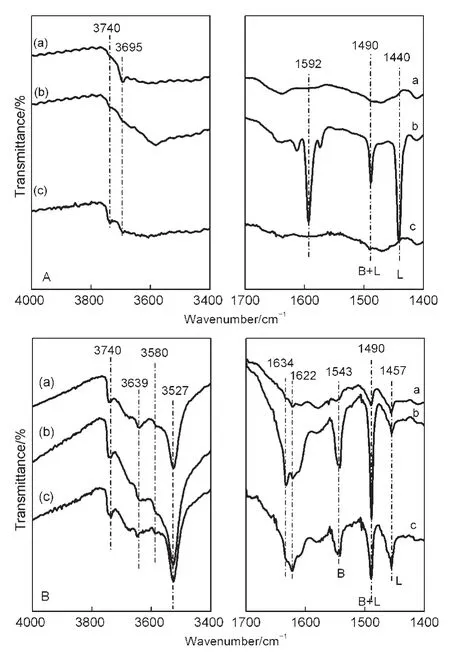
Fig.5 FTIR spectra of OH groups and pyridine adsorbed in the NaY(A)and CeY(B)zeolites
Fig.6(c)gives the FTIR spectra of cyclohexene adsorbed in the NaY and CeY zeolites.The bands appearing at 3002,1449,and 1439 cm-1(Fig.6A(c))indicate that the nature of cyclohexene adsorbed in NaY is weak/physical adsorption.The formation of alkenyl carbenium ions related to the band at 1520 cm-1and the disappearance of the band at 3002 cm-1(Fig.6B(c))suggest that cyclohexene can be activated by protonizations on the Brönsted acidic sites in the CeY zeolites.41The FTIR spectra in Fig.6(d)display that the modes of benzene adsorbed on NaY and CeY are both weak/physical adsorption.42
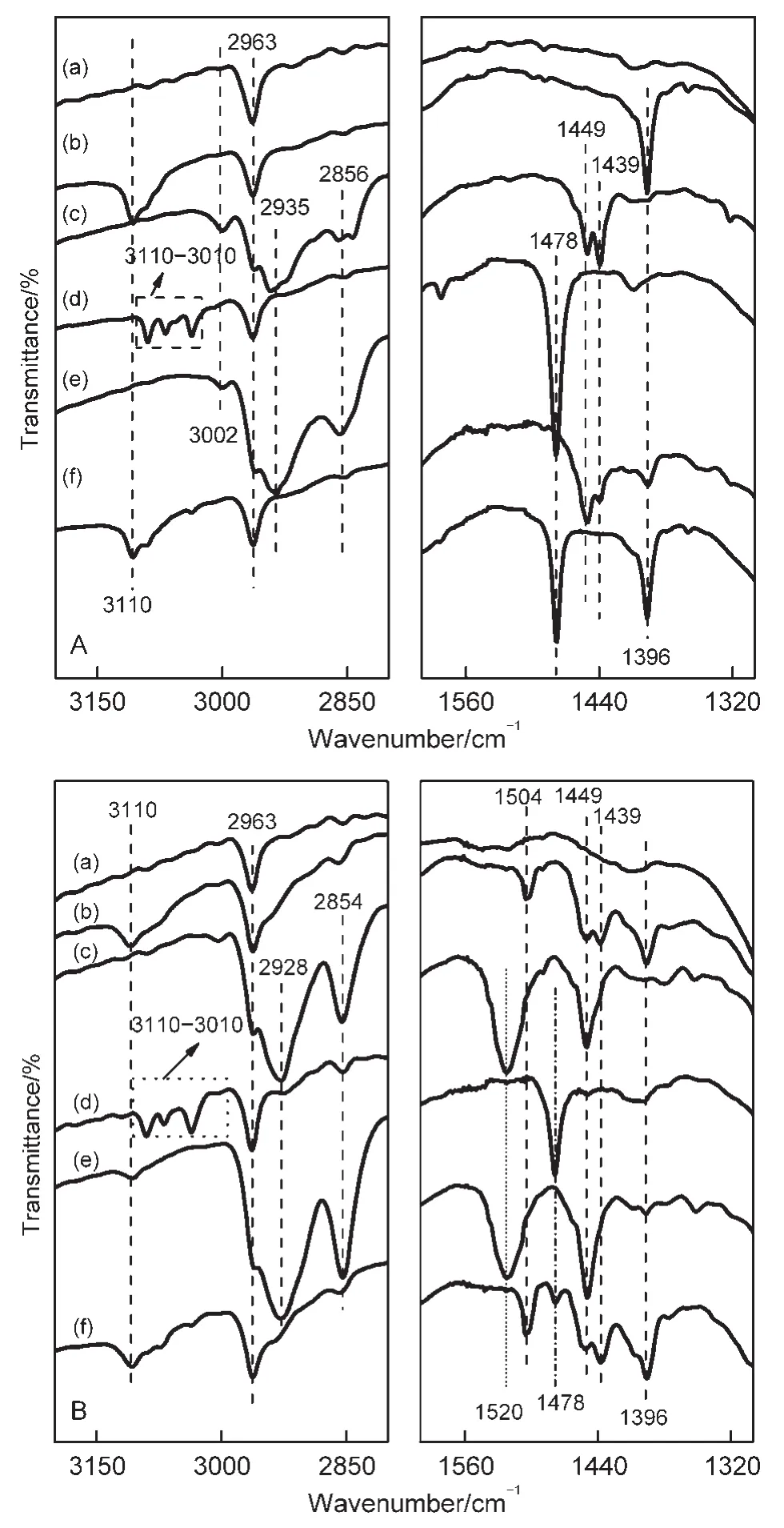
Fig.6 FTIR spectra of the fresh NaY(A)and CeY(B)samples(a),and adsorbed thiophene(b),cyclohexene(c),benzene(d),thiophene/cyclohexene(665 Pa/665 Pa)(e),and thiophene/benzene(665 Pa/665 Pa)(f)in NaY(A)and CeY(B)at RT for 1 h and then evacuating at RT for 0.5 h
The FTIR spectra of co-adsorption of thiophene and cyclohexene on the NaY and CeY zeolites are shown in Fig.6(e).The bands assigned to adsorbed cyclohexene and adsorbed thiophene are both exhibited in the spectra of co-adsorption of the two adsorbate on NaY(Fig.6A(e)),but the bands of the former are more prominent than that of the latter,implying that the adsorption intensity of cyclohexene is stronger than thiophene in NaY zeolites.This result suggests that NaY zeolites are not the ideal adsorbents for SADS from a gasoline containing olefines.The FTIR spectra of co-adsorption of cyclohexeneand thiophene in CeY(Fig.6B(e))show that the prior adsorption and the protonizations of olefin molecules on the Brönsted acid sites hinder the adsorption of thiophene molecules.This result illustrates that the coexisting olefinic compounds can depress the capability of removing thiophenic compounds of the CeY zeolites.
The results of co-adsorption of thiophene and benzene in NaY and CeY are shown in Fig.6(f).The strength of bands assigned to adsorbed benzene and thiophene are similar(Fig.6A(f)),implies that very strongly competitive adsorption between benzene and thiophene is present in NaY zeolites.But,from Fig.6B(f),it can be seen that the adsorption of thiophene is prior on the CeY sorbents rather than benzene,indicating that CeY zeolites are one kind of ideal sorbents for SADS technique for liquid fuels containing aromatics.
4 Conclusions
The results reported in this paper indicate that the desulfurization performance of CeY zeolites is affected both by competitive adsorption from aromatics and oligomerization reactions of olefinic and thiophenic compounds due to the strong Brönsted acidity on the surface of the adsorbents.
(1)The olefinic and aromatic hydrocarbons depress the desulfurization ability of CeY zeolites,but the effect mechanism is different.Besides,the effect of olefines is more significant than aromatics.
(2)The protonizations of olefinic or thiophenic molecules and oligomerizations due to the strong Brönsted acid sites in CeY zeolites are the unfavorable factor influencing the desulfurization capability of the adsorbents.
(3)The adsorption modes of thiophene on CeY zeolites are“direct coordinationviaS atoms”and“π-complexation mechanism”,but the former is the master one attributing to SADS.
(1)Yang,R.T.;Hernández-Maldonado,A.J.;Yang,F.H.Science2003,301(5629),79.doi:10.1126/science.1085088
(2)Velu,S.;Ma,X.L.;Song,C.S.;Namazian,M.;Sethuraman,S.;Venkataraman,G.Energy Fuels2005,19(3),1116.doi:10.1021/ef049800b
(3) Jeevanandam,P.;Klabunde,K.J.;Tetzler,S.H.Microporous Mesoporous Mat.2005,79(1),101.
(4) Nair,S.;Tatarchuk,B.J.Fuel2010,89(11),3218.doi:10.1016/j.fuel.2010.05.006
(5) Santos,A.L.;Reis,R.A.;Rossa,V.;Reis,M.M.;Costa,A.L.H.;Veloso,C.O.;Henriques,C.A.;Zotin,F.M.Z.;Paredes,M.L.L.;Silveira,E.B.;Chiaro,S.S.X.Mater.Lett.2012,83,158.doi:10.1016/j.matlet.2012.06.011
(6) Marín-Rosas,C.;Ramírez-Verduzco,L.F.;Murrieta-Guevara,F.R.;Hernández-Tapia,G.;Rodríguez-Otal,L.M.Ind.Eng.Chem.Res.2010,49(9),4372.doi:10.1021/ie901756b
(7) Seredych,M.;Bandosz,T.J.Fuel Process.Technol.2010,91(6),693.doi:10.1016/j.fuproc.2010.01.019
(8) Fallah,R.N.;Azizian,S.Fuel Process.Technol.2012,93(1),45.doi:10.1016/j.fuproc.2011.09.012
(9) Park,J.G.;Ko,C.H.;Yi,K.B.;Park,J.;Han,S.;Cho,S.;Kim,J.Appl.Catal.B:Environ.2008,81(3),244.
(10) Subhan,F.;Liu,B.;Zhang,Y.;Li,X.Fuel Process.Technol.2012,97,71.doi:10.1016/j.fuproc.2012.01.016
(11)Shao,X.C.;Duan,L.H.;Wu,Y.Y.;Qin,Y.C.;Yu,W.G.;Wang,Y.;Li,H.L.;Sun,Z.L.;Song,L.J.Acta Phys.-Chim.Sin.2012,28,1467.[邵新超,段林海,武玉叶,秦玉才,于文广,王 源,李怀雷,孙兆林,宋丽娟.物理化学学报,2012,28,1467.]doi:10.3866/PKU.WHXB201203312
(12)Shao,X.C.;Zhang,X.T.;Yu,W.G.;Wu,Y.Y.;Qin,Y.C.;Sun,Z.L.;Song,L.J.Appl.Surf.Sci.2012,263,1.doi:10.1016/j.apsusc.2012.07.142
(13) Yang,R.T.;Takahashi,A.;Yang,F.H.Ind.Eng.Chem.Res2001,40(26),6236.doi:10.1021/ie010729w
(14) Velu,S.;Ma,X.L.;Song,C.S.Ind.Eng.Chem.Res.2003,42(21),5293.doi:10.1021/ie020995p
(15) Hernández-Maldonado,A.J.;Yang,R.T.Ind.Eng.Chem.Res.2003,42(1),123.doi:10.1021/ie020728j
(16) Hernández-Maldonado,A.J.;Yang,R.T.J.Am.Chem.Soc.2004,126(4),992.
(17) Hernández-Maldonado,A.J.;Yang,R.T.Ind.Eng.Chem.Res.2004,43(4),1081.doi:10.1021/ie034206v
(18) Yang,R.T.;Hernández-Maldonado,A.J.Catal.Rev.-Sci.Eng.2004,46(2),111.doi:10.1081/CR-200032697
(19) Hernández-Maldonado,A.J.;Yang,F.H.;Qi,G.;Yang,R.T.Appl.Catal.B:Environ.2005,56(1),111.
(20) Tang,K.;Song,L.J.;Duan,L.H.;Li,X.Q.;Gui,J.Z.;Sun,Z.L.Fuel Process.Technol.2008,89(1),1.doi:10.1016/j.fuproc.2007.06.002
(21) Wang,H.G.;Jiang,H.;Xu,J.;Sun,Z.L.;Zhang,X.T.;Zhu,H.L.;Song,L.J.Acta Phys.-Chim.Sin.2008,24,1714.[王洪国,姜 恒,徐 静,孙兆林,张晓彤,朱赫礼,宋丽娟.物理化学学报,2008,24,1714.]doi:10.3866/PKU.WHXB20080933
(22) Ju,X.F.;Jin,L.L.;Ma,T.;Chen,X.L.;Song,L.J.Acta Phys.-Chim.Sin.2009,25,2256.[鞠秀芳,靳玲玲,马 涛,陈晓陆,宋丽娟.物理化学学报,2009,25,2256.]doi:10.3866/PKU.WHXB20091024
(23)Wang,W.Y.;Pan,M.X.;Qin,Y.C.;Wang,L.T.;Song,L.J.Acta Phys.-Chim.Sin.2011,27,1176.[王旺银,潘明雪,秦玉才,王凌涛,宋丽娟.物理化学学报,2011,27,1176.]doi:10.3866/PKU.WHXB20110442
(24) Lin,L.;Zhang,Y.;Zhang,H.;Lu,F.J.Colloid Interface Sci.2011,360,753.doi:10.1016/j.jcis.2011.04.075
(25) Wang,H.G.;Song,L.J.;Jiang,H.;Xu,J.;Jin,L.L.;Zhang,X.T.;Sun,Z.L.Fuel Process.Technol.2009,90(6),835.doi:10.1016/j.fuproc.2009.03.004
(26)Duan,L.H.;Gao,X.H.;Meng,X.H.;Zhang,H.T.;Wang,Q.;Qin,Y.C.;Zhang,X.T.;Song,L.J.J.Phys.Chem.C2012,116(49),25748.doi:10.1021/jp303040m
(27) Shi,Y.C.;Yang,X.J.;Tian,F.P.;Jia,C.Y.;Chen,Y.Y.J.Nat.Gas Chem.2012,21(4),421.doi:10.1016/S1003-9953(11)60385-X
(28) Chen,N.Y.;Mitchell,T.O.;Olson,D.H.;Pelrine,B.P.Ind.Eng.Chem.Prod.Res.Dev.1977,16(3),247.doi:10.1021/i360063a012
(29) Garcia,C.;Lercher,J.J.Phys.Chem.1992,96(6),2669.doi:10.1021/j100185a050
(30) Chica,A.;Strohmaier,K.;Iglesia,E.Langmuir2004,20(25),10982.doi:10.1021/la048320+
(31) Richardeau,D.;Joly,G.;Canaff,C.;Magnoux,P.;Guisnet,M.;Thomas,M.;Nicolaos,A.Appl.Catal.A:Gen.2004,263(1),49.doi:10.1016/j.apcata.2003.11.039
(32) Deangelis,B.A.;Appierto,G.J.Colloid Interface Sci.1975,53(1),14.doi:10.1016/0021-9797(75)90029-6
(33) Datka,J.;Sulikowski,B.;Gil,B.J.Phys.Chem.1996,100(27),11242.doi:10.1021/jp951523+
(34) Gil,B.;MierzyDska,K.;SzczerbiDska,M.;Datka,J.Microporous Mesoporous Mat.2007,99(3),328.doi:10.1016/j.micromeso.2006.09.025
(35) Rabo,J.A.;Angell,C.L.;Kasai,P.H.;Schoemaker,V.Discuss.Faraday Soc.1966,41,328.doi:10.1039/df9664100328
(36) Ward,J.W.J.Phys.Chem.1968,72(12),4211.doi:10.1021/j100858a046
(37) Layman,K.A.;Bussell,M.E.J.Phys.Chem.B2004,108(40),15791.doi:10.1021/jp047882z
(38) Garcia,C.L.;Lercher,J.A.J.Phys.Chem.1992,96(6),2669.doi:10.1021/j100185a050
(39) Zhang,X.T.;Yu,W.G.;Qin,Y.C.;Dong,S.W.;Pei,T.T.;Wang,L.;Song,L.J.Acta Phys.-Chim.Sin.2013,29,1273.[张晓彤,于文广,秦玉才,董世伟,裴婷婷,王 琳,宋丽娟.物理化学学报,2013,29,1273.]doi:10.3866/PKU.WHXB201303183
(40)Qin,Y.C.;Mo,Z.S.;Yu,W.G.;Dong,S.W.;Duan,L.H.;Gao,X.H.;Song,L.J.Appl.Surf.Sci.2014,292,5.doi:10.1016/j.apsusc.2013.11.036
(41) Yang,S.;Kondo,J.N.;Domen,K.Catal.Today2002,73(1),113.
(42) Kukulska-Zajac,E.;Kozyra,P.;Datka,J.Appl.Catal.A:Gen.2006,307(1),46.doi:10.1016/j.apcata.2006.03.005
