Nd3+/Yb3+离子共掺的氧化钛基上转换发光玻璃的热学和力学性能
2014-06-23于惠梅潘秀红张明辉余建定
于惠梅 潘秀红 张明辉 刘 岩 余建定
(1中国科学院上海硅酸盐研究所,上海200050;2美国宾州州立大学能源研究所,宾夕法尼亚州16802,美国)
1 Introduction
Upconversion from infrared to visible light in rare earth(RE)ions doped glasses attracted much attention for their potential use in infrared converters and upconversion short wavelength solid-state lasers.1,2The trivalent neodymium ion(Nd3+)was one of the most important active ions in the RE family due to its favorable energy level structure.Spectroscopic results demonstrated that the Nd3+ions were good candidates for upconversion luminescence.3,4Among the glassy materials,the fluoride glasses were notable due to its lower phonon energy,which can reduce the nonradiative loss caused by the multiphonon relaxation and thus realize strong upconversion luminescence.5,6Unfortunately,the fluoride glasses usually performed low mechanical property and thermal stability,and therefore were limited for practical utility.Oxide glasses,on the other hand,might be much better for practical applications because of their high thermal stability,chemical durability and mechanical property.
Titanium dioxide can be a good candidate matrix for upconversion generation because it has lower cutoff phonons of energy(600-700 cm-1).7,8However,it was difficult to form titanate bulk glass by traditional methods.Aerodynamic levitation is one of the containerless processing methods which can avoid melt contamination from containers and preclude the source for heterogeneous nucleation,resulting in deep undercooling for glass forming in the melt.It is a useful way to fabricate homogenous,high-purity and compact glasses.Therefore,in our previous work,this new containerless method by aerodynamic levitation was successfully used to prepare the Nd3+/Yb3+codoped TiO2-La2O3-ZrO2(TLZ)glasses.9Intense infrared-to-visible upconversion luminescence was observed when Nd3+and Yb3+ions were incorporated upon excitation of 980 nm laser.In this work,in order to estimate the overall performance of the titanate glass for practical application,thermal and mechanical properties of TLZ glasses incorporated with Nd3+and Yb3+ions are investigated by thermal differential analysis(DTA)and micro-hardness test.Besides,the upconversion luminescence property upon 808 nm laser excitation in the rare earth doped TLZ glasses has also been demonstrated.
2 Experimental
2.1 Preparation of Nd3+/Yb3+co-doped TLZ glasses
All the starting materials were of analytical grade and the host composition(molar fraction(x),%)of the titanate glass was 78TiO2-18La2O3-4ZrO2.Neodymium and ytterbium ions in the form of Nd2O3and Yb2O3were added.The concentration of Yb3+ion was fixed at 0.2%(molar fraction)while Nd3+concentration varied as 0.6%,1.2%,1.8%,and 2.4%(x),respectively.Raw materials with stoichiometric compositions were mixed and compressed into small rods.The rods were levitated in an aerodynamic levitation furnace and melted by a CO2laser with temperature of 1700°C.A pyrometer was utilized to measure the temperature of the sample.A high resolution charge-coupled device video camera equipped with telephoto objective lens was employed to obtain a magnified view of the sample.Then the CO2laser power was shut off and spherical sample with a diameter of~3 mm was obtained.The as-prepared spherical sample was polished to be 1.5 mm thick wafer for further measurements.Fig.1 shows the photo of Nd3+/Yb3+co-doped TLZ glass wafers with both sides well polished.Obviously,all the investigated TLZ samples performed high transparency in the region of visible light.
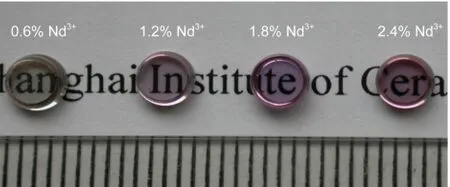
Fig.1 Photo of Nd3+/Yb3+co-doped TLZ glass wafers with both sides well polished
2.2 Characterization
Thermal differential analysis was carried out to characterize the thermal stability10-15by Netzsch STA 449C thermoanalyzer in the experiments.The amorphous structure of TLZ glasses prepared by levitation method has been identified by Bruker D8 Advance X-ray diffractometer(XRD).9,16The mechanical property was estimated by Vickers hardness test which was performed on an Akashi(AVK-A)hardness tester with an applied load of 1 kg for 10 s.Upconversion luminescence spectra were recorded by a spectrofluorometer(Fluorolog-3,Jobin Yvon,France)equipped with Hamamatsu R928 photomultiplier tube.An 808 nm continuous wave diode laser was used as the excitation source.
3 Results and discussion
3.1 Thermal stability
DTA was used to investigate the thermal stability of Nd3+/Yb3+co-doped TLZ glasses.Fig.2 shows the DTA curves at a heating rate(β)of 10 K · min-1.All the curves had similarshapes.The glass transition temperature(Tg),crystallization onset temperature(Tc),and crystallization peak temperature(Tp),for the TLZ glasses can be obtained from Fig.2.
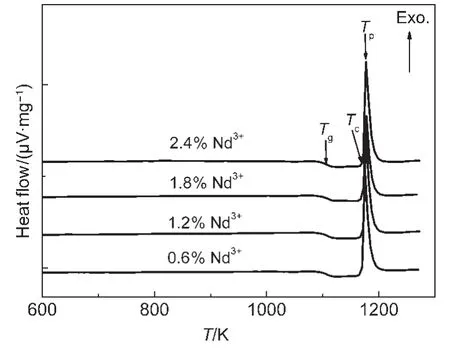
Fig.2 DTAcurves of Nd3+/Yb3+co-doped TLZ glass samples with a heating rate(β)of 10 K·min-1
It was obvious that the variation of Nd3+content had no significant effect on the values ofTg,Tc,andTpin the investigated scope.Tgwas estimated as high as~1093 K,much higher than that of fluoride or oxy-fluoride glasses which was usually lower than 700 K.13,14Therefore,the upconversion devices based on this glass might be used more widely for technological application.On the other hand,the values ofTcandTpwere 1168 and 1177 K,respectively.Similarly,these two values were also higher than those of fluoride or oxy-fluoride glasses.These results hinted a good thermal stability against high temperature.
The value of ΔT=Tc-Tg,which is often an important parameter to characterize the glass forming ability has also been calculated.Fig.3 shows the DTA curves of Nd3+/Yb3+co-doped TLZ glass samples at different heating rates.The obtained results are shown in Table 1.The calculated ΔTof present TLZ glasses was much lower than those of most traditional oxide glasses such as silicate or borate glass systems14,15and even lower than some heavy metal oxide glasses.16,17It indicated a comparatively poor glass forming ability for Nd3+/Yb3+co-doped TLZ glass.This makes it difficult to obtain bulk titanate glass by traditional melt-quenching method.Therefore,levitation method has to be introduced to prepare bulk titanate glass in present experiments,because overheated or undercooled melt was achieved more easily by this technique.Non-isothermal kinetic analyses were used in our experiments.18The heating rates were 5,10,15,and 20 K·min-1.The commonly used kinetic equation of solid thermal decomposition under non-isothermal conditions was given as follows:
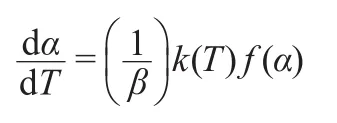
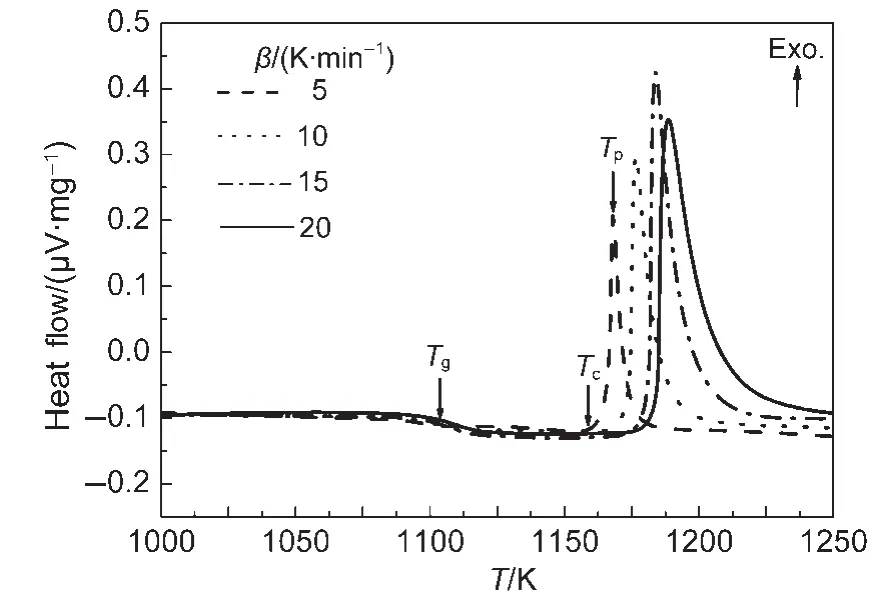
Fig.3 DTAcurves of Nd3+/Yb3+co-doped TLZ glass samples at different heating rates
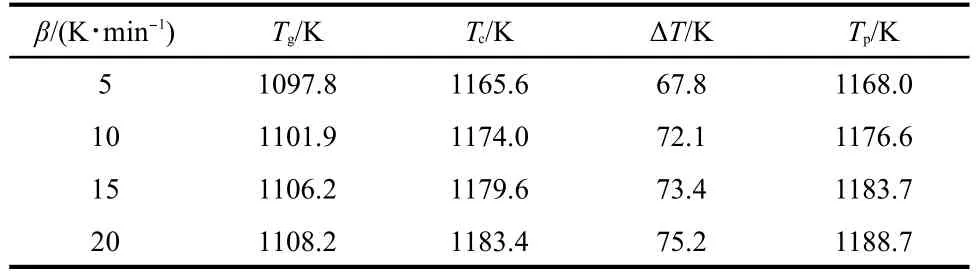
Table 1 Values of Tg,Tc,and Tpof the Nd3+/Yb3+co-doped TLZ glasses at different heating rates
Whereαwas the extent of reaction,k(T)=Ae-Ea/RT,Eawas the activation energy,Awas the pre-exponential factor.f(α)was the differential conversion function.Both Kissinger and Friedman methods can calculate the activation energy and lgAof the oxidation process under non-isothermal conditions.Fig.4 shows the average activation energy and lgAobtained by the Kissinger method for the crystallization of Nd3+/Yb3+co-doped TLZ glass.The Kissinger equation19was given as follows:
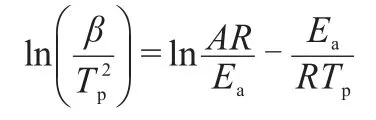
The average activation energy andAwere 325 kJ·mol-1and 6.02×1013s-1,respectively.The dependence ofEaand lgAobtained by Friedman analysis for the crystallization of Nd3+/Yb3+co-doped TLZ glass was shown in Fig.5.It can be concluded that large activation energy was required at the beginning of the reaction.As the extent of conversion enhancing,the precipitation was increasingly easy in the Nd3+/Yb3+co-doped TLZ glass.
3.2 Mechanical property
Vickers hardness was experimentally measured to evaluate the mechanical property of the Nd3+/Yb3+co-doped TLZ glasses.The Vickers hardness was determined using a micro-hard-ness tester equipped with a Vickers indenter.The indentation was examined by an optical microscopy.Fig.6 shows two typical optical micrographs of Vickers indentations for Nd3+/Yb3+co-doped TLZ glasses.Significant micro-cracks emanating from the corners of the indentations can be observed in both images.In Fig.6(a),no shear bands were observed,while in Fig.6(b)semi-circular shear band mark was visible on the one side of the indentation impression,suggesting an asymmetric deformation of the surface.It should be mentioned that,seldom shear band can be observed in TLZ glass with low Nd3+concentration.Nevertheless,they were observed frequently in TLZ glass with high Nd3+concentration.
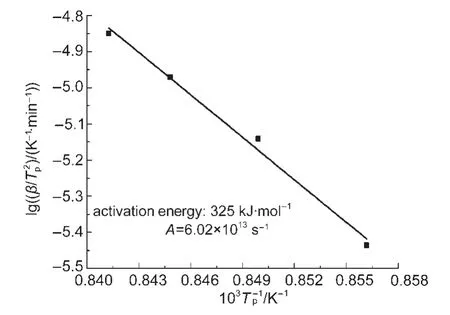
Fig.4 Average activation energy and lgAobtained by the Kissinger method for the crystallization of Nd3+/Yb3+co-doped TLZ glass
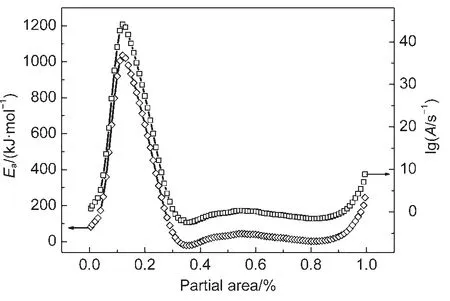
Fig.5 Dependence of Eaand lgAobtained by Friedman analysis for the crystallization of Nd3+/Yb3+co-doped TLZ glass
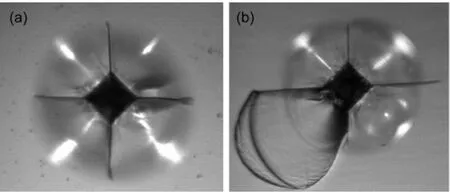
Fig.6 Two typical optical micrographs of Vickers indentations for Nd3+/Yb3+co-doped TLZ glasses
Quantitative value of Vickers hardness was averaged from three data points by the following standard formula:20Hv=1.854P/d2.Here,Hvwas the Vickers hardness,Pwas the applied load,anddwas the mean length of the two diagonal lines of the indentation.The measured values of Vickers hardness were listed in Table 2.No significant correlation seemed to exist between Nd3+concentration and the Vickers hardness since the variation of Nd3+concentration was very weak.However,theHvvalues were higher than 7.50 GPa for all the investigated TLZ glasses.This value was not only much higher than that of the ordinary fluoride glass(~2.0 GPa)21but also higher than that of some traditional oxide glass such as silicate or borate glass system.22Another important parameter characterizing the mechanical property of a material is fracture toughness,which describes the resistance of the material to crack propagation.The fracture toughnessKcfor present Nd3+/Yb3+co-doped TLZ glasses was determined by micro-indentation experiments with Vickers indenter.TheKcvalue was calculated using the following equation:23Kc=P(πb)-3/2(tgγ)-1,wherebwas the crack length,andγ=68°.The calculated results were also illustrated in Table 2,where one can see that theKcvalues of the TLZ glasses varyfrom 1.20 to 1.39 MPa·m1/2.Similarly,theKcvalues for the investigated rare earth doped TLZ glasses were higher than those of ordinary rare earth doped fluoride glasses(~0.60 MPa·m1/2).21The high Vickers hardness as well as high fracture toughness indicated a potential application for upconversion devices made of the Nd3+/Yb3+co-doped TLZ glasses.
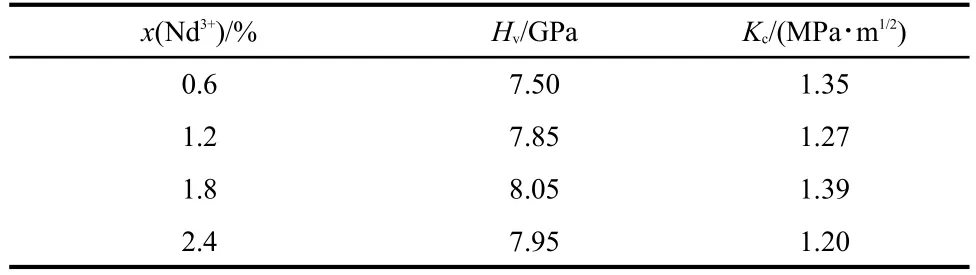
Table 2 Variation of Vickers hardness(Hv)and fracture toughness(Kc)with Nd3+concentrations(molar fraction)in the Nd3+/Yb3+co-doped TLZ glasses
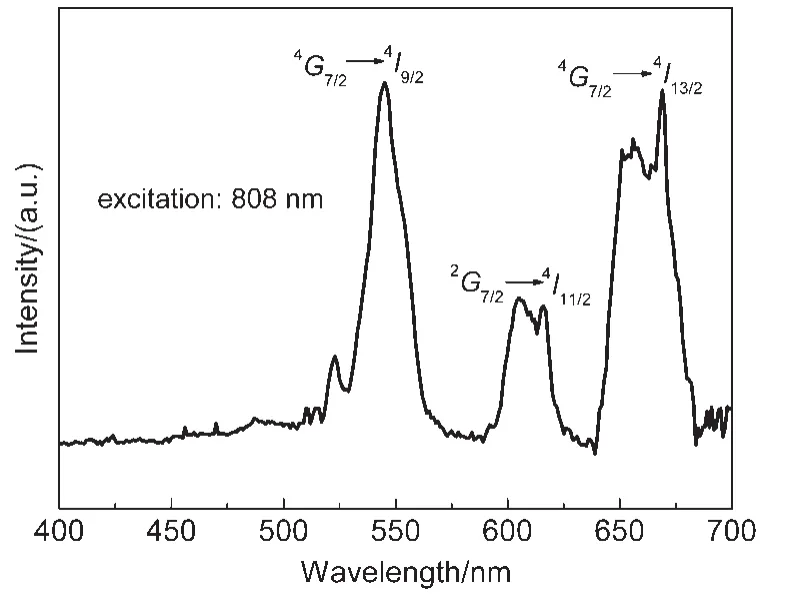
Fig.7 Upconversion emission spectrum of 1.2%(x)Nd3+/Yb3+co-doped TLZ glasses
3.3 Upconversion luminescence upon 808 nm laser excitation
The upconversion emission spectra of Nd3+/Yb3+co-doped TLZ glasses have been recorded at 808 nm excitations.All the spectra showed similar features except for an increase in intensities of the upconversion bands.Fig.7 shows the typical upconversion spectrum of Nd3+/Yb3+co-doped TLZ glass(for the sample of 1.2%(x)Nd3+ions).Three intense emission bands were observed centered at 545,605,and 669 nm,respectively,which were attributed to the Nd3+:4G7/2→4I9/2,2G7/2→4I11/2,and4G7/2→4I13/2transitions,respectively.It was clear that the emissions at 605 and 669 nm had significant shoulder peaks at 615 and 655 nm,respectively.They were possibly due to the energy level splitting of2G7/2and4G7/2levels.The peak intensity of the green emission at 545 nm was almost the same as that of the red emission at 669 nm.However,the band intensity of the orange emission at 605 nm was only a half or so,which was different from the result for 980 nm excitation where the orange emission intensity was much weaker than the green and red emission intensities.9This indicated that more Nd3+ions were populated to2G7/2level by 808 nm laser.
4 Conclusions
Nd3+/Yb3+co-doped TiO2-La2O3-ZrO2glasses were prepared by the levitation method.The glasses performed good thermal stability and high mechanical property.The average activation energyEaand the pre-exponential factorAobtained by means of Kissinger were 325 kJ·mol-1and 6.02×1013s-1,respectively.We also obtained the dependence ofEaand lgAon the extent of conversionacalculated by the Friedman method.Vickers hardness and fracture toughness were found higher than 7.50 GPa and 1.20 MPa·m1/2,respectively,indicating high mechanical property.Under 808 nm laser excitation,intensegreen(545 nm),orange(605 nm),and red(669 nm)upconversion emissions were observed,which were attributed to the Nd3+:4G7/2→4I9/2,2G7/2→4I11/2,and4G7/2→4I13/2transitions,respectively.
(1) Giri,N.K.;Singh,A.K.;Rai,S.B.J.Appl.Phys.2007,101,033102.doi:10.1063/1.2432305
(2) Silva,D.M.;Kassab,L.R.P.;Luthi,S.R.;Araujo,C.B.;Gomes,A.S.L.;Bell,M.J.V.Appl.Phys.Lett.2007,90,081913.doi:10.1063/1.2679798
(3) Fernandez,J.;Balda,R.;Mendioroz,A.;Sanz,M.;Adam,J.L.J.Non-Cryst.Solids2001,287,437.doi:10.1016/S0022-3093(01)00597-X
(4)Som,T.;Karmakar,B.J.Alloy.Compd.2009,476,383.doi:10.1016/j.jallcom.2008.09.006
(5) Feng,L.;Lai,B.;Wang,J.;Du,G.;Su,Q.J.Lumin.2010,130,2418.doi:10.1016/j.jlumin.2010.08.005
(6) Karmakar,B.J.Solid State Chem.2005,178,2663.doi:10.1016/j.jssc.2005.06.007
(7) Porto,S.P.S.;Fleury,P.A.;Damen,T.C.Phys.Rev.1967,154,522.doi:10.1103/PhysRev.154.522
(8) Mazza,T.;Barborini,E.;Piseri,P.;Milani,P.;Cattaneo,D.;Bassi,A.L.;Bottani,C.E.;Ducati,C.Phys.Rev.B2007,75,045416.doi:10.1103/PhysRevB.75.045416
(9)Pan,X.H.;Yu,J.;Liu,Y.;Zhang,M.H.J.Mater.Res.2011,26,2907.doi:10.1557/jmr.2011.365
(10) Plsko,A.;Liska,M.;Pagacova,J.J.Therm.Anal.Calorim.2012,108,505.doi:10.1007/s10973-011-1967-x
(11) Jaqueline,L.M.;Antonio,R.S.;Aurelio,H.R.;Beatriz,Z.;Carlos,G.Y.;Roberto,M.S.Thermochim.Acta2011,516,35.doi:10.1016/j.tca.2011.01.008
(12)Yu,H.M.;Qi,L.J.;Zhang,Q.H.;Jiang,D.Y.;Lu,C.L.J.Therm.Anal.Calorim.2011,106,47.doi:10.1007/s10973-010-1280-0
(13) Zhang,K.;Liu,Q.;Su,X.B.;Zhong,H.M.;Shi,Y.;Pan,Y.B.Acta Phys.-Chim.Sin.2011,27(8),2001.[张 孔,刘 茜,苏晓彬,钟红梅,石 云,潘裕柏.物理化学学报,2011,27(8),2001.]doi:10.3866/PKU.WHXB20110732
(14)Yu,D.W.;Zhu,M.Q.;Utigard,T.A.;Barati,M.Thermochim.Acta2014,575,1.doi:10.1016/j.tca.2013.10.015
(15)Abdel-Rehim,A.M.Thermochim.Acta2012,538,29.doi:10.1016/j.tca.2012.03.006
(16)Pan,X.H.;Yu,J.;Liu,Y.;Yoda,S.;Yu,H.M.;Zhang,M.H.;Ai,F.;Jin,F.;Jin,W.Q.J.Alloy.Compd.2011,509,7504.doi:10.1016/j.jallcom.2011.04.104
(17) Babu,P.;Seo,H.J.;Kesavulu,C.R.;Jang,K.H.;Jayasankar,C.K.J.Lumin.2009,129,444.doi:10.1016/j.jlumin.2008.11.014
(18) Koepke,C.;Piatkowski,D.;Wisniewski,K.;Naftaly,M.J.Non-Cryst.Solids2010,356,435.doi:10.1016/j.jnoncrysol.2009.12.012
(19)Abdel-Hameed,S.A.M.;El-kheshen,A.A.Ceram.Int.2003,29,265.doi:10.1016/S0272-8842(02)00114-1
(20)Xu,T.;Zhang,X.;Dai,S.;Nie,Q.;Shen,X.;Zhang,X.Physica B2007,389,242.doi:10.1016/j.physb.2006.06.156
(21) Simon,S.;Simon,V.Mater.Lett.2004,58,3778.doi:10.1016/j.matlet.2004.07.042
(22) Opfermann,J.J.Therm.Anal.Calorim.2000,60,641.doi:10.1023/A:1010167626551
(23) Hu,R.Z.;Gao,S.L.;Zhao,F.Q.;Shi,Q.Z.;Zhang,T.L.;Zhang,J.J.Thermal Analysis Kinetics;Sciences Press:Beijing,2008;pp 79-80.[胡荣祖,高胜利,赵凤起,史启祯,张同来,张建军.热分析动力学.北京:科学出版社,2008:79-80.]
(24) Lin,M.T.;Jiang,D.Y.;Li,L.;Lu,Z.L.;Lai,T.R.;Shi,J.L.Mater.Sci.Eng.A2003,351,9.doi:10.1016/S0921-5093(01)01772-5
(25) Delben,A.;Messaddeq,Y.;Caridade,M.D.;Aegerter,M.A.;Eiras,J.A.J.Non-Cryst.Solids1993,161,165.doi:10.1016/0022-3093(93)90691-P
(26) Sampaio,J.A.;Baesso,M.L.;Gama,S.;Coelho,A.A.;Eiras,J.A.;Santos,I.A.J.Non-Cryst.Solids2002,304,293.doi:10.1016/S0022-3093(02)01037-2
(27) Evans,A.G.;Charles,E.A.J.Am.Ceram.Soc.1976,59,371.doi:10.1111/jace.1976.59.issue-7-8
