Marine Pharmaceutical Industry Development in Developed Countries and Its Enlightenment
2014-05-15ZHANGLIjunHUANGTaikang
ZHANG LI-jun, HUANG Tai-kang,
(1.Research Center of Modern Social Pharmacy, Shenyang Pharmaceutical University, Shenyang 110016, China; 2.China Food and Drug Administration, Beijing 100810, China)
Marine Pharmaceutical Industry Development in Developed Countries and Its Enlightenment
ZHANG LI-jun1, HUANG Tai-kang1,2
(1.Research Center of Modern Social Pharmacy, Shenyang Pharmaceutical University, Shenyang 110016, China; 2.China Food and Drug Administration, Beijing 100810, China)
Objective To provide references for marine pharmaceutical industry development in China by analyzing the development modes of marine pharmaceutical industry in developed countries. Methods Comparative study was conducted. Results and Conclusion Experiences of marine pharmaceutical industry development in the US, Japan and the EU are discussed, and the enlightenment to marine pharmaceutical industry in China is analyzed.
marine pharmaceutical industry; scientific research and development; talent training
The ocean has become an increasingly critical area for human existence and development. Endowed with rich living marine resources, China boosts a favorable foundation and potential for developing marine pharmaceutical industry. However, the development of this industry is hampered by problems like small production scale, poor industryuniversity-research cooperation, insufficient proprietary intellectual property rights and the slow transformation of scientific research achievements into market products. These problems are the result of backward technologies of marine drugs and marine biological resources development, the complexity of marine drugs production and insufficient investment. At the symposium of the Political Bureau of the Communist Party of China Central Committee on July 30th 2013, XI Jin-ping, the General Secretary, pointed out that China would strive to better plan and supervise the advancement of marine industries, optimize and upgrade the marine industrial structure, and nurture strategic and emerging sectors so as to improve the quality of marine economy, boost economic growth and solidify the strategic status of marine industries. China has been increasingly aware of the significance of strategic marine industries and has accelerated the advancement of marine pharmaceutical industry. With new opportunities ahead, we should analyze and draw on experience of developed countries in their development modes in the field of marine pharmaceutical industry so as to facilitate the development of this industry in our own country.
1 The status quo of marine pharmaceutical industry in foreign countries
1.1 An overview
With the boom of global pharmaceutical products market and the increase of medical needs, all countries have shifted their focus from traditional pharmaceutical products to biotechnological pharmacy. The ocean, as the cradle of life and the treasury of resources, will undoubtedly become the core of biotechnological pharmacy and the focal point of the pharmaceutical industry. At the first International Symposium on Marine Medicine in the US in 1967, the US put forward the idea of “drugs from the sea”. Blue medicines have thus attracted worldwide attention. As a milestone of the development of marine pharmaceutical industry, this symposium ushered in an age of global cooperation in marine biological research[1]. The 1990s witnessed the wide clinical trials of natural marine products and the utilization of marine resources gradually became the focus of all governments. The United Nations Convention on the Law of the Sea came into effect in 1994. In an attempt to facilitate the development of marine pharmacy and microbial drugs, countries like the US, Russia, Japan and France have formulated a series of plans such as Sea SapphirePlan, Marine Biological Development Plan and Marine Biotechnology Research Plan[2]. Great investment was put into research and development. With the advent of the 21st century, human beings have entered into a golden age of exploiting and utilizing living marine resources. Numerous industries have been nurtured. Up to now, more than 20, 000 chemical compounds have been extracted from marine organisms[3]. These compounds are widely used in analgesia, anti-coagulation, anti-tumor, anti-bacterial, anticardiovascular diseases, anti-viral and anti-inflammatory treatments. Moreover, an increasing number of compounds are discovered each year and more than 200 have already been listed as patent compounds. At present, the industry scale of marine medicine amounts to several billions of US dollars worldwide. The following Table 1 shows the chemical structure and sources of some major marine medicines.
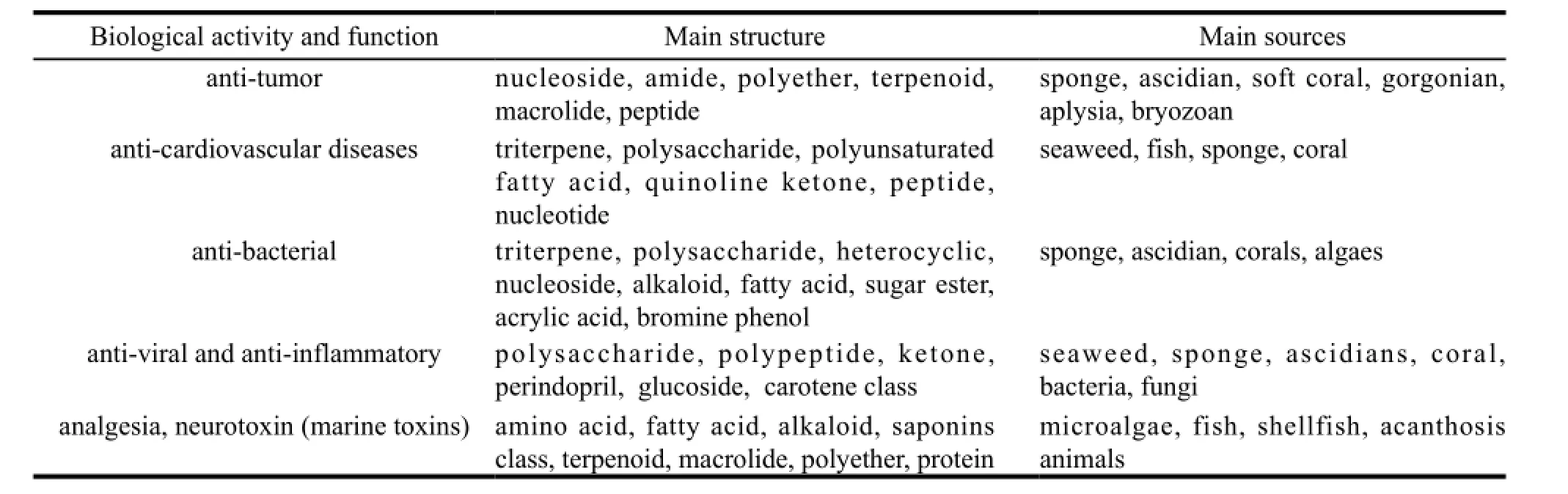
Table 1 Chemical structure and sources of major marine medicines
1.2 Analysis of the development modes of marine pharmaceutical industry in typical countries
1.2.1 The US
The first International Symposium on Marine Medicine was held in the US in 1967. Blue medicines have thus attracted worldwide attention. In the 1970s, American scientists of marine medicine technology conducted a rigorous R&D program of marine medicine. Featuring an absolute advantage in basic science and a solid theory foundation, the US has conducted wide and thorough studies in applied marine science with its major advantages in fields like marine medicines and has achieved remarkable progress in the industrialization of marine medicine. Endowed with cutting-edge science and technology and a solid economic basis, the US has achieved numerous results in developing high and new marine technologies especially in marine bio-medicine. As many as 1500 kinds of marine products are extracted each year. Of these products, 10% are with anti-cancer activity with more than 10 of them being put into clinical trials or pre-clinical research[4]. It is worthwhile to mention that a preparation extracted from Amydasincnsis cartilage has been proved effective in overcoming the side effects caused by chemotherapy and radiotherapy and in enhancing the immunity of patients. The Hawaii laboratories set up by the US government has attracted 3/5 of the scientists and engineers of marine research and development nationwide. Focusing on developing marine biotechnological products, these laboratories make an investment as high as 27 billion US dollars in high and new marine technologies each year.
Against the background of marine economy boom in the 1960s, Japan shifted its economic focus to oceanic exploitation and marine industrial development. In 1968, Japanese Marine Science and Technology Planning was promulgated. In the year of 1988, the earliest Marine Biotechnology Research Institute set up two laboratories, which successfully compounded “chitosan” from the cells of lower plants like algae and fungus. “Chitosan” was mainly used in detoxification and anti-cancer. Besides, Japan and other developed countries were very active in the development of marine bioactive peptides, which were extracted through biological enzymolysis technology and then made into highly nutritious food, functional foodand pharmaceutical intermediates. The first International Conference on Marine Technology was held in Japan in 1988, which was named as “The First Year of Marine Biotechnology”. The Japanese Marine Biotechnology Research Institute and Japan Marine Science and Technology Center invest as many as 0.1 billion US dollars in marine medicine research each year[5].
1.2.3 The EU
The Blue Book of Marine Integrated Policies of the EU pointed out that emerging marine industries included blue ocean technology industry, renewable energy industry, underwater technology and equipment and marine aquaculture. In the EU, 0.1 billion US dollars are invested in the study on the development and utilization of marine drugs. The US, Japan and the EU are pioneers in developing marine drugs. With robust economy, advanced science and technology and rich human resources, the EU stands in a quite favorable position in marine medicine research.
2 Successful experience of the development of marine pharmaceutical industry in developed countries
Based on the analysis of development modes of marine pharmaceutical industry in developed countries, a conclusion can be safely drawn: support from the government in terms of development policies, human resource and science and technology is a key to the success of the industry. The government should set up specialized industrial development agencies, promulgate national-level development policies and programs, provide special fund and train R&D personnel.
2.1 To promulgate national-level development policies and programs
Sound policies and programs of the government is a key to the success of marine pharmaceutical industry in developed countries. The American government has promulgated Ocean Blueprint in the 21st Century to include high and new technology industries such as marine pharmaceutical industry into its short-term objectives, and has passed the Future Biotechnology Investment and Expansion Act to encourage companies to increase investment in the exploitation and research of marine pharmaceutical industry. As for the Japanese government, they have drafted all kinds of policies to encourage the development of high and new marine technologies and marine medicine technologies. A series of policies and laws such as Japan Marine Development Plan, Basic Laws of the Ocean, Basic Ocean Plan Draft, and Medicine Law were continuously put into practice to supervise, safeguard and facilitate the healthy and sustainable development of marine pharmaceutical by providing supporting measures to protect the transformation of achievements and patent rights. The Blue Book of Integrated Marine Policies of the EU, the Instruction of Legal Protection of Biotechnological Inventions and European Life Science and Biotechnology Strategy will all guide and safeguard the development of biotechnology in the EU.
在国家确定的山东省用水总量控制目标内,根据《山东省水资源综合规划》、有关水量分配方案及各市实际用水情况,分解确定全省17市2015、2020、2030年分阶段用水总量控制目标及各年度用水总量控制目标,在此基础上,各市进一步分解到所辖县 (市、区)。取水审批机关审批的取水总量,不得超过本流域或行政区域的取水许可总量控制指标,在审批的取水总量已达到取水许可总量控制指标的流域和行政区域,不得再审批新增取水。
UK Natural Environment Research Council has published Marine Science and Technology Planning of 2025. As a national program to meet the challenge of marine changes, it consists of 10 topics, including biological diversity and biological system function. In 2003, the Australian government published the Australian Marine Industry Development Strategy, in which it pointed out that emerging marine industry would be an important area and would play a critical role in economic growth. The Australian government defined emerging marine industry as marine biotechnology, chemicals, seabed minerals, marine alternative energies and sea water desalinization. Based on the above analysis and China’s current situation, we should promulgate national-level industrial development policies and laws as well as specific development programs facilitate and safeguard the development of marine pharmaceutical industry.
2.2 To establish an effective mechanism for investment and financing
Marine pharmaceutical industry features high risk, high investment and long pay-off period. As a result, sufficient fund is critical to the development of the industry. Advanced countries of marine pharmaceutical industry all pay great attention to financial investment in order to facilitate the research and development of relative technologies and products. Nevertheless, the subject of investment in China is still the government, which cannot guarantee the sustainable development of this industry. The government should publish effective policies and increase investment, establish a multi-level relationship between market and capital and continuously perfect banks’indirect financing system and the system of absorbing foreign capital. The government should also encourage companies to increase financial investment, attractrisk investment and credit capital to promote industrial development. Moreover, companies can realize their growth through high-end technology transfer, shortening new product commercialization process and timely withdrawal from fund circulation[6].
2.3 To establish professional managerial and coordinating organizations
In developed countries, managerial organizations for emerging marine industries are very common. These organizations aim to coordinate the relationship between the government and companies, increase people’s awareness of emerging marine industries such as marine pharmaceutical industry and of the value and economic impact of these industries, coordinate internal contradictions, strengthen the relationship between marine development companies, R&D departments and other resources, create a better environment for mutual cooperation. These organizations are critical to the development of marine pharmaceutical industry and marine economy as a whole. In China, emerging marine industries, marine pharmaceutical industry in particular, is far from mature and advanced. As a result, we should learn from those advanced countries to establish relevant managerial and coordinating organizations so as to conduct in-depth research and promulgate industrial development planning, to further facilitate the integration of marine science and technology resources, to accelerate the industrialization of high and new marine technologies, the transformation efficiency of scientific and technological achievements and to promote the rapid growth of marine pharmaceutical industry and marine industries as a whole[7].
2.4 To lay more stress on the nurturing of marine high-tech talents
Future competition between countries is the competition of comprehensive national strength and the competition of high-tech talents. In A Strategy for American Innovation: Driving towards Sustainable Growth and Quality Jobs in 2009, the American president Barack Obama pointed out that America would strengthen the ability to learn and utilize new science and technologies of the next generation in order to maintain the country’s high creativity. In the same year, the Comprehensive Strategies to Strengthen Basic Scientific Ability was published in Japan in order to cultivate more talents to promote Japan’s international competitiveness. The Australian government has also published several programs such as To Inspire Creativity: Innovation Agenda of the 21st Century and To Construct Australia’s Research Power in an attempt to train and attract more talents. These developed countries have paid great attention to training talents and arousing their initiative as well. Favorable living and working environment and remuneration incentive is provided to these talents, who will then create greater economic value in future. As a typical technology intensive high-tech industry, marine pharmaceutical industry needs more professionals. Marine pharmaceutical companies should spare no efforts in absorbing highly qualified and educated talents in order to secure their competitiveness. As a matter of fact, the development of marine pharmaceutical industry in China is severely hampered by shortage of high-end talents. In order to find a suitable training and managerial mode of this industry, we should learn from developed countries in training qualified scientists and technicians and borrow their advanced managerial expertise. Moreover, we should absorb more high-end technical talents and establish better innovation and entrepreneurship platforms for these talents while stressing on the nurturing of managerial professionals of ourselves. Finally, we should provide better environment and incentive mechanism for these talents so that they can tap their full potential and make greater contribution to the development of marine pharmaceutical industry.
2.5 To enhance technological innovation capability
Marine pharmaceutical industry in China started much later than developed countries. As a result, our technological capability lags far behind that of other countries. The level of industrialization of marine pharmaceutical technology is very low and there are very few companies with competent and independent innovation capability. All the above facts have hampered the rapid development of marine pharmaceutical industry in China. As a knowledge and technology intensive industry, marine pharmaceutical industry is characterized with high technology, high investment, high risk, and high return. It is without doubt that technological innovation is the core impetus. At present, most technological innovations and patents of this industry in China come from developed countries and most of our products are imitations which constitute the major problem in the development of marine drugs. As a result, we should spare no efforts to enhance technological innovation capability especially that of key companies and key research institutes.
2.6 To strengthen international cooperation
Cooperation among different countries can eliminate the cask effect and achieve a win-win result. Bilateral and multilateral cooperation in technological research, personnel exchange and equipment utilization plays a very significant role in advancing high-tech industries such as marine pharmaceutical industry. For example, the US and Japan have partly realized resource sharing through joint utilization of equipments and experimental facilities. Against this backdrop, we should borrow advanced technological theories from developed countries and strengthen the cooperation with other countries based on our strength in this industry as to achieve the large scale and prosperity of marine pharmaceutical industry.
To sum up, we are in an unfavorable position in the development of marine pharmaceutical industry compared with developed countries and regions. Due to different national conditions and industrial development stages, different countries have taken their own ways. However, a country can achieve fast development in marine pharmaceutical industry through establishing professional managerial and coordinating organizations, promulgating national-level development policies and planning, nurturing high-tech marine talents, establishing effective mechanism for investment and financing, enhancing technological innovation capability and strengthening international cooperation. We should learn from developed countries and take measures based on our own situation so as to maintain a healthy, sustainable, rapid and efficient development of marine pharmaceutical industry.
[1] ZHANG Chao-yi, HU Miao-shen. Marine Drug Development in the Eyes of Entrepreneurs [J]. Shandong Medical Industry, 2000, 5: 38.
[2] CHEN Sheng-ping. On “Integration” and “Combination” in the Course of Scientific Research Management--on the Case of Marine Biotechnique Subject in 863 Plan [J]. Research and Development Management, 2001, 5: 72-75.
[3] CHEN Xi, CHEN Xiu-xia, CHEN Qiang, et al. The Research of Marine Bioactive Substances [J]. Fujian Agricultural Science and Technology, 2012, 2: 83-86.
[4] SHI Qing-wen, HUO Chang-hong, LI Li-geng, et al. Review on the Research of Marine Natural Products [J]. Chinese Medicinal Herb, 2009, 40 (11): 1687-1695.
[5] CHEN Lai-cheng. The General Situation of the Development of Marine Biological Technology Abroad [J]. Progress in Biotechnology, 1994, 14 (6): 11-20.
[6] ZHONG Wen-wen. Comparison and Reference of the Strategic Development of Emerging Marine Industries at Home and Abroad [J]. Journal of Ocean University of China (Social Science Edition), 2013, 3: 12-16.
[7] TAN Song-hua, WANG Jian. Innovative Choices of Talent Training Mode in the New Age [J]. Higher Education in China, 2012, 6 (4): 14.
Author’s information: ZHANG Li-jun. Major research area: Social pharmacy. Tel: 0580-2281666, E-mail: max2088@sina.com
猜你喜欢
杂志排行
亚洲社会药学杂志的其它文章
- Study on the Establishment of Efficiency Evaluation System of Drug Supervision and Sampling Test
- New Version of GDP in EU and Its Enlightenment
- FDA Practices: GMP Quality System Inspection and Evaluation
- Family Economic Risk Caused by Five Major Chronic Diseases among Urban Residents: A Comparative Study Based on the Data from 9 Cities in China
- Three Therapeutic Regimens in Treatment of Community-acquired Pneumonia: A Cost-effectiveness Analysis
- Pharmacovigilance System for Pharmaceutical Enterprises in EU: Regulation and Its Enlightenment
