Protective Effects of Oyster Extract Against Hepatic Tissue Injury in Alcoholic Liver Diseases
2014-04-20ZHANGCuipingLIXiaoyuJINGXueZHANGBoZHANGQiNIUQinghuiWANGJianjunandTIANZibin
ZHANG Cuiping, LI Xiaoyu, JING Xue, ZHANG Bo, ZHANG Qi, NIU Qinghui, WANG Jianjun, and TIAN Zibin
Department of Digestive Diseases, the Affiliated Hospital of Medical College, Qingdao University, Qingdao 266003, P. R. China
Protective Effects of Oyster Extract Against Hepatic Tissue Injury in Alcoholic Liver Diseases
ZHANG Cuiping*, LI Xiaoyu, JING Xue, ZHANG Bo, ZHANG Qi, NIU Qinghui, WANG Jianjun, and TIAN Zibin
Department of Digestive Diseases, the Affiliated Hospital of Medical College, Qingdao University, Qingdao 266003, P. R. China
Oyster extract is an effective bioactivity component. It has abundant nutritional value and antiviral, antitumor and immune defense functions. The role of oyster extract in treating liver injury has been paid more attention. We use Wistar rats to make alcoholic liver disease model through injecting alcohol into rats’ stomachs. These rats were randomly divided into five groups: model group, control group, low-dose, middle-dose and high-dose experimental group with a dose of 0.12 g kg−1, 0.40 g kg−1, and 1.20 g kg−1alcoholic. After nine weeks, serum biomarkers (ALT, AST, TG and TCHO), malondialdehyde (MDA), glutathione (GSH), C3a, C5a, IL-17, TNF-ɑ, anti-MAA-HAS IgG, CD3+, CD4+, CD8+ , NK cell activation and zinc content were assessed. The results showed that the serum biomarkers(ALT, AST, TG and TCHO), MDA content, anti-MAA-HSA IgG, serum C3a, C5a IL-17 and TNF-ɑ levels of oyster extract treatment groups were significantly decreased in comparison with model group. On the contrary, GSH showed adverse trend. Serum CD3+, CD4+ and NK cell activation were significantly increased in middle-dose group and high-dose group compared with model group, and there was decrease of CD8+ activity in high-dose group. Plasma Zn level was decreased in model group compared with that in control group. Meanwhile, Mean plasma Zn levels increased dramatically following the dose increase of a given oyster extract.
oyster extract; liver diseases; alcohol; lipid peroxidation
1 Introduction
Alcohol, a natural product, has been available for human consumption for thousands of years. A small amount of alcohol consumption may be beneficial for preventing and reducing the mortality rate of coronary heart disease and ischemic stroke, but it should also be noted that alcohol is toxic to almost every organ of the body. Long term alcohol abuse can cause alcoholic liver diseases (ALD). It is a main reason of serious liver diseases in western countries (Diehl et al., 2002; Mann et al., 2003). In China, alcohol abuse has been considered the second leading cause of hepatic lesion inferior virus hepatitis (Zhuang, 2003). In addition to directly causing liver disease, alcohol consumption is a common comorbid condition with other chronic liver diseases and may exacerbate liver injury, particularly in nonalcoholic fatty liver disease, chronic viral hepatitis, hereditary hemochromatosis, and autoimmune liver diseases. This synergism can result in increased hepatic inflammation and accelerated rates of fibrosis, with more rapid and earlier development of cirrhosis, and also increase the risk for liver cancer and death from liver disease (Lee et al., 2012). The most common alcoholic liver disease is alcoholic fatty liver, which is caused by accumulation of triglyceride and other factors. Alcoholic fatty liver can affect liver function, and even develop into alcoholic cirrhosis. Progression of alcohol-induced liver disease (ALD) is a multifactorial process that involves a number of genetic, nutritional and environmental factors. The possible mechanisms in the pathogenesis of ALD include oxidative stress (Cohen et al., 2011), metabolism of cholesterol, triglycerides, phospholipids (Fernando et al., 2010), and inf l ammatory mediators or other toxic agents (Purohit et al., 2004).
Oyster is a kind of Lamellibranchia mollusk, commonly known as ‘Hailizi’ or ‘Hao’ in China. It is rich in glycogen, taurine, vitamin B, microelement zinc, essential amino acid and is on the medicine food homology list announced by the National Health Ministry. Oyster extract is an effective bioactivity component, which is extracted from high-quality oyster, scallop, surf clam and so on from Jiao Zhou Bay with zymohydrolysis. It retains high purity and has no side effects. It has great nutritional value and antiviral, antitumor, immune defense functions. This study aimed to investigate the probable protective mechanism of the oyster extract on the alcoholic liverdiseases, which may be useful for exploiting the ocean resources further.
2 Materials and Methods
2.1 Chemicals
Oyster extract (taurine ganbo, provided by Qingdao Dongyi Science and Technology Development Company, batch number QS370222020116) was a dose of 0.4 g/ granule. HRP-labeled Rabbit anti-Rat IgG (H+L) was obtained from Beijing Biosynthesis Biotechnology Co., LET. Other reagents were purchased from Qingdao Wolsen Biotechnology Co., LET.
2.2 Animals and Protocol
Healthy male Wistar rats, weighing (232.8±19.9) g, were purchased from the Experimental Animal Center of Tongji Medical of Huazhong University of Science &Technology. They were living under a controlled condition (12 h of light/dark cycles and 23±2℃), and were given commercial standard rat chow and water. The rats were randomly divided into 5 groups, and each group had 15 rats. Group 1: the model group, given 50% dehydrated alcohol (analytical pure) into the stomach, at a dose of 8 mL kg−1per day for 2 weeks, 10 mL kg−1per day for 2 weeks and 15 mL kg−1per day for 4 weeks. Group 2: control group, healthy control rats received distilled water for 8 weeks. Group 3, 4, 5: treatment groups, given oyster extract at a dose of 0.12, 0.40, 1.20 g kg−1per day for 8 weeks separately. And then, all groups were fed normally for another week. Rats were anesthetized with ethylether. Blood was drawn directly from the left atrium of the heart, and hepatic tissues were collected for biochemical analysis and histological tests. All animals were handled in accordance with all applicable government regulations concerning laboratory animals.
2.3 Biochemical Factors Evaluation
Serum biomarkers of liver function including alanine aminotransferase (ALT), aspatate aminotransferase (AST), triglyceride (TG), and total cholesterol (TCHO) were measured by Hitachi 7600-210 automatic biochemistry analyzer. The serum levels of C3a, C5a, IL-17 and TNF-α were measured by Enzyme-Linked Immunosorbent Assay (ELISA) according to the directions.
2.4 Measurement of Lipid Peroxidation
The rats’ liver tissues were removed and rapidly homogenized in ice-cold saline. Tissue homogenates were centrifuged at 3000 r min−1for 10 min at 4 to remove℃crude fractions. Then the supernatants were collected. Lipid peroxidation, measured as glutathione (GSH) and malondialdehyde (MDA), were determined by TBA colorimetry and DTNB colorimetry, according to the procedure provided, respectively.
2.5 Measurement of Plasma Zinc Level
Separation of plasma and serum was achieved by centrifugation. Plasma zinc levels were measured after mixing plasma with an ionization buffer (Na+: 25 mEqL−1+ K+: 50 mEqL−1, plasma: buffer=1:3), by using atomic absorption spectrophotometry (Perkin-Elmer Model 560 –A.A.S.). Standards for zinc were Zn spectrosols by B.D.H.
2.6 Circulating IgG Against Human Serum Albumin Modif i ed by Malondialdhyde-Acetaldehyde (MAA-HSA)
Circulating IgG against MAA-HSA was measured by ELISA according to Vidali et al. (Vidali et al., 2008; Xu et al., 1998; Rigamonti et al., 2003; Rolla et al., 2000). MAA-protein adducts were generated by reacting 1 mg mL−1of human serum albumin (HSA) with 1 mmol L−1of acetaldehyde and 1 mmol L−1MDA, obtained by the acid hydrolysis of MDA-bis-dimethylacetal as previously described. Polystyrene microwell plates for ELISA were coated for 16 h at 4℃ with 0.05 mg mL−1of either modif i ed or native HSA solubilized in 0.1 mol L−1bicarbonate buffer, pH 9.6. After incubation, the solutions were removed and replaced by 0.3 mL of coating buffer containing 3% bovine serum albumin in PBS, pH 7.4. The plates were further incubated for 1 h at 37℃ to block non-specif i c binding sites. Then the coated wells were washed 3 times with PBS containing 0.25% TritonX-100. The sera of the patients were diluted 1:50 with the coating buffer and added in duplicate as aliquots of 0.20 mL to the appropriate wells and incubated 1 hour at 37℃. After washing the plates 3 times with PBS 0.25% TritonX-100, peroxidase-linked rabbit antihuman IgG (dilution 1:5000) were added and incubated for 1 h at 37℃. The antibody binding was revealed by the addition of 0.10 mL of TMB coloration solution. After 15 min, the reaction was stopped by adding 50 μL 1 mmol L−1H2SO4, and absorbance was measured at 450 nm using a BIO- RAD microplate reader (Model 550).
2.7 Assay Methods of Peripheral Blood T Cell Subsets and NK Cell Activity
Peripheral blood T-cell (CD3+, CD4+, CD8+) and NK cell (CD16+CD56+) were assayed by Flow cytometry instrument. Mononuclear cells were extracted with lymphocyte separation fluid from 1mL peripheral venous blood with heparin anticoagulation. After adjusted to a cell density of 1×106mL−1, these cells were labeled with appropriate fluorescein labeled monoclonal antibodies according to the manufacturer’s instructions, and subjected for FCM analysis. We used antibodies listed below: APC-anti-hCD4, FITC-anti-hCD8, PE-anti-hCD3, FITC -anti-hCD4, FITC-anti-hCD3, PE-anti-hCD56 and PE-anti-hCD25 (eBioscience). All the experiment is then repeated three times in order to guarantee the system stability.
2.8 Histological Analysis
Liver tissues were fixed in 10% formalin solution (pH 7.0), embedded in paraffin. Sections were cut into 5 μmand stained with haematoxylin and eosin. The histopathological lipid accumulation changes of the liver were observed by light microscopy. Every visual field was observed carefully. Pathological changes were quantified using a visual grading system. Each section was quantized by ‘-(0 point), +(1 point), ++(2 point), +++(3 point), ++++(4 point)’ (Zhang et al., 2006).
2.9 Analytical Methods
All measurement data were expressed as mean ± SD and analyzed with the SPSS 11.5 statistics software. The measurement data were analyzed by variance (One-Way ANOVA), and the pathological changes of five groups were analyzed by Mann-Whitney Test, with P<0.05 being considered statistically significant.
3 Results and Discussion
3.1 The Protective Effect of Oyster Extract on the Serum Biochemical Data (ALT, AST, TG and TCHO)
In our experiment, we found that Oyster extract could help reduce the serum contents of ALT, AST, TG and TCHO, and showed protective effect on liver function. ALT and AST were measured to determine the concentration of intracellular hepatic enzymes that had leaked into the circulation and served as a marker of hepatocyte injury. The effects of oyster extract on serum biochemical of liver are presented in Fig.1. Alcohol was found to induce a more significant increase of ALT, AST, TG and TCHO in model group than in control group. The plasma level of ALT, AST, TG and TCHO were all more significantly decreased in treatment groups with oyster extract than in model group and showed a downward trend from low-dose to high-dose oyster extract group.
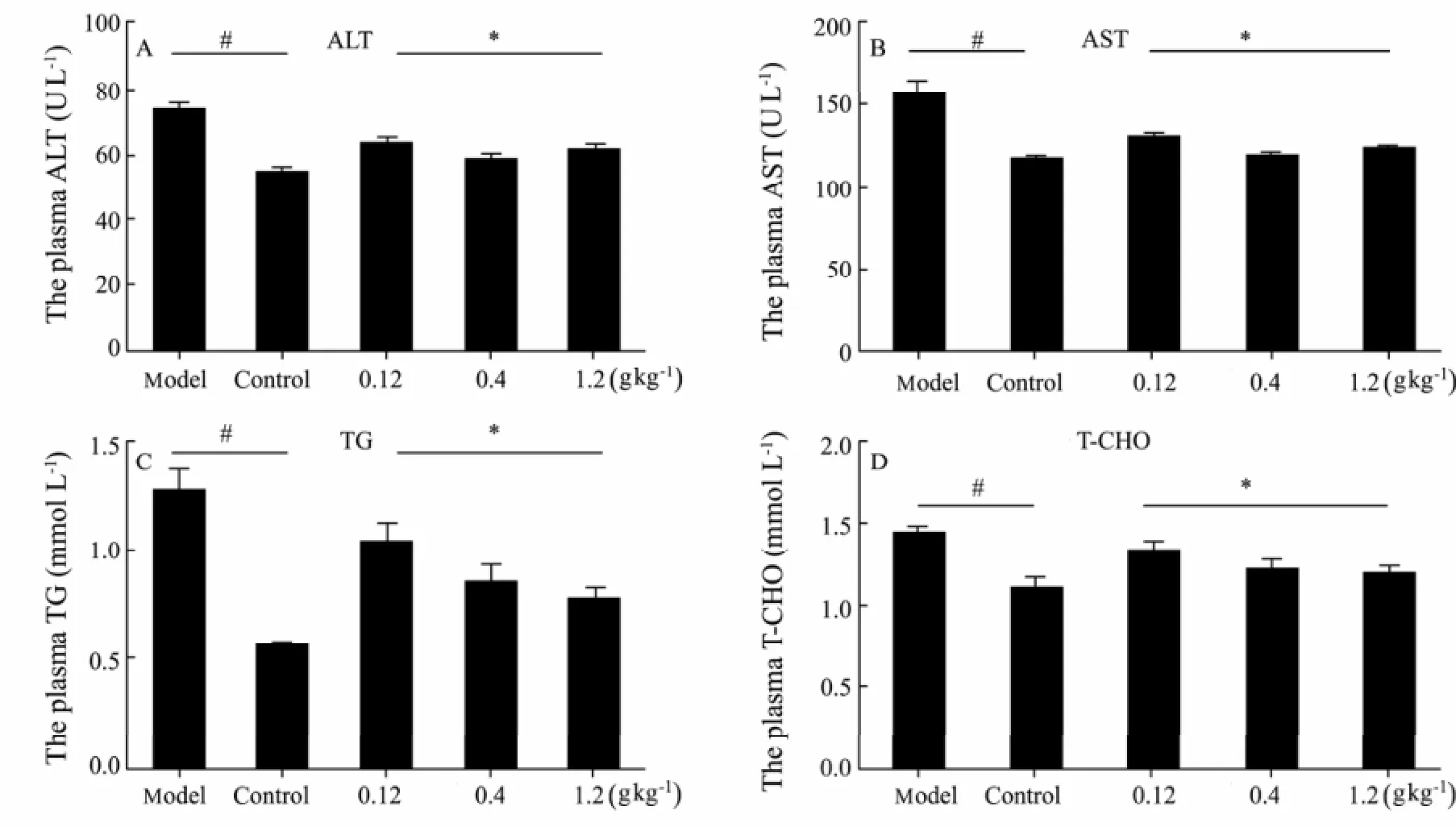
Fig.1 Effect of Oyster extract on the serum Biochemical analysis (ALT, AST, TG and TCHO) in male Wistar rats (mean±SD, n=15). ∗: compared with the model group, P<0.05; #: compared with the model group, P<0.01.
3.2 The Effect of Oyster Extract on Plasma C3a, C5a, IL-17, and TNF-α
Both cellular and circulating components of the innate immune system are activated by exposure to ethanol. For example, ethanol exposure enhances toll-like receptor-4 (TLR-4)-dependent cytokine expression by Kupffer cells, likely due to dysregulation of redox signaling. Similarly, complement activation in response to ethanol leads to increased production of the anaphylatoxins, C3a and C5a, and activation C3a receptor and C5a receptor. Complement activation thus contributes to increased inflammatory cytokine production and can influence redox signaling. Immune dysregulations plays an important role in alcoholic liver diseases (Lemmers et al., 2009; Thomas et al., 2006; Jeong et al., 2008; Duryee et al., 2008). It has been identified that the complement system has been implicated in liver regeneration after toxic injury (Bykov et al., 2006; Pritchard et al., 2007; Strey et al., 2003). In mice lacking C3 (C3-/- mice) alcohol-induced liver steatosis is absent or strongly reduced after chronic or acute alcohol exposure. This suggests that the complement system and its component C3 contribute to the development of alcohol-induced fatty liver and its consequences (Bykov et al., 2006). It has been identified that C3 contributes primarily to the accumulation of triglyceride in the liver, whereas C5 is involved in inf l ammation and injury to hepatocytes (Pritchard et al., 2007). Some data indicate that C3a and C5a, two potent inflammatory mediators of the innate immune response, contribute essentially to the early priming stages of hepatocyte regeneration (Strey et al., 2003; Bykov et al., 2007; Markiewski et al., 2004). In our experiment, plasma C3a and C5a were increased more significantly in model group than in control group. In treatment groups, plasma C3a and C5awere more decreased than in model group and showed a down- ward trend from low-dose to high-dose oyster extract group. That is to say, Oyster extract can protect liver from alcohol injury so it less needs to reduce regeneration.
Tumor necrosis factor-alpha (TNF-α) is a proinflammatory cytokine that is mostly produced by monocyte-macrophages under appropriate stimuli, particularly in response to bacterial endotoxins. Alcohol-induced endotoxemia enhances Kupffer cell TNF-α production, which mediates, among other disorders, alcoholic inflammatory liver disease (alcoholic hepatitis) (Zhou et al., 2003). Clinical and animal studies implicate that TNF-α is a major mediator in the pathogenesis of alcoholic liver disease (Vidali et al., 2008). Alcohol-induced reduction in mitogen-activated protein kinase (MAPK) phosphatase-1 MKP-1 expression in the liver leads to increased MAPK activity and increased TNF-a production (Ajakaiye et al., 2011). It has been reported that serum TNF-α concentrations are higher in patients with alcoholic liver disease than in alcoholics without liver disease, moderate drinkers, or abstainers (Latvala et al., 2005). The distribution of serum TNF-α levels in relation to alcohol consumption is distinct from trends observed with serum interleukin (IL)-8, a proinflammatory cytokine also involved in the pathogenesis of alcoholic hepatitis (Jaeschke et al., 2002). Interleukin-17 (IL-17A) is a cytokine secreted exclusively by activated T-cells. The evolving IL-17 family of ligands and receptors may play an important role in the homeostasis of tissues in health and disease beyond the immune system. High expression of IL-17 and IL-17RE is associated with poor prognosis of hepatocellular carcinoma (Liao et al., 2013). Increased Th17 cells and IL-17 contribute to immune activation and disease aggravation in patients with chronic hepatitis B virus infection (Yang et al., 2013). IL-17 plays an important role in inducing liver inflammation via stimulating multiple types of liver nonparenchymal cells to produce proinflammatory cytokines, such as TNF-α, and is significantly elevated in alcoholic liver disease (Lafdil et al., 2010). In alcoholic hepatitis, liver infiltration with IL-17-secreting cell infiltrates is a key feature that might contribute to liver neutrophil recruitment (Lemmers et al., 2009).
Our experimental datas indicated that oyster extract could increase the immune function. As Fig.2 reveals, plasma C3a and C5a were increased significantly in model group than in control group. In treatment groups, plasma C3a and C5a were more decreased than in model group and showed a downward trend from low-dose to high-dose oyster extract group. IL-17 and TNF-α alterations in the normal control group and low-dose, middle-dose and high-dose oyster extract group were significantly lower than in the model group. Oyster extract may attenuate liver inflammation via IL-17 and TNF-α and decrease alcohol toxicity.
3.3 The Different Changes of MDA and GSH of Five Groups in Liver Tissue
Several studies have identif i ed that accumulation of lipids in the liver is a critical early stage in the development of ALD (Teli et al., 1995), and it makes the liver susceptible to inf l ammatory mediators or other toxic agents (Purohit et al., 2004). It is generally accepted that increasing lipid peroxidation is a major pathogenic mechanism in ethanol-induced hepatotoxicity. However, alcoholic steatosis is a reversible stage, and reduction of steatosis will likely slow down the progression of alco-holic liver disease (Lakshman et al., 2004). Our previous studies have shown that taurine could decrease alcohol-induced hepatic steatosis and lipid peroxidation (Kerial et al., 1999; Wu et al., 2009). Oyster extract contains abundant taurine, which can explain why oyster extract can reduced alcohol-induced hepatic steatosis and lipid peroxidation.
The GSH plays a key role in protection against hydrogen peroxide and is a major hepatoprotective agent against liver injury, including lipid peroxidation. The importance of GSH homeostasis in preventing alcoholmediated oxidative injury has been shown in the observation that the stimulation of GSH re-synthesis in rats by supplementation with either of the GSH precursors L-2-oxothiazolidine-4-carboxylic acid or N-acetylcysteine prevents liver injury in the enteral alcohol model (Iimuro et al., 2000; Ronis et al., 2005). The degree of liver injury is associated with reduction of hepatic GSH levels. SAMe is a precursor for the synthesis of GSH, which plays a key role in protection against oxidative stress and is a major hepatoprotective agent against liver injury, including lipid peroxidation. GSH is also lacking in alcoholic subjects.
Oyster extract is helpful against the alcoholic liver injury. The GSH plays an important role in protect against alcoholic liver disease. The MDA is a crucial production of lipid peroxidation. MDA and GSH contents show the effects of oyster extract on antioxidative activity in liver tissue. In our experiment, we found MDA contents of the liver tissue in model group were higher than that in control group (bP<0.01), which means alcohol could cause lipid peroxidation in liver tissue. MDA contents of the liver tissue in middle-dose and high-dose treatment group were significantly decreased compared to that in model group (bP<0.01), which means oyster extract could abate the degree of lipid peroxidation. On the contrary, the GSH contents of the liver were increased in middle-dose and high-dose treatment group compared with the model group as seen in Table 1.

Table 1 The MDA and GSH contents of the liver tissues (mean±SD, n = 15) (aP < 0.05,bP < 0.01)
3.4 The Plasma Zinc Level of all Groups
Zinc is involved in the function of over 200 enzymes and 500 transcription factors. Zinc def i ciency is one of the most consistently observed nutritional/biochemical manifestations in alcoholic liver disease. Clinical studies demonstrated that zinc concentrations in both serum and liver were signif i cantly reduced in patients with alcoholic liver disease (Rodrıguez-Moreno et al., 1997). Zinc deprivation signif i cantly suppressed the DNA-binding activities of HNF-4α and PPAR-α, and led to lipid accumulation in HepG2 cells, which was partially recovered by zinc supplementation (Kang et al., 2009). Some animal studies showed that dietary zinc supplementation attenuates alcohol-induced liver injury (Zhou et al., 2005). In our study, a decrease in mean plasma Zn level was observed in alcohol-induced liver injury group (model group) in comparison with the healthy control group. That is to say, alcohol-induced liver injury group (model group) presented Zinc deficiency.
Zinc supplementation enhanced enzymes involved in antioxidant defense and alcohol metabolism. Superoxide dismutase activity was signif i cantly reduced by alcohol exposure, which was normalized by zinc supplementation (Kang et al., 2008). Previous study have provided that hepatocyte apoptosis occurred in the liver after longterm ethanol exposure and was attenuated by zinc supplementation, which might through inhibition of oxidative stress and death-receptor signaling (Zhou et al., 2008; Agnieszka et al., 2005). These studies showed that zinc supplementation partially attenuated alcohol-induced lipid accumulation and liver injury. Oyster extract contains taurine and zinc mainly that can reduce the lipid accumulation and inhibit oxidative stress and anti lipid peroxidation.
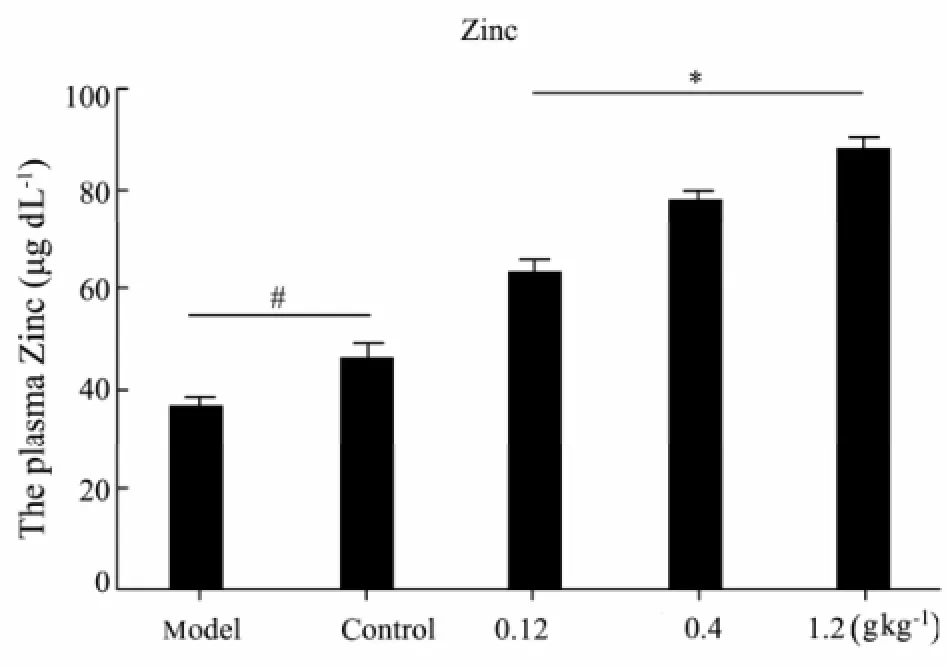
Fig.3 Plasma Zinc level of all groups (mean± SD, n = 15).∗: compared with the model group, P < 0.01;#: compared with the model group, P < 0.05.
We found mean plasma Zn levels in experimental groups increased dramatically with the dose increase of given oyster extract. Zinc participates as enzymatic cofactors in the maintenance and regulation of many physiological procedures. Some animal studies show that dietary zinc supplementation attenuates alcohol-induced liver injury. Zinc supplementation enhances enzymes involved in antioxidant defense and alcohol metabolism. As Fig.3 reveals, a decrease in mean plasma Zn level was observed in model group as compared with the control group (P<0.01). Meanwhile, Mean plasma Zn levels increased dramatically following the dose increase of a given oyster extract (P<0.05, Fig.3). Moreover, the increase in zinc levels was followed by elevation of the GSH and reduction of the MDA in the liver tissue, showing that oyster extract supplementation could partially attenuate alcohol-induced lipid accumulation and liver injury.
3.5 The Plasma Titers of Circulating IgG Against MAA-HSA
Reactive oxygen species (ROS) are produced by normal cellular metabolism with beneficial effects such as cytotoxicity against bacteria and other pathogens. However, these reactive species also may affect cells of the host organism, by leading to the oxidation of cellular macromolecules, such as lipids, protein or DNA, inhibiting normal function (Cohen et al., 2011). Oxidative stress from ethanol exposure results from increased generation of reactive oxygen species and decreased hepatocellular antioxidant activity, including changes in the thioredoxin/peroxiredoxin family of proteins. Experimental and clinical studies increasingly show that oxidative damage induced by ethanol contributes in many ways to the pathogenesis of alcohol hepatoxicity. Most of the alcohol is oxygenolysised by the liver. During the metabolic process, alcohol is oxygenized into acetaldehyde, and 10%–20% of the acetaldehyde is catalysis by hepatocyte microsomal ethanol oxidizing system, then magnanimous free radical is produced. The MDA is a very important production of lipid peroxidation, which can combine with acetaldehyde and protein to form mixed MDA-acetaldehyde-protein (MAA-protein) adducts (Worrall et al., 2001). MAA-protein adducts can cause immune responses against oxidative stress-derived antigens. It is found that elevated titers of IgG towards adducts originate from the reaction between liver proteins and lipid peroxidation products such as MDA and 4-hydroxyno- nenal (Mmottaran et al., 2002; Tuma, 2002; Tuma et al., 2003; Duryee et al., 2007).
In our study, we also found oyster extract was helpful against lipid peroxidation. In model group, a signif i cant increase of circulating IgG against MAA-HSA was detected compared to control group (P<0.01). As shown in Fig.4, in middle-dose and high-dose treatment groups with oyster extract for 8 weeks, circulating IgG showed a significant decrease trend compared to model group (P<0.01), which meant the degree of lipid peroxidation was reduced.
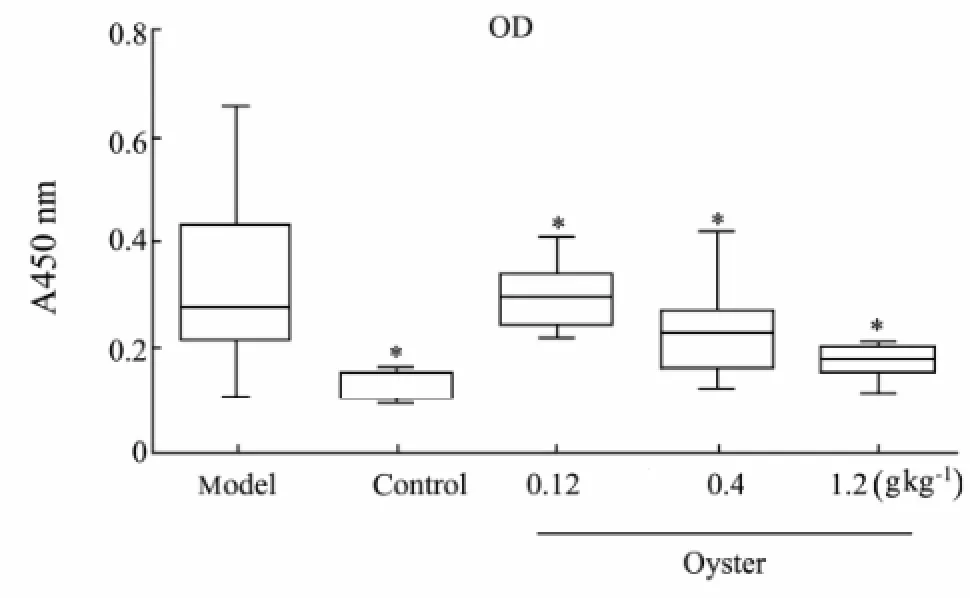
Fig.4 Plasma titers of Circulating IgG against MAA-HSA (mean±SD, n=15).
3.6 The Changes of T-cell sub-Group and NK Cell Activity of all Groups
Natural Killer (NK) cell is a component of the innate immune system with increased presence in the liver compared to other organs and has been reported to participate in the inflammatory processes during hepatic diseases. Accumulating evidence supports an antifibrotic role of NK cells mainly via an inhibitory effect on hepatic stellate cells by inducing apoptosis and via production of interferon-gamma. Therefore, downregulation of NK cells during most types of liver injury may facilitate liver fibrosis (Notas et al., 2009; Laso et al., 2010).
Oyster extract was also useful for cellular immunity. In model group, serum CD3+, CD4+ and NK cell activation were more significantly reduced than that in control group (aP<0.05). After treated with middle-dose and highdose oyster extracts, serum CD3+, CD4+ and NK cell activation increased compared with the model group (aP<0.05 orbP<0.01). In the meanwhile, there was a decrease tendency of CD8+ activity in high-dose treatment group compared to model group (bP<0.01). The results meant oyster extract could promote the activation of CD3+, CD4+ and NK cell and inhibited the activation of CD8+ cell as the contents of oyster extract increased.
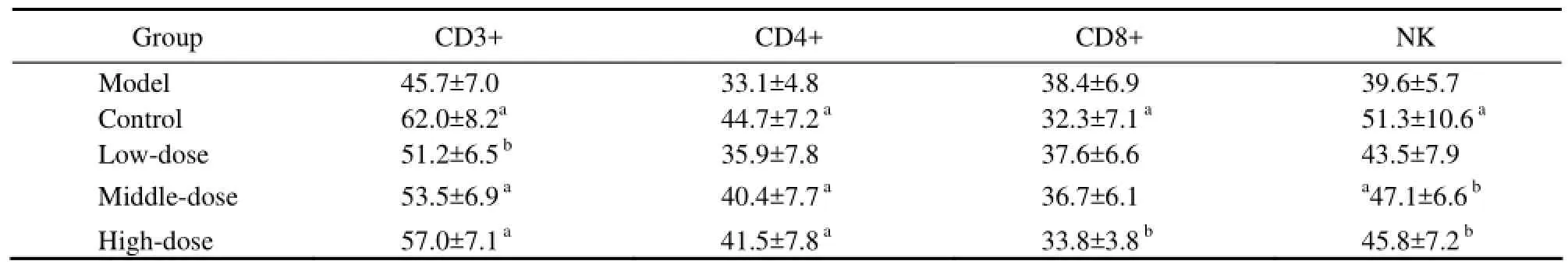
Table 2 Changes of T-cell sub-group and NK cell activity of all groups in the experiment (%) (aP<0.05,bP<0.01)
3.7 Histological Changes of All Groups
Looking through the microscope, we could see that alcohol caused a more serious liver lipid accumulation in model group than in control group, and the degree of liver lipid accumulation was decreased by oyster extract supplementation in middle-dose and high-dose treatment groups. The scores of liver histological analysis of different groups are showed in Table 3 and Fig.5.
4 Conclusions
Alcohol abuse is widely accepted as a risk factor for health damage and a social problem. Generally, alcohol is metabolized in the liver. When the alcohol concentration is in excess, the liver would accumulate more hepatic NADH. As a result, more fatty acids and triglycerides would be synthesized. It is known that ALD includes alcoholic fatty liver, alcoholic hepatitis, alcoholic fi brosisand alcoholic cirrhosis, which can occur alone or together. The progression of liver injury consequent to chronic alcohol abuse is a multifactorial event that involves a number of genetic and environmental factors, and both parenchymal and nonparenchymal cells resident in the liver. Alcohol consumption alters metabolism of cholesterol, triglycerides, and phospholipids that could contribute to the development of fatty liver, which also indicate that fatty liver precedes oxidative stress, activation of the innate immune system and inf l ammation (Fernando et al., 2010).
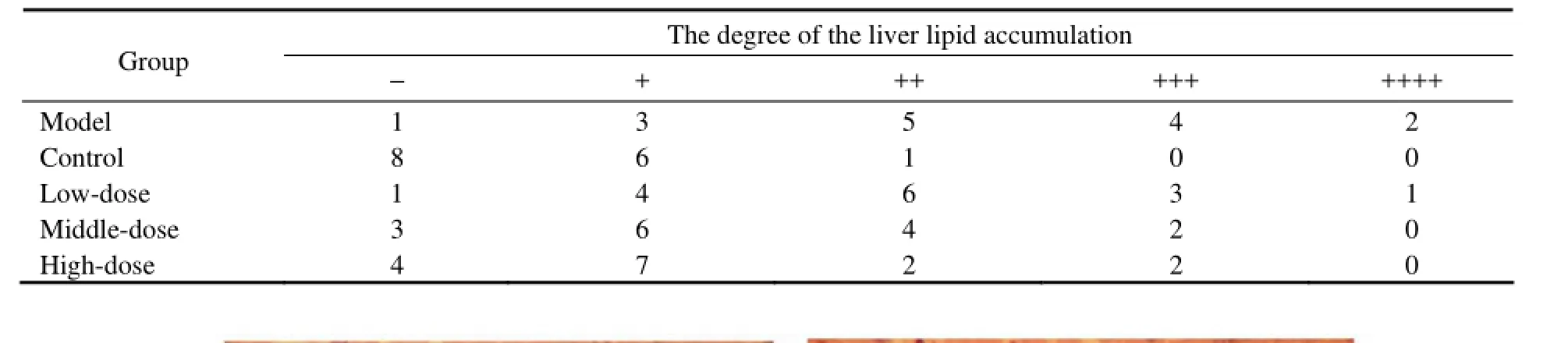
Table 3 Histological changes of all groups
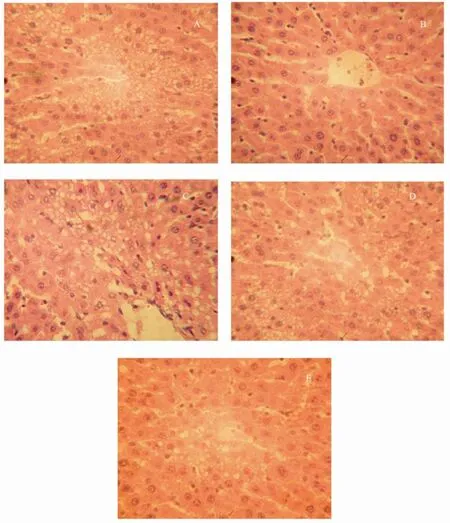
Fig.5 Effect of oyster extract on liver lipid accumulation in Wistar rats. These photomicrographs of hepatic cells show different degrees of steatosis in each group: model group (A), control group (B), low-dose(C), middle-dose(D) and high-dose treatment group (E). (H.E stain ×400).
In our study, we found that alcohol could induce liver injury, which was followed by a significant increase of ALT, AST, TG and TCHO in model group compared with control group. The plasma level of ALT, AST, TG and TCHO were all more significantly decreased in treatment groups with oyster extract than in model group and showed a downward trend from low-dose to high-dose oyster extract groups. Oyster extract can increase serum CD3+, CD4+ and NK cell activation significantly, which is likely because oyster extract can decrease alcohol-induced hepatic steatosis and lipid peroxidation, andreduce the MDA of the liver tissue and the titers of IgG against MDA-HSA. Furthermore, histological observations showed that taurine was capable of not only preventing but actually reversing the patho-morphological changes of ALD, such as changes in fat deposition and inf l ammatory cell inf i ltration.
Oyster extract can decline ethanol-induced oxidative stress, activation of the innate immune system interaction and inf l ammation, for example serum C3a, C5a, IL-17 and TNF-α levels. Meanwhile, mean plasma Zn levels increased dramatically with the dose increase of a given oyster extract, and reversing the patho-morphological changes of ALD was showed by histological observations. All of these results prove that the oyster extract can keep hepatic cells from the alcohol injury. It is confirmed that oyster extract provides an effective and safe treatment for alcoholic liver diseases.
Acknowledgements
This work was financially supported by Grants from Qingdao Technology Office, Qingdao Technology Developing Plan, 10-3-3-3-11-NSH and 03-3-hh-09. We would like to thank Mr. Jianjun Wang from Qingdao Dongyi Science and Technology Development Company for providing the oyster extract.
Agnieszka, S. C., Jadwiga, D., and Agnieszka, B. J., 2005. Apoptosis of blood mononuclear cells in alcoholic liver cirrhosis: The inf l uence of in vitro ethanol treatment and zinc supplementation. Toxicology, 212: 124-134.
Ajakaiye, M. A., Jacob, A., Wu, R., Zhou, M., Ji, Y., Dong, W., Wang, Z., Qiang, X., Chaung, W. W., Nicastro, J., Coppa, G. F., and Wang, P., 2011. Upregulation of kupffer cell a2A-adrenoceptors and downregulation of MKP-1 mediate hepatic injury in chronic alcohol exposure. Biochemical and Biophysical Research Communications, 409 (3): 406-11.
Bykov, I., Junnikkala, S., Pekna, M., Lindros, K. O., and Meri, S., 2007. Effect of chronic ethanol consumption on the expression of complement components and acute- phase proteins in liver. Clinical Immunology, 124: 213- 220.
Bykov, I., Junnikkala, S., Pekna, M., Lindros, K. O., and Meri, S., 2006. Complement C3 contributes to ethanol- induced liver steatosis in mice. Annals of Medicine, 38: 280-286.
Cohen, J. I., Chen, X., and Nagy, L. E., 2011. Redox signaling and the innate immune system in alcoholic liver disease. Antioxid Redox Signal, 15 (2): 523-34.
Diehl, A. M., 2002. Liver Disease in Alcohol Abuser, Clinical Perspective. Alcohol, 27: 721.
Duryee, M. J., Klassen, L. W., and Thiele, G. M., 2007. Immunological response in alcoholic liver disease. World Journal of Gastroenterology, 13 (37): 4938-4946.
Duryee, M. J., Klassen, L. W., Jones, B. L., Willis, M. S., and Tuma, D. J., 2008. Thiele GM. Increased immuno- genicity to P815 cells modified with malondialdehyde and acetaldehyde. International Immunopharmacology, 8: 1112-1118.
Fernando, H., Kondraganti, S., Bhopale, K. K., Volk, D. E., Neerathilingam, M., Kaphalia, B. S., Luxon, B. A., Boor, P. J., and Shakeel Ansari, G. A., 2010.1H and31P NMR lipidome of ethanol-induced fatty liver. Alcoholism: Clinical and Experimental Research, 34 (11): 1937-1947.
Iimuro, Y., Bradford, B. U., Yamashina, S., Rusyn, I., Nakagami, M., and Enomoto, N., 2000. The glutathione precursor L-2-oxothiazolidine-4-carboxylic acid protects against liver injury due to chronic enteral ethanol exposure in the rat. Hepatology, 31: 391-398.
Jaeschke, H., 2002. Neutrophil-mediated tissue injury in alcoholic hepatitis. Alcohol, 27 (1): 23-27.
Jeong, W. L., and Gao, B., 2008. Innate immunity and alcoholic liver fibrosis. Joural of Gastroenterology and Hepatology, 23: 112-118.
Kang, X., Song, Z., McClain, C. J., Kang, Y. J., and Zhou, Z., 2008. Zinc supplementation enhances hepatic regeneration by preserving hepatocyte nuclear factor-α in mice subjected to a long-term alcohol administration. American Journal of Pathology, 172: 916-925.
Kang, X., Zhong, W., Liu, J., Song, Z., McClain, C. J., and Kang, Y. J., 2009. Zinc supplementation reverses alcoholinduced steatosis in mice through reactivating hepatocyte nuclear factor-4α and peroxisome proliferator-activated receptor-α. Hepatology, 50 (8): 1-10.
Kerial, M. D. J., Waterf i eld, C. J., Kenyon, S. H., Asker, D. S., and Timbrell, J. A., 1999. Reversal of ethanol-induced hepatic steatosis and lipid peroxidation by taurine: A study in rats. Alcohol and Alcoholism, 34: 529-541.
Lafdil, F., Miller, A. M., Ki, S. H., and Gao, B., 2010. Th17 cells and their associated cytokines in liver diseases. Cellular and Molecular Immunology, 7 (4): 250-254.
Lakshman, M. R., 2004. Some novel insights into the pathogenesis of alcoholic steatosis. Alcohol, 34: 45-48.
Laso, F. J., Almeida, J., Torres, E., Vaquero, J., Marcos, M., and Orfao, A., 2010. Chronic alcohol consumption is associated with an increased cytotoxic Prof i le of circulating lymphocytes that may be related with the development of liver injury. Alcoholism: Clinical and Experimental Research, 34 (5): 876-885.
Latvala, J., Hietala, J., Koivisto, H., Jarvi, K., Anttila, P., and Niemela, O., 2005. Immune responses to ethanol metabolites and cytokine profiles differentiate alcoholics with or without liver disease. American Journal of Gastroenterology, 100: 1303-1310.
Lee, M., and Kowdley, K. V., 2012. Alcohol’s effect on other chronic liver diseases. Clinical Liver Disease, 16 (4): 827-837.
Lemmers, A., Moreno, C., Gustot, T., Marechal, R., Degre, D., Demetter, P., de Nadai, P., Geerts, A., Quertinmont, E., Vercruysse, V., Le Moine, O., and Deviere, J., 2009. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology, 49 (2): 646-657.
Lemmers, A., Moreno, C., Gustot, T., Maréchal, R., Degré, D., Demetter, P., de Nadai, P., Geerts, A., Quertinmont, E., Vercruysse, V., Le Moine, O., and Devière, J., 2009. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology, 49 (2): 646-57.
Liao, R., Sun, J., Wu, H., Yi, Y., Wang, J. X., He, H. W., Cai, X. Y., Zhou, J., Cheng, Y. F., Fan, J., and Qiu, S. J., 2013. High expression of IL-17 and IL-17RE associate with poor prognosis of hepatocellular carcinoma. Journal of Experimental and Clinical Cancer Research, 11: 32-33.
Mann, R. E., Smart, R. G., and Govoni, R., 2003. The epidemiology of alcoholic liver disease. Alcohol Research and Health, 27 (3): 209-219.
Markiewski, M. M., Mastellos, D., Tudoran, R., DeAngelis, R.A., Strey, C. W., Franchini, S., Wetsel, R. A., Erdei, A., and Lambris, J. D. L., 2004. C3a and C3b activation products of the third component of complement (C3) are critical for normal liver recovery after toxic injury. Journal of Immunology, 173: 747-754.
Mmottaran, E., Stewart, S. F., Rolla, R., Vay, D., Cipriani, V., and Moretti, M., 2002. Lipid peroxidation contributes to immune reactions associated with alcoholic liver disease. Free Radical Biology and Medicine, 32 (1): 38- 45.
Notas, G., Kisseleva, T., and Brenner, D., 2009. NK and NKT cells in liver injury and fibrosis. Clinical Immuno- logy, 130: 16-26.
Pritchard, M. T., McMullen, M. R., Stavitsky, A. B., Cohen, J. I., Lin, F., and Medof, M. E. N., 2007. Different contributions of C3, C5, and decay-accelerating factor to ethanol-induced fatty liver in mice. Gastroentrology, 132: 1117-1126.
Purohit, V., Russo, D., and Coates, P. M., 2004. Role of fatty liver, dietary fatty acid supplements, and obesity in the progression of alcoholic liver disease: Introduction and summary of the symposium. Alcohol, 34: 3-8.
Rigamonti, C., Mottaran, E., Reale, E., Rolla, R., Cipriani, V., and Capelli, F., 2003. Moderate alcohol consumption increases oxidative stress in patients with chronic hepatitis C. Hepatology, 38 (1): 42-49.
Rodrıguez-Moreno, F., Gonzalez-Reimers, E., Santolaria-Fernandez, F., Galindo-Martın, L., Hernandez-Torres, O., Batista-Lopez, N., and Molina-perez, M., 1997. Zinc, Copper, Manganese, and Iron in chronic alcoholic liver disease. Alcohol, 14: 39-44. Rolla, R., Vay, D., Mottaran, E., Parodi, M., Traverso, N., and Arico, S., 2000. Detection of circulating antibodies against malondialdehyde-acetaldehyde adducts in patients with alcohol-induced liver disease. Hepatology, 314 (20): 878-884.
Ronis, M. J. J., Butura, A., Sampey, B. P., Prior, R. L., Korourian, S., and Albano, E., 2005. Effects of N-acetyl cysteine on ethanol-induced hepatotoxicity in rats fed via total enteral nutrition. Free Radical Biology and Medicine, 6: 616-630.
Strey, C. W., Markiewski, M., Mastellos, D., Tudoran, R., Spruce, L. A., Greenbaum, L. E., and Lambris, J. D., 2003. The proinf l ammatory mediators C3a and C5a are essential for liver regeneration. The Journal of Experimental Medicine, 198: 913-923.
Teli, M. R., Day, C. P., Burt, A. D., Bennett, M. K., and James, O. F., 1995. Determinants of progression to cirrhosis or fi brosis in pure alcoholic fatty liver. Lancet, 346: 987-999.
Thomas, J. W., Robert, T. C., and Elizabeth, J. K., 2006. Alcohol and inf l ammation and immune responses: Summary of the 2005 Alcohol and Immunology Research Interest Group (AIRIG) meeting. Alcohol, 38: 121-125.
Tuma, D.J., 2002. Role of Malondialdehyde-acetaldhyde adducts in liver injury. Free Radical Biology and Medicine, 32 (4): 303-308.
Tuma, D. J., and Casey, C. A., 2003. Dangerous byproducts of alcohol breakdown focus on adducts. Alcohol Research and Health, 27 (4): 285.
Vidali, M., Hietala, J., Occhino, G., Ivaldi, A., Sutti, S., Niemelä, O., and Albano, E., 2008. Immune responses against oxidative stress-derived antigens are associated with increased circulating tumor necrosis factor-alpha in heavy drinkers. Free Radical Biology and Medicine, 45 (3): 306-311.
Vidali, M., Hietala, J., Occhino, G., Ivaldi, A., Sutti, S., and Niemelä, O., 2008. Immune responses against oxidative stress-derived antigens are associated with increased circulating tumor necrosis factor-α in heavy drinkers. Free Radical Biology and Medicine, 45: 306-3119.
Worrall, S., and Thiele, G. M., 2001. Protein modif i cation in ethanol toxicity. Adverse Drug Reactions and Toxicolo- gical Reviews, 20: 133-159.
Wu, G. F., Yang, J. C., Sun, C. G., Luan, X. H., Shi, J., and Hu, J. M., 2009. Effect of taurine on alcoholic liver disease in rats. Amino Acids, 36: 457-464.
Xu, D. S., Thiele, G. M., Beckenhauer, J. L., Klassen, L. W., Sorrell, M. F., and Tuma, D. J., 1998. Detection of circulating antibodies to malondialdehyde-acetaldehyde adducts in ethanol-fed rats. Gastroenterrology, 115: 682-692.
Yang, B., Wang, Y., Zhao, C., Yan, W., Che, H., Shen, C., and Zhao, M., 2013. Increased Th17 cells and IL-17 contribute to immune activation and disease aggravation in patients with chronic hepatitis B virus infection. Immunology Letters, 149 (1-2): 41-49.
Zhang, C. P., Li, Y. L., Zhang, M. S., Tian, Z. B., and Kong, X. J., 2006. Protective effect and the anti-lipidperoxidation of oyster extract on hepatic injury induced by alcohol in mice. Chinese Journal of Clinical Rehabilitation, 10 (43): 82.
Zhou, Z., Wang, L., Song, Z., Lambert, J. C., McClain, C. J., and Kang, Y. J., 2003. A critical involvement of oxidative stress in acute alcohol-induced hepatic TNF-α production. American Journal of Pathology, 163: 1137-1146.
Zhou, Z., Wang, L., Song, Z., Saari, J. T., McClain, C. J., and Kang, Y. J., 2005. Zinc supplementation prevents alcoholic liver injury in mice through attenuation of oxidative stress. American Journal of Pathology, 166: 1681-1690.
Zhou, Z. X., Liu, J., and Song, Z.Y., 2008. Zinc supplementation inhibits apoptosis in mice subjected to a long-term ethanol exposure. Experimental Biology and Medicine, 5: 540-548.
Zhuang, H., 2003. Epidemiology of alcoholic liver disease. Chinese Journal of Hepatology, 11 (11): 689.
(Edited by Ji Dechun)
(Received July 30, 2013; revised October 13, 2013; accepted October 20, 2013)
© Ocean University of China, Science Press and Springer-Verlag Berlin Heidelberg 2014
* Corresponding author. Tel: 0086-532-82911304
E-mail: jianyilu16@yahoo.com.cn
杂志排行
Journal of Ocean University of China的其它文章
- Wave Effect on the Ocean Circulations Through Mass Transport and Wave-Induced Pumping
- A Study of Transport and Impact Strength of Fukushima Nuclear Pollutants in the North Pacific Surface
- Annual and Interannual Variability of Scatterometer Ocean Surface Wind over the South China Sea
- A Method for Sea Surface Wind Field Retrieval from SAR Image Mode Data
- Sedimentary Characteristics of Relict Deposits on the Western South Yellow Sea
- Use of Different Mooring Models on Global Response Analysis of an Innovative Deep Draft Platform
