Class A scavenger receptor activation inhibits endoplasmic reticulum stress-induced autophagy in macrophage
2014-04-18HnpengHungXioyuLiYnZhungNnLiXudongZhuJinHuJingjingBenQingYngHuiBiQiChen
Hnpeng Hung,Xioyu Li,,✉,Yn Zhung,Nn Li,Xudong Zhu,Jin Hu,Jingjing Ben, Qing Yng,Hui Bi,Qi Chen,
aAtherosclerosis Research Centre,Laboratory of Molecular Intervention for Cardiovascular Diseases,Nanjing Medical University,Nanjing,Jiangsu 210029,China;
bState Key Laboratory of Reproductive Medicine,Nanjing Medical University,Nanjing,Jiangsu 210029,China.
Class A scavenger receptor activation inhibits endoplasmic reticulum stress-induced autophagy in macrophage
Hanpeng Huanga,Xiaoyu Lia,b,✉,Yan Zhuangb,Nan Lia,Xudong Zhub,Jin Hua,Jingjing Bena, Qing Yanga,Hui Baia,Qi Chena,b
aAtherosclerosis Research Centre,Laboratory of Molecular Intervention for Cardiovascular Diseases,Nanjing Medical University,Nanjing,Jiangsu 210029,China;
bState Key Laboratory of Reproductive Medicine,Nanjing Medical University,Nanjing,Jiangsu 210029,China.
Macrophage death in advanced atherosclerosis promotes plaque necrosis and destabilization.Involvement of autophagy in bulk degradation of cellular components has been recognized recently as an important mechanism for cell survival under endoplasmic reticulum(ER)stress.We previously found that the engagement of class A scavenger receptor(SR-A)triggered JNK-dependent apoptosis in ER-stressed macrophages.However,pro-apoptotic mechanisms mediated by SR-A are not fully understood.Therefore,we sought to see if SR-A mediated apoptosis was associated with autophagy in macrophages.Here,we showed that fucoidan inhibited microtubule-associated protein light chain 3-phospholipid conjugates(LC3-II)formation as well as the number of autophagosomes under ER stress.The inhibition of LC3-II formation was paralleled by the activation of the mTOR pathway,and the inhibition of mTOR allowed LC3-II induction in macrophages treated with thapsigargin plus fucoidan.Furthermore, apoptosis induced by fucoidan was prevented under ER stress by the mTOR inhibitor.We propose that fucoidan, a SR-A agonist,may contribute to macrophage apoptosis during ER stress by inhibiting autophagy.
SR-A,autophagy,ER stress,apoptosis,macrophage
INTRODUCTION
Autophagy,or‘‘self eating’’,serves as a dynamic recycling system that produces new building blocks and energy for cellular renovation and homeostasis[1]. The process of autophagy is characterized by the formation of double-membrane vesicles known as autophagosomes,which is mediated by the Atg12-Atg5-Atg16 complex and microtubule-associated protein light chain 3-phospholipid conjugates(LC3-II)[2,3]. The outer membrane of the autophagosome fuses with the lysosome,and cytoplasm-derived materials are degraded in the autolysosome.To date,autophagy has been implicated in various physio-pathological processes,including cell death,cell survival and tumorigenesis[4,5].Accumulating evidence indicated that autophagy may serve as a cell death mechanism under certain cellular scenarios and this autophagydependent cell death has been defined as‘‘autophagic cell death’’or‘‘type II programmed cell death’’[6]. Intriguingly,autophagy has also been shown as a pro-survival mechanism against cell death in response to a variety of stimuli including oxidative stress,metabolic stress and endoplasmic reticulum(ER)stress[7,8].In cardiomyocytes,low baseline levels of regulated autophagy are beneficial to maintaining cardiac structure and function,but uncontrolled or excessive autophagy can cause extensive self-destruction and cell death[9].Macrophage apoptosis is a critical process in the formation of necrotic cores in vulnerable atherosclerotic plaques[10].In vitro and in vivo studies indicated that macrophage apoptosis in advanced atheromata is triggered by a combination of ER stress and the engagement of class A scavenger receptor(SRA)[11],which together induce death through a rise in cytosolic calcium and the activation of toll-like receptor-4(TLR4)[12,13].We previously found that the engagement of SR-A triggered JNK-dependent apoptosis in ER-stressed macrophages[14].However, whether autophagy is involved in SR-A-mediated apoptosis in macrophage has not been defined.
In this study,we demonstrated that fucoidan,a ligand for SR-A[15],could inhibit ER stress induced autophagy by activating the mammalian target of rapamycin(mTOR)pathway.Our observations suggest that the suppression of ER stress induced autophagy may contribute to a mechanism of SRA-engaged macrophage apoptosis.
MATERIALS AND METHODS
Reagents and plasmid constructs
Polyclonal antibodies against phospho-Akt(Ser 473), phospho-mTOR,phospho-p70 S6 kinase and caspase-3 were obtained from Cell Signaling Technology (Danvers,MA,USA).Anti-LC3 polyclonal antibody was purchased from Santa Cruz Biotechnology(Santa Cruz,CA,USA).Thapsigargin(Tg),3-methyladenine (3-MA)and rapmycin were supplied by Sigma(St. Louis,MO,USA).Roswell Park Memorial Institute medium 1640(RPMI-1640),pcDNA3.1-EGFP vector, fetal calf serum(FCS),glutamine,penicillin,streptomycin,G418 and LipofectamineTM2000 were obtained from Invitrogen(Carlsbad,CA,USA).The construction of pcDNA3.1-EGFP-LC3 plasmid was described previously[16].MLC3 sequence from pCMV.SPORT6.1-mL C3 was subcloned to pcDNA3.1-EGFP vector.
Cell culture
RAW264.7 cells were obtained from the American Type Culture Collection(ATCC,Manassas,VA, USA),and cultured in RPMI-1640 containing 10% (v/v)foetal calf serum(FCS),supplemented with 2 mmol/L glutamine,100 U/mL penicillin and 100 μg/mL streptomycin.RAW264.7 cells were transfected with plasmid pcDNA3.1-GFP-LC3.PcDNA3.1-GFP-LC3 recombinant eukaryotic expression vector was gifted by professor Zhigang He at Harvard University.RAW264.7 cells were transfected by pcDNA3.1-GFP-LC3 plasmid using Effectene Transfection Reagent.GFP-LC3 stably expressing cells were selected and maintained in G418(600 mg/mL).
SR-A-/-mice,congenic to 129/ICR strain,were used in this study.The characterization of SR-A-/-mice was described previously[17].All aspects of the animal care and experimental protocols were in accordance with the guidelines for the‘‘Principles of Laboratory Animal Care’’and approved by the Experimental Animal Care and Use Committee of the authors′affiliated institution.Peritoneal macrophages(PM) were harvested 4 days after thioglycollate was injected into the mouse peritoneal cavity.Cells were washed with chilled phosphate-buffered saline(PBS)(pH 7.4),and macrophages were resuspended in RPMI 1640 containing 10%FCS,100 U/mL penicillin and 100 μg/mL streptomycin,and plated on 60-mm round Petri dishes.After a 2 hour incubation at 37°C,5% CO2non-adherent cells were removed,and the remaining adherent cells were cultured in RPMI 1640 containing 10%FCS,100 U/mL penicillin and 100 μg/mL streptomycin.
Western blotting assays
Cells were washed twice with PBS and lysed in lysis buffer(50 mmol/L Tris-HCl,pH 7.6,0.15 mol/L NaCl,1%Triton X-100,5 mmol/L EDTA,1 mmol/L sodium orthovanadate,10 μg/mL leupeptin and 10 μg/mL aprotinin).Cell lysates were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis(SDS-PAGE).Proteins were transferred to polyvinylidene difluoride(PVDF)membrane and blocked for 30 minutes in blocking buffer(Tris-buffered saline,pH 7.6,0.05%Tween and 3%non-fat dry milk).After incubation with primary antibody diluted in blocking buffer for 60 minutes and washing, blot was incubated for 30 minutes with appropriate secondary anti-IgG-horseradish peroxidase conjugate. The membrane was washed 3 times for 10 minutes each and developed with SuperSignal chemiluminescent substrate(Pierce,Rockford,IL,USA).
Immunofluorescence analysis
RAW264.7 cells were grown on coverslips for 24 hours at 37°C.After fixation with 3%paraformaldehyde in PBS for 15 minutes at room temperature, cells were permeabilized with 0.1%Nonidet P-40, PBS for 5 minutes,and blocked with 2%BSA, 0.01%Tween 20 and PBS(PBST-BSA)for 30 minutes.The antibody against nuclei(DAPI,Sigma)inPBST-BSA was incubated with cells for 1 minute and each coverslip was then washed 3 times for 10 minutes.Morphologic observation was performed with a Zeiss LSM 710 META confocal microscope.Twochannel optical images(DAPI and GFP)were collected with sequential scanning mode(405-and 488-nm excitation,respectively and 450-and 522-nm emission, respectively)of the Zeiss LSM 710 META confocal system.Cells containing 3 or more GFP-LC3 dots were defined as autophagy-positive cells.Pictures were obtained using sequential scanning,and the exposure settings and gain of laser were kept the same for each condition.
Apoptosis assay
After treatment,RAW264.7 cells were washed, resuspended in the staining buffer,and examined with the Annexin V-FITC and propidium iodide(PI)apoptosis kit(Biouniquer Technology Co.,Ltd,Shanghai, China)according to the manufacturer′s instructions. Stained cells were detected by FACS(FACSCalibur; BD Biosciences,CA,USA).Annexin V-positive and PI-negative cells were regarded as apoptotic cells.
Statistical analysis
Results were expressed as mean±S.D.Statistical significance between groups was assessed by one-way analysis of variance(ANOVA)followed by Student-Newman-Keuls(SNK)post-hoc test.The effect of autophagy on apoptosis was a one-way ANOVA design;however,the variances were unequal,and the Welch test was performed followed by a Tamhane post-hoc test.P<0.05 was considered statistically significant.
RESULTS
Fucoidan inhibits ER stress-induced autophagy in macrophages
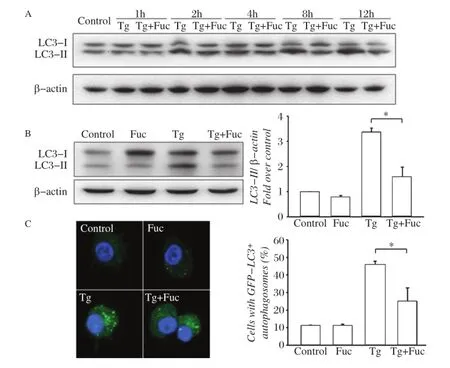
Fig.1Fucoidan inhibits thapsigargin(Tg)-induced autophagy.A:RAW264.7 cells were left untreated or incubated with 0.5 μmol/L Tg or 0.5 μmol/L Tg plus 25 μg/mL fucoidan for 1,2,4,8 or 12 hours.Cell lysates were subjected to Western blotting and detected by antibodies against LC3 and β-actin.B RAW264.7 cells were incubated for 12 hours with the indicated reagents,alone or in combination with 25 μg/mL fucoidan and 0.5 μmol/L Tg.Cell lysates were applied to Western blotting and detected by antibodies against LC3 and β-actin(left).The ratio of LC3-II to β-actin density was calculated using the ImageJ software and set to 1 for control(right).Non-treated cells were as control.Results were expressed as mean± S.D.of triplicate samples.*P<0.01 compared with the Tg treated group.C:GFP-LC3-RAW264.7 cells were incubated for 12 hours with the indicated reagents,alone or in combination:25 μg/mL fucoidan and 0.5 μmol/L Tg.GFP-LC3 fluorescence images and quantitative analyses are shown in the left and right panels,respectively.Results were expressed as mean±SD of triplicate samples.*P<0.01 compared with the Tg treated group.
ER stress results in autophagy in cells[7].Tg was used to induce ER stress in RAW264.7 cell and autophagosome formation was assessed by followingthe phospholipid conjugation of protein LC3-I(cytosolic form)to LC3-II(autophagosomal membranebound form)[2,18].Time-course experiments revealed that LC3 type II,an indicator of autophagosome formation,was increased after 2-hour treatment by Tg in RAW264.7 cells.When fucoidan was simultaneously added to cells,LC3 II accumulation was reduced at 8 hours and maximal inhibition was seen at 12 hours after treatment(Fig.1A).As shown inFig.1B,Tginduced LC3 II accumulation was decreased by 48.5%± 13.7%by treatment with fucoidan for 12 hours,whereas single fucoidan treatment did not change LC3 II expression in macrophages. Moreover,Tg treatment led to a redistribution of GFP-LC3 from a diffuse distribution to a punctuate distribution in GFP-LC3-expressing RAW264.7 cells, which was inhibited by 52.2%± 8.2%by co-incubation of fucoidan(Fig.1C).These data suggested that Tg-induced autophagic response in macrophages could be inhibited by simultaneous treatment with fucoidan.
Inhibition of autophagy promotes apoptosis in macrophages
To identify the role of autophagy in apoptosis,we treated RAW264.7 cell with Tg alone or Tg plus 3-MA,an inhibitor of autophagy.As shown inFig.2, treatment with Tg alone insignificantly increased apoptosis in macrophages.However,simultaneous treatment with 3-MA caused a dramatic increase in apoptotic cells,indicating that the inhibition of autophagy may promote apoptosis in macrophages.
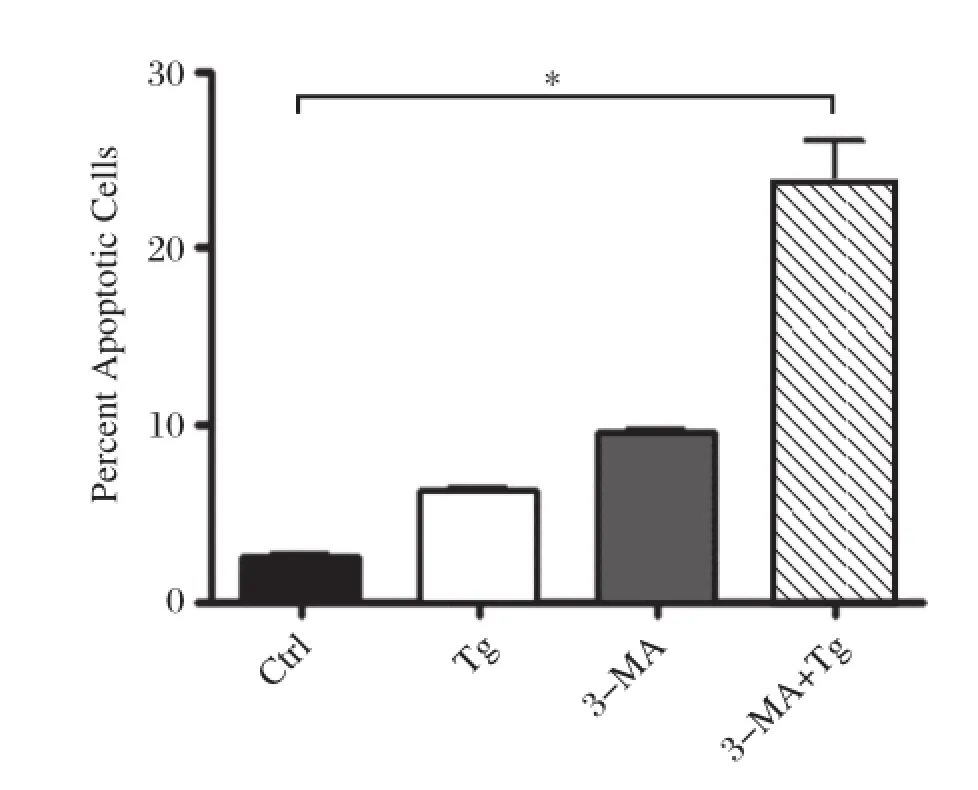
Fig.2The inhibition of autophagy increases apoptosis in RAW264.7 cells.RAW264.7 cells were incubated with 10 mmol/L 3-MA,0.5 μmol/L Tg,or 10 mmol/L 3-MA plus 0.5 μmol/L Tg for 12 hours,respectively.Non-treated cells were used as a control.Cells were stained with annexin V and propidium iodide(PI)and analyzed by FACS.Results were expressed as mean± SD N=5,*P<0.01 compared with control.
Fucoidan activates the mTOR pathway through SR-A
Autophagy is negatively regulated by the mTOR pathway in response to stress signals[19,20].Akt,a serine/threonine kinase,can activate mTOR and p70 S6 kinase(S6K).To understand the molecular mechanism whereby fucoidan inhibited autophagy in macrophages,we analyzed these signaling molecules activities.It was found that fucoidan or fucoidan plus Tg treatment induced AKT,mTOR and p70S6K phosphorylation in macrophages at 2 and 4 hours while single Tg treatment had no effect on this pathway. Phosphorylation of AKT,mTOR and p70S6K was obviously activated by fucoidan or Tg plus fucoidan at 4 hours(Fig.3A).These results revealed that fucoidan may activate the AKT/mTOR/p70S6K pathway in macrophages.
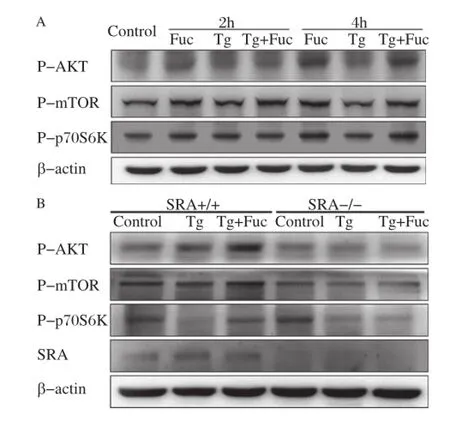
Fig.3SR-A is required for fucoidan-induced activation of the mTOR pathway.A:RAW264.7 cells were incubated with the indicated reagents,alone or in combination:25 μg/mL fucoidan and 0.5 μmol/L Tg for 2 and 4 hours.Cell lysates were applied to Western blotting and detected by antibodies against p-AKT,p-mTOR,p-p70S6K and β-actin. B:Wildtype(WT),and SRA-/-macrophages were left untreated or treated with 0.5 μmol/L Tg or 0.5 μmol/L Tg plus 25 μg/mL fucoidan for 12 hours.Cell lysates were subjected to Western blotting and detected by antibodies against p-AKT,p-mTOR,p-p70S6K and β-actin.
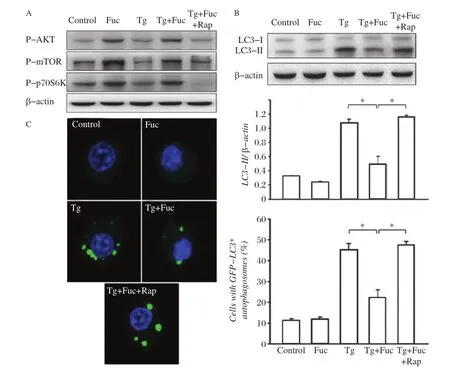
Fig.4The inhibition of the mTOR pathway restores fucoidan-inhibited autophagy.A:RAW264.7 cells were incubated for 4 hours with the indicated reagents,alone or in combination:25 μg/mL fucoidan,0.5 μmol/L Tg and 10 μmol/L Rap.Cell lysates were subjected to Western blotting and detected by antibodies against p-AKT,p-mTOR,p-p70S6K and β-actin.B:RAW264.7 cells were incubated for 12 hours with the indicated reagents, alone or in combination:25 μg/mL fucoidan,0.5 μmol/L Tg and 10 μmol/L Rap.Cell lysates were applied to Western blotting and detected by antibodies against LC3 and β-actin.The ratio of LC3-II to β-actin density was calculated using the ImageJ software and set to 1 for control.Non-treated cells were as control.Results were expressed as mean±SD of triplicate samples.*P<0.01.C:GFP-LC3-RAW264.7 cells were incubated for 12 hours with the indicated reagents,alone or in combination:25 μg/mL fucoidan,0.5 μmol/L Tg and 10 μmol/L Rap.GFP-LC3 fluorescence images and quantitation analyses are shown in the left and right panels,respectively.Results were expressed as mean±SD of triplicate samples.*P<0.01.
To determine whether the activation of the AKT/ mTOR/p70S6K pathway by fucoidan was mediated through SR-A,PMs from SR-A knockout and wildtype mice were used for the experiments.In wildtype macrophages,AKT,mTOR and p70S6K were markedly activated by treatment with Tg plus fucoidan compared with Tg treatment.However,these effects were abolished in the SR-A deficient macrophages(Fig. 3B),indicating that SR-A would be requisite for fucoidan-induced activation of the mTOR pathway in macrophages.
Blockage of the mTOR pathway restores ER stress-induced autophagy
To observe the role of mTOR in Tg-induced autophagy,we further used rapamycin,a pharmacologic inhibitor of mTOR,to treat macrophages.We found that rapamycin had a potent inhibitory effect on the activation of the mTOR pathway induced by Tg plus fucoidan treatment(Fig.4A).Moreover,rapamycin restored LC3-II to the level similar to treatment with Tg alone(Fig.4B). Consistently,the blockage of the mTOR pathway by rapamycin also restored the occurrence of autophagosomes in GFP-LC3 cells that was significantly inhibited by treatment with Tg plus fucoidan.These results suggested that rapamycin antagonized the inhibitory effect of fucoidan on Tg induced autophagy in macrophages.
Blockage of the mTOR pathway inhibits fucoidan-Tg induced macrophage apoptosis
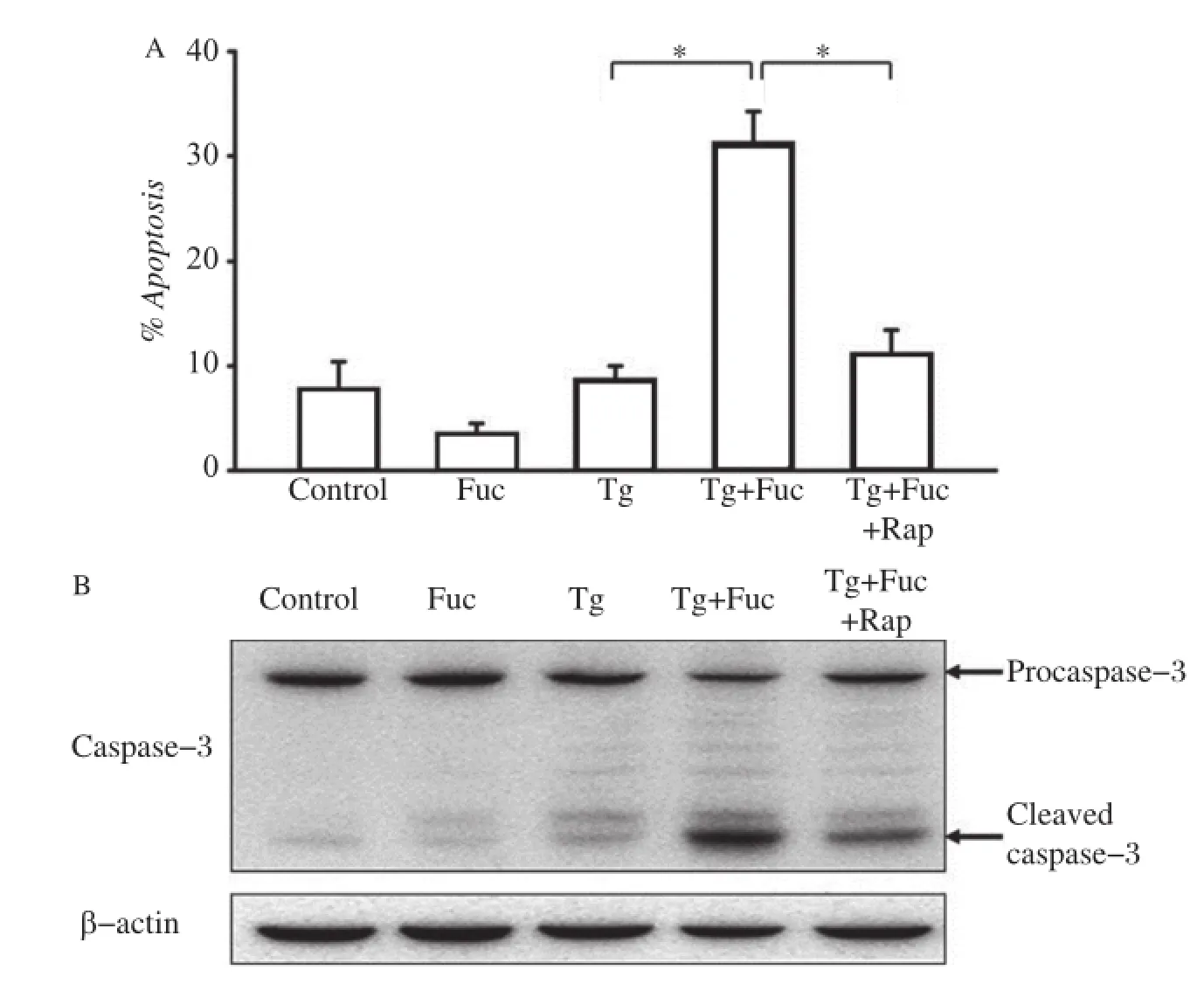
Fig.5The inhibition of the mTOR pathway on cell apoptosis.A:RAW264.7 cells were incubated for 12 hours with the indicated reagents, alone or in combination:25 μg/mL fucoidan,0.5 μmol/L Tg and 10 μmol/L Rap.Cells were stained with annexin V and PI and analyzed by FACS.The apoptotic cells(the annexinV-positive and PI-negative cells)were indicated as the percentage of gated cells.Results were expressed as mean±SD of triplicate samples.*P<0.01.B:RAW264.7 cells were incubated for 12 hours with the indicated reagents,alone or in combination:25 μg/mL fucoidan, 0.5 μmol/L Tg and 10 μmol/L Rap.Cell lysates were applied to Western blotting and detected by antibodies against Caspase-3 and β-actin.
We further examined whether the blockage of the mTOR pathway was able to mitigate macrophage apoptosis induced by the co-addition of Tg and fucoidan.As shown inFig.5A,treatment with Tg or fucoidan alone did not dramatically impact on macrophage apoptosis.It caused a low production of cleaved caspase-3,a pivotal aspargine protease in the apoptotic response(Fig.5B).However,the combined application of these two reagents led to a marked increase in macrophage apoptosis and the production of cleaved caspase-3,consistent with our previous study[14]. Accordingly,additional rapamycin treatment significantly inhibited cell apoptosis and caspase-3 cleavage induced by Tg plus fucoidan,which indicated that the inhibition of the mTOR pathway may prevent ER stress-dependent apoptosis.
DISCUSSION
Fucoidan shows antitumor activity by inducing apoptosis in cultured human cancer cells[21-23]. Moreover,long time treatment with fucoidan at high concentration can induce autophagy and suppress cell proliferation in AGS human gastric cancer cells[24]. However,it appears that the roles of autophagy in regulating cell death are highly dependent on cell type and stimulus.For instance,fucoidan itself cannot induce autophagy or apoptosis in macrophage[14].Macrophage apoptosis is triggered by ER stress in combination with the engagement of SR-A,but not either stimulus alone[11].We found that fucoidan could inhibit ER stress trigged autophagy mediated by SR-A in macrophage. This is consistent with the finding that pattern recognition receptors(PRRs)such as nucleotide-binding oligomerization domain-like receptor(NLR)C4 NLRC4 and NLRP4 have an inhibitory effect on autophagy[25].In contrast,the activation of quintessential PRRs like toll such as toll receptor 4(TLR4)and TLR7 can induce autophagy in RAW264.7 cells to defend against pathogen invasion[26].
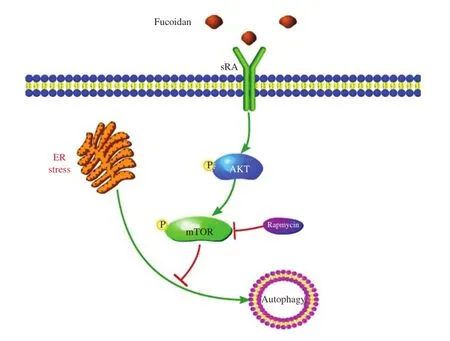
Fig.6A model for the effects of the SR-A pathway on ER-stress induced autophagy in macrophage.
Three distinct forms of autophagy have been identified,including macroautophagy,microautophagy and chaperone-mediated autophagy.Macroautophagy is thought to be the major type of autophagy.Autophagy,by means of self-cannibalization,may contribute to cell survival or death depending on the threshold level[27-29].Autophagy can protect against cell death during nutrient starvation and other stressors[30].Mild ER stress inhibits neuronal death by promoting autophagy in drosophila and mouse models of Parkinson′s disease[31].The induction of autophagy by ER stress under therapeutic dosage before ischemia is cardioprective for rats[32]whereas high levels of autophagy promote cell death[33].Apoptosis and autophagy have been shown to act in synergy and also to counter each other,since they are not mutually exclusive pathways at all[6].The inhibition of differentiation-induced autophagy leads to monocyte apoptosis[34].In the present study,we showed that thapasigargin induced a significant increase in the formation of autophagosomes but little apoptosis.Moreover,the co-addition of thapasigargin and fucoidan compromised this process and markedly induced apoptosis.In this case,autophagy does not lead to cell death,but instead acts to reduce apoptosis by creating a cellular milieu in which survival is favored.Autophagy might serve as a cell survival mechanism by maintain ER function through the consumption of protein aggregates and misfolded proteins,thus limiting ER stress response and subsequent apoptosis[35].Accordingly,the suppression of autophagy promoted cell apoptosis.Interestingly,Liao et al.have demonstrated that blocking autophagy rendered macrophagesmoresusceptibletocelldeathandpromotedplaquenecrosis in mice[36].Itis interesting that wehave also proved that the internalization of SR-A and its ligand complex into cells is negatively regulated by the interaction of SR-A by glucose-regulated protein 78 (GRP78),a chaperon of ER stress[37].Thus,the anti-ER stress propertyof fucoidan/SR-A mayinvolve binding of SR-A to GRP78 in macrophage.
Several cell-signaling pathways contribute to the regulation of autophagy,which are cell type-specific and signal-dependent[38-40].The mTOR pathway is one of the most important autophagy regulators,which negatively control autophagy[19,20,41].In addition,mTOR has been found to have a pleiotropic function in the regulation of cell apoptosis.It works as an apoptosis inhibitor[42,43]or an apoptosis inducer[44]under different conditions.Therefore,mTOR may play an important role in regulating the cross-talk between autophagy and apoptosis.By activating the mTOR pathway via SR-A,fucoidan alone or in combination with Tg could consequently inhibit Tg-induced autophagy.It is worth noting that the inhibition of the mTOR pathway by rapamycin treatment could rescue autophagy response and reduce apoptosis in macrophage even in the presence of Tg plus fucoidan.Our observations support the notion that SR-A and the mTOR pathway are key elements in regulating a balance between autophagy and the apoptotic responses in macrophage.
Macrophage apoptosis plays an important role in the pathogenesis of many diseases.Macrophage apoptosis in early atherosclerotic lesions would limit plague development through a negative regulation of inflammation[45].In advanced atherosclerosis,macrophage apoptosis coupled with defective phagocytic clearance of dead cells leads to plaque necrosis[46].SR-A functions in mediating macrophage apoptosis and cleaning up of these apoptotic cells.We showed that fucoidan,a well-defined nonlipoprotein ligand for SR-A,could promote macrophage apoptosis by repressing ER stressor triggered autophagy.Multiple ER stressorsand SR-A ligands are known to exist in atheromata. Athero-relevant ER stressors include oxidant stress, peroxynitrite,insulin resistance,glucosamine,saturated fatty acids,hypoxia,homocyteine,oxidized phospholipids,oxysterols and serum starvation[47].SR-A ligands that trigger macrophage apoptosis during ER stress include modified forms of LDL,advanced glycation end products(AGEs),β-amyloid and anionic phospholipids,as well as pathogens and pathogen-associated molecules[14].Our in vitro results imply that the ligands of SR-A may have an antagonistic effect on ER stress trigged by ER stressors.Whether it takes place in in vivo pathophysiological situations needs to be validated. Our previous studies revealed an unique signal motif in the cytoplasm domain that mediates the internalization of SR-A[48]and demonstrated that SR-A-induced apoptosis is mainly through the caveolae route,which is linked to p38 kinase and JNK signaling[14].It is possible that SR-A engaged macrophage apoptosis is regulated by the p38 and JNK pathways directly.The impact of SR-A on autophagy may constitute a supplementary regulatory mechanism for macrophage apoptosis.In atherosclerosis,once macrophage apoptosis is triggered, the consequence of apoptosis,whether beneficial by suppressing cellularity in early lesions,or detrimental by contributing to necrotic core formation in advanced lesion,is likely dependent on the efficiency of phagocytes.The apoptotic macrophages that failed to be cleared by defective efferocytosis accumulate eventually and build up necrotic debris promotes inflammation, plaque instability,and acute thrombosis.
In summary,we report that fuciodan is essential for the activation of the mTOR pathway and the inhibition of autophagic response under conditions of ER stress in macrophages,which results in apoptosis.A postulated model for fucoidan regulation of autophagy is shown inFig.6.The inhibitory effect of fucoidan on autophagy could be abrogated by blocking the mTOR pathway,and thus,Tg-fucoidan induced apoptosis was prevented.Fucoidan/SR-A may contribute to macrophage apoptosis during ER stress by suppressing autophagy through its regulation of the mTOR pathway.
Acknowledgements
This work was supported by the National Basic Research Program(973)Grant(No.2012CB517503 and No.2011CB503903)and National Natural Science Foundation of China(No.81230070 and No. 81070120)to Qi Chen,the National Natural Science Foundation of China Grant(No.81000118)to Jingjing Ben and the National Natural Science Foundation of China Grant(No.81100857)to Xiaoyu Li.
[1] Mizushima N,Komatsu M.Autophagy:Renovation of cells and tissues.Cell 2011;147:728-41.
[2] Kabeya Y,Mizushima N,Ueno T,Yamamoto A,Kirisako T,Noda T,et al.Lc3,a mammalian homologue of yeast apg8p,is localized in autophagosome membranes after processing.EMBO J 2000;19:5720-8.
[3] Levine B,Kroemer G.Autophagy in the pathogenesis of disease.Cell 2008;132:27-42.
[4] Klionsky DJ.Autophagy:From phenomenology to molecular understanding in less than a decade.Nat Rev Mol Cell Biol 2007;8:931-7.
[5] Wu YT,Tan HL,Huang Q,Ong CN,Shen HM.Activation of the pi3k-akt-mTOR signaling pathway promotes necrotic cell death via suppression of autophagy.Autophagy 2009;5:824-34.
[6] Eisenberg-Lerner A,Bialik S,Simon HU,Kimchi A.Life and death partners:Apoptosis,autophagy and the crosstalk between them.Cell Death Differ 2009;16:966-75.
[7] Ogata M,Hino S,Saito A,Morikawa K,Kondo S, Kanemoto S,et al.Autophagy is activated for cell survival after endoplasmic reticulum stress.Mol Cell Biol 2006;26: 9220-31.
[8] Sakiyama T,Musch MW,Ropeleski MJ,Tsubouchi H, Chang EB.Glutamine increases autophagy under basal and stressed conditions in intestinal epithelial cells. Gastroenterology 2009;136:924-32.
[9] Takemura G,Miyata S,Kawase Y,Okada H,Maruyama R,Fujiwara H.Autophagic degeneration and death of cardiomyocytes in heart failure.Autophagy 2006;2: 212-4.
[10]Feng B,Zhang D,Kuriakose G,Devlin CM,Kockx M, Tabas I.Niemann-pick c heterozygosity confers resistance to lesional necrosis and macrophage apoptosis in murine atherosclerosis.Proc Natl Acad Sci U S A 2003;100: 10423-8.
[11]Devries-Seimon T,Li Y,Yao PM,Stone E,Wang Y, Davis RJ,et al.Cholesterol-induced macrophage apoptosis requires er stress pathways and engagement of the type a scavenger receptor.J Cell Biol 2005;171:61-73.
[12]Seimon TA,Obstfeld A,Moore KJ,Golenbock DT, Tabas I.Combinatorial pattern recognition receptor signaling alters the balance of life and death in macrophages. Proc Natl Acad Sci U S A 2006;103:19794-9.
[13]Lim WS,Timmins JM,Seimon TA,Sadler A,Kolodgie FD,Virmani R,et al.Signal transducer and activator of transcription-1 is critical for apoptosis in macrophages subjected to endoplasmic reticulum stress in vitro and in advanced atherosclerotic lesions in vivo.Circulation 2008;117:940-51.
[14]Zhu XD,Zhuang Y,Ben JJ,Qian LL,Huang HP,Bai H, et al.Caveolae-dependent endocytosis is required for class a macrophage scavenger receptor-mediated apoptosis in macrophages.J Biol Chem 2011;286:8231-9.
[15]Liang CP,Han S,Okamoto H,Carnemolla R,Tabas I, Accili D,et al.Increased cd36 protein as a response to defective insulin signaling in macrophages.J Clin Invest 2004;113:764-73.
[16]Zhuang Yan BJ,Bai Hui,Chen Qi.Establishment of a stable gfp-lc3-expressed raw264.7 cell line.Acta Univ Med Nanjing 2009;29:6.
[17]Suzuki H,Kurihara Y,Takeya M,Kamada N,Kataoka M, Jishage K,et al.A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection.Nature 1997;386:292-6.
[18]Tanida I,Minematsu-Ikeguchi N,Ueno T,Kominami E. Lysosomal turnover,but not a cellular level,of endogenous lc3 is a marker for autophagy.Autophagy 2005;1:84-91.
[19]Jung CH,Ro SH,Cao J,Otto NM,Kim DH.mTOR regulation of autophagy.FEBS Let 2010;584:1287-1295.
[20]Sudarsanam S,Johnson DE.Functional consequences of mTOR inhibition.Curr Opin Drug Discov Devel 2010;13: 31-40.
[21]Aisa Y,Miyakawa Y,Nakazato T,Shibata H,Saito K, Ikeda Y,Kizaki M.Fucoidan induces apoptosis of human hs-sultan cells accompanied by activation of caspase-3 and down-regulation of erk pathways.Am J Hematol 2005;78:7-14.
[22]Nagamine T,Hayakawa K,Kusakabe T,Takada H, Nakazato K,Hisanaga E,Iha M.Inhibitory effect of fucoidan on huh7 hepatoma cells through downregulation of cxcl12.Nutr Cancer 2009;61:340-7.
[23]Yamasaki-Miyamoto Y,Yamasaki M,Tachibana H, Yamada K.Fucoidan induces apoptosis through activation of caspase-8 on human breast cancer mcf-7 cells.J Agric Food Chem 2009;57:8677-82.
[24]Park HS,Kim GY,Nam TJ,Deuk Kim N,Hyun Choi Y. Antiproliferative activity of fucoidan was associated with the induction of apoptosis and autophagy in ags human gastric cancer cells.J Food Sci 2011;76:T77-83.
[25]Jounai N,Kobiyama K,Shiina M,Ogata K,Ishii KJ, Takeshita F.Nlrp4 negatively regulates autophagic processes through an association with beclin1.J Immunol 2011;186:1646-55.
[26]Mihalache CC,Simon HU.Autophagy regulation in macrophages and neutrophils.Exp Cell Res 2012;318: 1187-92.
[27]Kroemer G,Levine B.Autophagic cell death:The story of a misnomer.Nat Rev Mol Cell Biol 2008;9:1004-10.
[28]Kourtis N,Tavernarakis N.Autophagy and cell death in model organisms.Cell Death Differ 2009;16:21-30.
[29]Levine B,Yuan J.Autophagy in cell death:An innocent convict?J Clin Invest 2005;115:2679-88.
[30]Mizushima N,Levine B,Cuervo AM,Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature 2008;451:1069-75.
[31]Fouillet A,Levet C,Virgone A,Robin M,Dourlen P, Rieusset J,et al.Er stress inhibits neuronal death by promoting autophagy.Autophagy 2012;8:915-26.
[32]Petrovski G,Das S,Juhasz B,Kertesz A,Tosaki A,Das DK.Cardioprotection by endoplasmic reticulum stressinduced autophagy.Antioxid Redox Signal 2011;14: 2191-200.
[33]Maiuri MC,Zalckvar E,Kimchi A,Kroemer G.Self-eating and self-killing:Crosstalk between autophagy and apoptosis.Nat Rev Mol Cell Biol 2007;8:741-52.
[34]Zhang Y,Morgan MJ,Chen K,Choksi S,Liu ZG. Induction of autophagy is essential for monocyte-macrophage differentiation.Blood 2012;119:2895-905.
[35]Ding WX,Ni HM,Gao W,Hou YF,Melan MA,Chen X, Stolz DB,Shao ZM,Yin XM.Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival.J Biol Chem 2007;282:4702-10.
[36]Liao X,Sluimer JC,Wang Y,Subramanian M,Brown K, Pattison JS,et al.Macrophage autophagy plays a protective role in advanced atherosclerosis.Cell Metab 2012; 15:545-553.
[37]Ben J,Gao S,Zhu X,Zheng Y,Zhuang Y,Bai H,et al. Glucose-regulated protein 78 inhibits scavenger receptor a-mediated internalization of acetylated low density lipoprotein.J Mol Cell Cardiol 2009;47:646-55.
[38]Codogno P,Meijer AJ.Autophagy and signaling:Their role in cell survival and cell death.Cell Death Differ 2005;12(S2):1509-18.
[39]He C,Klionsky DJ.Regulation mechanisms and signaling pathways of autophagy.Annu Rev Genet 2009;43:67-93.
[40]Yang Z,Klionsky DJ.Mammalian autophagy:Core molecular machinery and signaling regulation.Curr Opin Cell Biol 2010;22:124-31.
[41]Chang YY,Juhasz G,Goraksha-Hicks P,Arsham AM, Mallin DR,Muller LK,Neufeld TP.Nutrient-dependent regulation of autophagy through the target of rapamycin pathway.Biochem Soc Trans 2009;37:232-6.
[42]Beuvink I,Boulay A,Fumagalli S,Zilbermann F,Ruetz S,O′Reilly T,et al.The mTOR inhibitor rad001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation.Cell 2005; 120:747-59.
[43]Teachey DT,Obzut DA,Cooperman J,Fang J,Carroll M, Choi JK,et al.The mTOR inhibitor cci-779 induces apoptosis and inhibits growth in preclinical models of primary adult human all.Blood 2006;107:1149-55.
[44]Castedo M,Ferri KF,Blanco J,Roumier T,Larochette N, Barretina J,et al.Human immunodeficiency virus 1 envelope glycoprotein complex-induced apoptosis involves mammalian target of rapamycin/fkbp12-rapamycin-associated protein-mediated p53 phosphorylation. J Exp Med 2001;194:1097-110.
[45]Babaev VR,Chew JD,Ding L,Davis S,Breyer MD, Breyer RM,et al.Macrophage ep4 deficiency increases apoptosis and suppresses early atherosclerosis.Cell Metab 2008;8:492-501.
[46]Tabas I.Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis:The importance of lesion stage and phagocytic efficiency.Arterioscler Thromb Vasc Biol 2005;25:2255-64.
[47]Seimon T,Tabas I.Mechanisms and consequences of macrophage apoptosis in atherosclerosis.J Lipid Res 2009;50 Suppl:S382-7.
[48]Chen Y,Wang X,Ben J,Yue S,et al.The di-leucine motif contributes to class a scavenger receptor-mediated internalization of acetylated lipoproteins.Arterioscler Thromb Vasc Biol 2006;26:1317-22.
Received 10 July 2013,Revised 21 August 2013,Accepted 20 October 2013,Epub 12 December 2013
✉Corresponding author:Xiaoyu Li,MD,Ph.D,Atherosclerosis Research Centre,Laboratory of Molecular Intervention with Cardiovascular Diseases,Nanjing Medical University,Hanzhong Road140,Nanjing,Jiangsu 210029,China.Tel/Fax:+86-25-86862967/+86-25-86862735,E-mail:xyli@njmu.edu.cn.
The authors reported no conflict of interests.
ⓒ2014 by the Journal of Biomedical Research.All rights reserved.
10.7555/JBR.28.20130105
杂志排行
THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Standardized training for resident doctors in China
- Metabolic regulation by protein tyrosine phosphatases
- Apolipoprotein B100 quality control and the regulation of hepatic very low density lipoprotein secretion
- A genetic variant in pseudogene E2F3P1contributes to prognosis of hepatocellular carcinoma
- Expression of human hepatic lipase negatively impacts apolipoprotein A-I production in primary hepatocytes from Lipc-null mice
- Correlation of obstructive sleep apnea hypopnea syndrome with metabolic syndrome in snorers
