植物矿质养分吸收的长距离反馈调节研究进展
2014-04-08熊长明田晓莉
熊长明, 王 晔, 田晓莉
(中国农业大学作物化学控制研究中心, 植物生理学与生物化学国家重点实验室,北京 100193)
土壤矿质养分的时空波动广泛存在,植物已进化出精细的局部和长距离调节系统对此做出响应,以维持体内矿质养分的稳态。局部调节包括根毛和侧根增生等根系形态结构的变化[1-6]及一些养分运输载体的诱导表达[7-9]。长距离调节则由根-冠调节和冠-根调节组成,根系感受到周围介质养分的波动后首先通过木质部向地上部传递信号,并触发一系列响应; 随后地上部产生信号并由韧皮部向下运输,对根系生长和养分吸收速率进行反馈调节,使根系的吸收能力与地上部的养分需求得以匹配[10]。


1 养分吸收的长距离反馈调节现象普遍存在
嫁接和分根是研究矿质养分长距离反馈调节的常用方法,养分感受和信号系统发生缺陷的突变体也为探明养分稳态的反馈调节提供了手段。
尽管有少量相反的报道[23],但绝大多数研究证明矿质养分吸收的长距离反馈调节现象是普遍存在的。
向部分根系供Pi,植株可能通过提高其养分吸收速率(与整根供Pi相比)部分或全部补偿缺Pi部分根系的损失[26-27]。植株对Pi的需求通过供Pi部分根系得到满足后,缺Pi部分根系Pi转运蛋白的表达不再受低Pi诱导[28-30]。Mt4是蒺藜苜蓿根系的Pi-饥饿诱导基因(PSI),在分根试验中部分根系供Pi后发生系统下调[31]。pho1突变体根系的木质部Pi载入发生缺陷,地上部的Pi水平较低[31],导致其根系Mt4基因在充足供Pi时也不发生下调[32-33]。Grevilleacrithmifolia(银桦)的排根起始和生长以及根系的Pi净吸收速率也均受到系统反馈调节[34]。

分根试验也广泛用于研究缺Fe响应的调节机制。车前草[40]、 黄瓜[41]、 拟南芥[42-43]、 番茄(Lycopersiconesculentum)[44]和木本植物小金海棠[45]等植物的部分根系处于缺Fe条件下时,可诱导另一部分供Fe根系的Fe(III)还原酶活性和/或质子分泌增强。豌豆耐低铁基因型(Santi)作为接穗可以诱导不耐低铁基因型(Parafield)砧木的缺铁响应,使其Fe(III)还原酶活性维持在较高水平[46]。拟南芥frd3突变体的木质部Fe装载(由Fe(III)柠檬酸盐螯合物介导)发生缺陷,在充足供Fe条件下叶片仍处于缺Fe状态[47],此时根系中的缺铁响应也不能下调[48-50],提示来自于地上部的缺Fe信号在持续调节根系吸铁基因的表达。
植株去顶或去叶后,发现根系的一些缺素响应减弱或消失[3, 41, 51, 56], 说明根系的吸收能力依赖于地上部对缺素的响应,并受到冠-根信号的介导。
2 长距离反馈信号的作用模式尚不明确
虽然已经证明了地上部可以发送长距离冠-根信号调控根系的缺素响应,但地上部是在缺素条件下发出促进信号/需求信号、 还是在养分充足条件下发出抑制信号/供应信号尚未得到一致的结论。


地上部控制根系缺Fe响应的信号机制得到较多研究。Vert等(2003)曾提出拟南芥地上部调控根系Fe吸收机制的促进模型和抑制模型[43]。促进模型(the promotive model)是指在缺Fe条件下叶片产生促进信号传递至根系,诱导Fe吸收基因的表达,在Fe充足条件下则不产生信号; 抑制模型(the repressive model)是指在供Fe充足条件下地上部产生抑制信号,传递至根系后抑制Fe吸收响应,在缺Fe条件下则不产生信号。
Grusak和Pezeshgi(1996)将豌豆突变体dgl(正常供铁条件下根部缺铁响应机制仍然处于激活状态,导致植株体内的Fe 超量积累)和野生型DGV共同嫁接在野生型砧木上,发现充足供Fe时野生型砧木的Fe(III)还原速率也能在dgl突变体接穗的诱导下得到提高,他们认为这种现象是由dgl突变体向下传递的促进信号所致[55]。Enomoto等(2007)的去叶试验也证明地上部在缺Fe条件下向根系传递促进信号,他们发现烟草根系编码Fe运输载体的基因NtIRT1和编码Fe(III)还原酶的基因NtFRO1在缺Fe条件下被诱导,但去掉叶片3 h后它们的表达快速下降,并逐渐降低到充足供Fe下的水平[56]。
拟南芥opt3-2突变体韧皮部中的肽-Fe螯合物运输存在缺陷[57]。García等(2012)向处于缺Fe状态的野生型植株叶面喷施FeSO4,发现根系的Fe(III)还原酶活性下降数倍,吸铁基因的表达几乎被阻断; 但opt3-2突变体的Fe(III)还原酶活性和吸铁基因表达仍然维持在与缺Fe处理相当的水平。他们据此推断,叶面施Fe后产生了特异的抑制信号以阻止根系的缺Fe响应,而非减弱或阻断了地上部的促进信号向根系传递[58]。
Lucena等(2006)对Fe吸收反馈调节的促进模型和抑制模型进行了整合,提出地上部既可向根系发出促进信号(乙烯或促进乙烯合成的物质,如生长素或乙烯前体ACC),也可发出抑制信号(与韧皮部中的Fe有关); 根系可将两类信号进行整合,最终决定是诱导还是抑制吸铁基因的表达[59]。
3 长距离反馈信号的鉴定取得一定进展
3.1 养分或它们的同化物/代谢物信号

分根试验表明,缺Pi侧根系PSI基因的上调受到抑制,很可能由供Pi侧运输而来的Pi介导[28, 32, 70]。拟南芥PHO2基因编码一个位于叶片韧皮部的Pi转运蛋白,pho2突变体的Pi吸收能力和根-冠转运能力提高,导致地上部的Pi过量积累,暗示Pi的负反馈调节功能缺失[71]。pho1突变体根系木质部的Pi载入发生缺陷,地上部的Pi水平较低,其根系中PSI的下调同时受到破坏[32-33]。但Pi本身可能不负责这种系统下调,因为在蒺藜苜蓿的分根系统中,缺Pi部分根系Mt4的下调早于Pi浓度的上升,而且Pi流量的减少不影响系统抑制[32]。当然,也可能是根系和地上部之间Pi的动态循环(而不是Pi浓度自身)为反馈调节提供了信号[35]。
充足供Fe的蓖麻植株,其叶片Fe浓度和韧皮部中Fe复合物的水平高于缺Fe植株; 叶面供应Fe-EDTA可降低根系的缺Fe响应(质子泌出和Fe(III)还原酶活性提高)。因此,韧皮部中的Fe复合物可能控制植物根系对Fe有效性的响应[72]。拟南芥opt3-2突变可能影响韧皮部中Fe-肽螯合物的运输[73]; 番茄chln突变导致烟草胺(NA,氨基羧酸类Fe螯合剂)无法合成[74],而NA可以与Fe(II)形成稳定的复合体在韧皮部运输[75-77]; 豌豆dgl突变体在韧皮部中不形成野生型具有的Fe-肽螯合物[78-80]。上述这些突变体根系的缺Fe响应在供Fe条件下均比较强,据此推测与肽或NA形成复合物的Fe可能作为韧皮部信号下调根系吸Fe基因的表达。最近的叶面57Fe标记试验表明,突变体opt3-2、chln和dgl根系中积累的57Fe与各自的野生型相似[58],说明韧皮部中的总Fe含量不是根系缺Fe响应的抑制信号,为上述Fe复合物作为信号物质的推测提供了旁证。
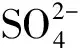
3.2 地上部衍生的碳水化合物信号
Pitman(1972)的遮光试验表明,K+自根系向地上部的运转量与根系中的糖浓度有关,推测地上部可能通过向根系供应能量来反馈调节K+向地上部的运输[81]。
糖类物质对Pi饥饿响应的调节得到很多研究的肯定。根中糖浓度的增加早于磷胁迫响应基因(PSR)的诱导[82]。在缺Pi条件下,采用黑暗或环割处理抑制蔗糖的生物合成和转运,使根中PSI的表达降低[83]。pho3突变体的SUC2基因(编码一个在韧皮部表达的蔗糖运输载体)发生缺陷,在低Pi条件下其根系的酸性磷酸酶(ACP)活性和PSI基因表达减弱[84-85]。Lei等(2011)构建了一个SUC2基因过表达的拟南芥突变体hps1,其在Pi吸收、 转运和根系构型等多方面表现出低Pi响应[86]。缺Pi白羽扇豆排根中的几个PSR以依赖光合作用的方式上调[82]。外源施用糖类物质对根系形态和Pi运输载体等PSR有放大作用[83-89]。
3.3 植物激素信号
有研究表明,CK可抑制Pi饥饿响应[102],但外源CK处理不能模拟恢复供Pi的抑制效应[70],因而对CK作为Pi长距离信号的作用提出了质疑。
拟南芥lpr1(抗低Pi)突变体的侧根形成在Pi饥饿条件下减少,而该突变体是Auxin极性运输所需要的BIG的等位基因系[104],表明自上而下运输的Auxin可能也与根系的Pi饥饿响应有关。应用野生型和Auxin响应突变体进行的研究发现,根系中Auxin的重新分布激发了Pi饥饿诱导的根系发育[105]。但其他不依赖于Auxin的信号途径也参与了这些响应[104]。
大量应用突变体、 去顶(去除Auxin产生的主要源器官)、 环割(阻断Auxin的向基运输)、 添加Auxin及其极性运输抑制剂的研究为Auxin作为系统信号参与缺Fe响应提供了证据。拟南芥突变体aux1-7的生长素极性运输有缺陷,该突变体的转录因子FIT及其靶标基因Fe(III)还原酶的表达不受缺Fe诱导[106]。大豆缺Fe植株去掉茎顶端、 或在初生叶下方的茎部施用IAA运输抑制剂CFM,根系的Fe(III)还原酶活性降低,恢复到与供Fe处理相当的水平[41]。缺Fe导致红三叶草根系的IAA积累和Fe(III)还原酶活性的提高,而在茎部施用IAA运输抑制剂TIBA后根系的IAA积累减少,还原酶活性也受到显著抑制[107]。最近Wu等(2012)的系列试验证明,IAA自上而下的长距离运输确实介导了根系的缺Fe响应,如茎环割完全抑制了根系中的IAA积累,同时阻止了根系中质子泌出和Fe(III)还原酶活性的上调; 去掉茎尖抑制了根中的质子泌出和Fe(III)还原酶活性的上调,但向去顶植株的顶端补充NAA可以恢复这两种生理响应[108]。
Auxin作为长距离冠-根信号调控根系缺Fe响应也受到一些质疑。去顶和施用CFM不能降低黄瓜缺Fe植株根系的Fe(III)还原酶活性[41],烟草去顶不影响根系中NtIRT1和NtFRO1的表达[56]。Schmidt等(2000)应用多种突变体的研究表明,主要植物激素(包括Auxin)均未参与拟南芥的缺Fe响应[109]。Bacaicoa等(2011)则推测,IAA调节缺Fe响应的作用可能是次级的,而且是非必需的[110]。
3.4 新的长距离信号物质MicroRNA
在韧皮部汁液中鉴定出MicroRNAs(miRNAs)的事实,提示这类物质具有作为长距离信号分子的潜力[111-112]。目前,几种主要矿质养分缺乏时均发现韧皮部中有miRNAs上调现象。miR395、 miR398和miR399的表达分别在S、 Cu和Pi缺乏时上调[113-116]。最近在油菜(Brassicarapus)韧皮部汁液中检测到了miR395、 miR398和miR399,而且它们的丰度在植物分别处于S、 Cu和Pi饥饿时增加[108]。油菜缺Fe时韧皮部中的miR158b上调[117],拟南芥中也发现同样的现象[118]。借助谷氨酰合成酶抑制剂MSX,Gifford等(2008)鉴定出一个响应有机N的miR167/ARF8调节模型,该模型调节侧根起始和伸长,使根系与有机N进行匹配[66]。miR393/AFB3(生长素受体)模型则通过调节主根和侧根的生长对N作出响应[119]。但目前还没有直接证据表明miR167或miR393可以从地上部运输至根系。
miR399对Pi饥饿响应的调节得到最系统和广泛的研究。miR399在缺Pi组织中特异上调[114, 120-122],直接引起一种泛素结合E2酶基因PHO2 mRNA的裂解,从而促进Pi吸收和向地上部的运输。过表达miR399的拟南芥和水稻在地上部过量积累Pi,在充足供Pi条件下出现Pi中毒现象[120, 124]。由于在油菜和南瓜的韧皮部汁液中也检测到miR399[123, 126],因此推测长距离信号系统在不同植物种类中可能是保守的。采用嵌合基因(启动子-报告基因)的分析表明,miR399和PHO2主要在维管组织中表达[121]。嫁接研究证明,野生型与过表达miR399的拟南芥或烟草互相嫁接,接穗中的miR399能通过嫁接位点向野生型砧木移动并抑制砧木中PHO2的表达,从而激活Pi的吸收和运输[123, 127]。着生菌根的苜蓿植株,其叶片中成熟miR399及其前体含量均高于无菌根植株[125],并且部分成熟miR399可被转运至菌根中组成miR399-PHO2 信号途径。
由于miR399既可以在地上部表达、 也可以在根系表达,它在冠-根之间移动的生物学意义曾受到质疑。但时间动态分析表明,Pi缺乏条件下miR399在地上部的表达早于根系,因此它的系统移动确实属于Pi缺乏的早期响应[127]。
4 长距离反馈信号的作用靶标得到初步研究
地上部产生的系统反馈信号需要由根系感受器接收,然后引发一系列生理生化反应乃至根系形态变化对养分缺乏作出响应。但反馈信号的调控靶标、 相关调控网络的上、 下游组分研究总体上比较薄弱,目前仅有一些初步进展。
上文提到的PHO2被鉴定为miR399的下游靶标[121-122]。Pi缺乏条件下,miR399的上调抑制了PHO2的表达,解除了PHO2对Pi吸收的抑制。pho2突变体的Pi吸收和运输受到促进,导致地上部过量积累Pi出现Pi中毒现象[71, 121]。已知miR399 在Pi饥饿信号途径中的上游元件可能为转录因子PHR1[122],同为反馈信号的蔗糖也位于miR399 的上游[128]。PHO2的下游分子元件尚不清楚,推测泛素介导的蛋白质水解或功能修饰可能参与其中[13]。
5 研究展望
至今,人们对养分长距离反馈调节机制的了解还很有限。已知的养分/代谢物信号是直接调控根系吸收能力还是通过激发其他次级信号发挥调节作用尚不可知。此外,笔者推测抑制信号和促进信号共存比较接近反馈调节的本质,但目前缺乏足够的试验证据。
因此,鉴定潜在的长距离信号物质、 揭示其作用模式仍然是养分吸收反馈调节领域需要面对的长期挑战,应用养分感受或信号传导途径发生缺陷的突变体有望加快这方面的进展。另外,反馈信号的产生部位、 运输机制、 作用靶标及此后的调控网络都是该领域未来的研究重点。
参考文献:
[1] Drew M C. Comparison of the effects of a localized supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley[J]. New Phytologist, 1975, 75(3): 479-490.
[2] Zhang H, Jennings A, Barlow P Wetal. Dual pathways for regulation of root branching by nitrate[J]. Proceedings of the National Academy of Science of the United States of America, 1999, 96(11): 6529-6534.
[3] Ruffel S, Krouk G, Ristova Detal. Nitrogen economics of root foraging: Transitive closure of the nitrate-cytokinin relay and distinct systemic signaling for N supply vs. demand[J]. Proceedings of the National Academy of Science of the United States of America, 2011, 108(45): 18524-18529.
[4] Vance C P, Uhde-Stone C, Allan D L. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource[J]. New Phytologist, 2003, 157(3): 423-447.
[5] Lynch J P. Root phenes for enhanced soil exploration and phosphorus aiquisition: tools for future crops[J]. Plant Physiology, 2011, 156(3): 1041-1049.
[6] Peret B, Clement M, Nussaume Letal. Root developmental adaptation to phosphate starvation: better safe than sorry[J]. Trends in Plant Science, 2011, 16(8): 442-450.
[7] Zhuo D, Okamoto M, Vidmar J Jetal. Regulation of a putative high-affinity nitrate transporter (Nrt2; 1At) in roots ofArabidopsisthaliana[J]. Plant Journal, 1999, 17(5): 563-568.

[9] Rouached H, Wirtz M, Alary Retal. Differential regulation of the expression of two high-affinity sulfate transporters, SULTR1.1 and SULTR1.2, in Arabidopsis[J]. Plant Physiology, 2008, 147(2): 897-911.
[10] Lough T J, Lucas W J. Integrative plant biology: Role of phloem long-distance macromolecular trafficking[J]. Annual Review of Plant Biology, 2006, 57: 203-232.
[11] Girin T, EI-Kafafi E S, Widiez Tetal. Identification of Arabidopsis mutants impaired in the systemic regulation of root nitrate uptake by the nitrogen status of the plant[J]. Plant Physiology, 2010, 153(3): 1250-1260.
[12] Ho C H, Lin S H, Hu H Cetal. CHL1 functions as a nitrate sensor in plants[J]. Cell, 2009, 138: 1184-1194.
[13] Chiou T J, Lin S I. Signaling network in sensing phosphate availability in plants[J]. Annual Review of Plant Biology, 2011, 62: 185-206.
[14] Liu L L, Ren H M, Chen L Qetal. A protein kinase, calcineurin B-like protein-interacting protein kinase9, interacts with calcium sensor calcineurin B-like protein3 and regulates potassium homeostasis under low-potassium stress in Arabidopsis[J]. Plant Physiology, 2013, 161(1): 266-277.
[15] Remans T, Nacry P, Pervent Metal. The Arabidopsis NRT1.1 transporter participates in the signaling pathway triggering root colonization of nitrate-rich patches[J]. Proceedings of the National Academy of Science of the United States of America, 2006, 103(50): 19206-19211.
[16] Gojon A, Krouk G, Perrine-Walker Fetal. Nitrate transceptor(s) in plants[J]. Journal of Experimental Botany, 2011, 62(7): 2299-2308.
[17] Krouk G, Lacombe B, Bielach Aetal. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants[J]. Developmental Cell, 2010, 18(6): 927-937.
[18] Schmidt W. Iron solutions: acquisition strategies and signaling pathways in plants[J]. Trends in Plant Science, 2003, 8(4): 188-193.
[19] Desnos T. Root branching responses to phosphate and nitrate[J]. Current Opinion in Plant Biology, 2008, 11(1): 82-87.
[20] Hermans C, Hammond J P, White P Jetal. How do plants respond to nutrient shortage by biomass allocation[J]. Trends in Plant Science, 2006, 11(12): 610-617.
[21] Gojon A, Nacry P, Davidian J C. Root uptake regulation: A central process for NPS homeostasis in plants[J]. Current Opinion in Plant Biology, 2009, 12(3): 328-338.
[22] Liu T Y, Chang C Y, Lin T J. The long-distance signaling of mineral macro-nutrients[J]. Current Opinion in Plant Biology, 2009, 12(3): 312-319.
[23] Martinez-Cordero M A, Martinez V, Rubio F. High-affinity K+uptake in pepper plants[J]. Journal of Experimental Botany, 2005, 56(416): 1553-1562.
[24] Ruffel S, Freixes S, Balzergue Setal. Systemic signaling of the plant nitrogen status triggers specific transcriptome responses depending on the nitrogen source inMedicagotruncatula[J]. Plant Physiology, 2008, 146(4): 2020-2035.

[26] Drew M C, Saker L R. Nutrient supply and the growth of the seminal root system in barley. II. Compensatory increases in growth of lateral roots and in tates of phosphate uptake in response to a localized supply of phosphate[J]. Journal of Experimental Botany, 1978, 29(2): 435-451.
[27] Lefebvre D D, Glass A D M. Regulation of phosphate influx in barley roots: effects of phosphate deprivation and reduction of influx with provision of orthophosphate[J]. Physiologia Plantarum, 1982, 54(2): 199-206.
[28] Liu C, Muchhal U S, Uthappa Metal. Tomato phosphate transporter gene are differentially regulated in plant tissues by phosphorus[J]. Plant Physiology, 1998, 116(1): 91-99.
[29] Chiou T-J, Liu H, Harrison M J. The spatial expression patterns of a phosphate transporter (MtPT1) fromMedicagotruncatulaindicate a role in phosphate transport at the root/soil interface[J]. Plant Journal, 2001, 25(3): 281-293.
[30] Smith F W, Mudge S R, Rae A Letal. Phosphate transport in plants[J]. Plant and Soil, 2003, 248(1-2): 71-83.
[31] Poirier Y, Thoma S, Somerville Cetal. A mutant of Arabidopsis deficient in xylem loading of phosphate[J]. Plant Physiology, 1991, 97(3): 1087-1093.
[32] Burleigh S H, Harrison M J. The down-regulation of Mt4-like genes by phosphate fertilization occurs systemically and involves phosphate translocation to the shoots[J]. Plant Physiology, 1999, 119(1): 241-248.
[33] Martin A C, del Pozo J, Iglesias Jetal. Influence of cytokinins on the expression of phosphate starvation responsive genes in Arabidopsis[J]. Plant Journal, 2000, 24(5): 559-567.
[34] Shane M W, Lambers H. Systemic suppression of cluster-root formation and net P-uptake rates inGrevilleacrithmifoliaat elevated P supply: a proteacean with resistance for developing symptoms of ‘P toxicity’[J]. Journal of Experimental Botany, 2006, 57(2): 413-423.
[35] Drew M C, Saker L R. Uptake and long-distance transport of phosphate, potassium and chloride in relation to internal ion concentrations in barley: evidence of non-allosteric regulation[J]. Planta, 1984, 160(6): 500-507.
[36] Drew M C, Webb J, Saker L R. Regulation of K+uptake and transport to the xylem in barley roots: K+distribution determined by probe X-ray microanalysis of frozen-hydrated cells[J]. Journal of Experimental Botany, 1990, 41(7): 815-825.
[37] Engels C, Marschner H. Root to shoot translocation of macronutrient in relation to shoot demand in maize (ZeamaysL.) grown at different root zone temperatures[J]. Journal of Plant Nutrition and Soil Science, 1992, 155(2): 121-128.
[38] Li B, Wang Y, Zhang Z Yetal. Cotton shoot plays a major role in mediating senescence induced by potassium deficiency[J]. Journal of Plant Physiology, 2012, 169(4): 327-335.
[39] 王晔, 田晓莉. 棉花幼苗钾吸收的系统反馈调节初步研究[J]. 作物学报, 2013, 39(10): 1843-1848.
Wang Y, Tian X L. Systematic feedback regulation of K+uptake in cotton at seedling stage[J]. Acta Agronomica Sinica, 2013, 39(10): 1843-1848.
[40] Schmidt W, Boomgaarden B, Ahrens V. Reduction of root iron inPlantagolanceolataduring recovery from Fe deficiency[J]. Physiologia Plantarum, 1996, 98(3): 587-593.
[41] Li C, Zhu X, Zhang F. Role of shoot in regulation of iron deficiency responses in cucumber and bean plants[J]. Journal of Plant Nutrition, 2000, 23(11-12): 1809-1818.
[42] Schikora A, Schmidt W. Iron stress-induced changes in root epidermal cell fate are regulated independently from physiological responses to low iron availability[J]. Plant Physiology, 2001, 125(4): 1679-1687.
[43] Vert G A, Briat J F, Curie C. Dual regulation of theArabidopsishigh-affinity root iron uptake system by local and long-distance signals[J]. Plant Physiology, 2003, 132(2): 796-804.
[44] Schmidt W, Michalke W, Schikora A. Proton pumping by tomato roots. Effect of Fe deficiency and hormones on the activity and distribution of plasma membrane H+-ATPase in thizodermal cells[J]. Plant Cell and Environment, 2003, 26(3): 361-370.
[45] Wu T, Zhang H, Wang Yetal. Induction of root Fe (Ⅲ) reductase activity and proton extrusion by iron deficiency is mediated by auxin-based systemic signalling in Malusxiaojinensis[J]. Journal of Experimental Botany, 2012, 63(2): 859-870.
[46] Kabir A H, Paltridge N G, Roessner Uetal. Mechanisms associated with Fe-deficiency tolerance and signaling in shoots ofPisumsativum[J]. Physiologia Plantarum, 2012, 147(3): 381-395.
[47] Durrett T P, Gassmann W, Rogers E E. The FRD3-mediated efflux of citrate into the root vasculature is neessary for efficient iron translocation[J]. Plant Physiology, 2007, l44(1): 197-205.
[48] Delhaize E. A metal-accumulator mutant ofArabidopsisthaliana[J]. Plant Physiology, 1996, 111(3): 849-855.
[49] Yi Y, Guerinot M L. Genetic evidence that induction of root Fe (Ⅲ) chelate raductase activity is necessary for iron uptake under iron deficiency[J]. Plant Journal, 1996, 10(5): 835-844.
[50] Rogers E E, Guerinot M L. FRD3, a member of the multidrug and toxin efflux family, controls iron deficiency responses in Arabidopsis[J]. Plant Cell, 2002, 14(8): 1787-1799.
[51] Landsberg E C. Organic acid synthesis and release of hydrogen ions in response to Fe deficiency stress of mono- and dicotyledonous plant species[J]. Journal of Plant Nutrition, 198l, 3(1-4): 579-591.
[52] Lappartient A G, Vidmar J J, Leustek Tetal. Touraine B. Inter-organ signaling in plants: regulation of ATP sulfurylase and sulfate transporter genes expression in roots mediated by phloem-translocated compound[J]. Plant Journal, 1999, 18(1): 89-95.
[53] Jeudy C, Ruffel S, Freixes Setal. Adaptation ofMedicagotruncatulato nitrogen limitation is modulated via local and systemic nodle developmental responses[J]. New Phytologist, 2010, 185(3): 817-828.
[54] Miller A J, Fan X, Shen Qetal. Amino acids and nitrate as signals for the regulation of nitrogen acquisition[J]. Journal of Experimental Botany, 2008, 59(1): 111-119.
[55] Grusak M A, Pezeshgi S. Shoot-to-root signal transmission regulates root Fe (Ⅲ) reductaseactivity in thedglmutant of pea[J]. Plant Physiology, 1996, 110(1): 329-334.
[56] Enomoto Y, Hodoshima H, Shimada Hetal. Long-distance signals positively regulate the expression of iron uptake genes in tobacco roots[J]. Planta, 2007, 227(1): 81-89.
[57] Stacey M G, Patel A, McClain W Eetal. TheArabidopsisAtOPT3 protein functions in metal homeostasis and movement of iron to developing seeds[J]. Plant Physiology, 2008, 146(2): 589-601.
[58] Garcia M J, Romera F J, Stacey M Getal. Shoot to root communication is necessary to control the expression of iron-acquisition genes in Strategy I plants[J]. Planta, 2012, 237(1): 65-75.
[59] Lucena C, Waters B M, Romera F Jetal. Ethylene could influence ferric reductase, iron transporter, and H+-ATPase gene expression by affectingFER(orFER-like) gene activity[J]. Journal of Experimental Botany, 2006, 57(15): 4145-4154.
[60] Cooper H D, Clarkson D T. Cycling of amino-nitrogen and other nutrients between shoots and roots in cereals? Possible mechanism integrating shoot and root in the regulation of nutrient uptake[J]. Journal of Experimental Botany, 1989, 40(7): 753-762.
[61] Imsande J, Touraine B. N demand and the regulation of nitrate uptake[J]. Plant Physiology, 1994, 105(1): 3-7.
[62] Miller A J, Fan X, Orsel Metal. Nitrate transport and signaling[J]. Journal of Experimental Botany, 2007, 58(9): 2297-2306.

[64] Forde B G. Nitrate transporters in plants: structure, function and regulation[J]. Biochimica et Biophysica Acta-Biomembranes, 2000, 1465(1-2): 219-235.
[65] Forde B G, Lea P J. Glutamate in plants: metabolism, regulation, and signaling[J]. Journal of Experimental Botany, 2007, 58(9): 2339-2358.
[66] Gifford M L, Dean A, Gutierrez R Aetal. Cell-specific nitrogen responses mediate developmental plasticity[J]. Proceedings of the National Academy of Science of the United States of America, 2008, 105(2): 803-808.
[67] Beuve N, Rispail N, Laine Petal. Putative role of r-aminobutyric acid (GABA) as a long-distance signal in up-regulation of nitrate uptake inBrassicanapusL[J]. Plant Cell and Environment, 2004, 27: 1035-1046.
[68] Kinnersley A M, Lin F. Receptor modifiers indicate that 4-aminobutyric acid (GABA) is a potential modulator of ion transport in plants[J]. Plant Growth Regulation, 2000, 32(1): 65-76.
[69] Forde B G. Local and long-range signaling pathways regulating plant responses to nitrate[J]. Annual Review of Plant Biology, 2002, 53: 203-224
[70] Franco-Zorrilla J M, Martin A C, Leyva Aetal. Interaction between phosphate-starvation, sugar and cytokinin signaling in Arabidopsis and the role of cytokinin receptors CRE1/AHK4 and AHK3[J]. Plant Physiology, 2005, 138(2): 847-857.
[71] Delhaize E, Randall P. Characterization of a phosphate-accumulator mutant ofArabidopsisthaliana[J]. Plant Physiology, 1995, 107(1): 207-213.
[72] Maas F M, van de Wetering D A, van Beusichem M Letal. Characterization of phloem iron and its possible role in the regulation of Fe-efficiency reactions[J]. Plant Physiology, 1988, 87(1): 167-171.
[73] Stacey M G, Patel A, McClain W Eetal. The Arabidopsis AtOPT3 protein functions in metal homeostasis and movement of iron to developing seeds[J]. Plant Physiology, 2008, 146(2): 589-601.
[74] Ling H Q, Koch G, Baumleiin Hetal. Map-based cloning of chloronerva, a gene involved in iron uptake of higher plants encoding nicotianamine synthase[J]. Proceedings of the National Academy of Science of the United States of America, 1999, 96(12): 7098-7103.
[75] von Wiren N, Klair S, Bansal Setal. Nicotianamine chelates both Fe (Ⅲ) and Fe (Ⅱ). Implications for metal transport in plants[J]. Plant Physiology, 1999, 119(3): 1107-1114.
[76] Pich A, Manteuffel R, Hillmer Setal. Fe homeostasis in plant cells: does nicotianamine play multiple roles in the regulation of cytoplasmic Fe concentration[J]. Planta, 2001, 213(6): 967-976.
[77] Koike S, Inoue H, Mizuno Detal. OsYSL2 is a rice metal-nicotianamine transporter that is regulated by iron and expressed in the phloem[J]. Plant Journal, 2004, 39(3): 415-424.
[78] Gottschalk W G. Improvement of the selection value of genedglthrough recombination[J]. Pisum Newslett, 1987, 19: 9-11.
[79] Marentes E, Stephens B W, Grusak M A. Characterization of a phloem mobile chelator involved in the phloem transport of iron from vegetative tissues to developing seeds of pea[C]. Iron Nutrition and Interactions in Plants Symposium, 1997.
[80] Marentes E, Grusak M A. Iron transport and storage within the seed coat and embryo of developing seeds of pea (PisumsstivumL.)[J]. Seed Science Research, 1998, 8(3): 367-375.
[81] Pitman M G. Uptake and transport of ions in barley seedlings. Ⅲ.Correlation between transport to the shoot and relactive growth rate[J]. Australian Journal of Biological Sciences, 1972, 25(5): 905-920.
[82] Hammond J P, White P J. Sucrose transport in the phloem: integrating root responses to phosphorus starvation[J]. Journal of Experimental Botany, 2008, 59(1): 93-109.
[83] Liu J, Samac D A, Bucciarelli Betal. Signaling of phosphorus dificiency-induced gene expression in white lupin requires sugar and phloem transport[J]. Plant Journal, 2005, 41(2): 257-268.
[84] Zakhleniuk O V, Raines C A, Lioyd J C. Pho3: a phosphorus deficient mutant ofArabidopsisthaliana(L.) Heynh[J]. Planta, 2001, 212(4): 529-534.
[85] Lioyd J C, Zakhleniuk O V. Responses of primary and secondary metabolism to sugar accumulation revealed by microarray expression analysis of theArabidopsismutant,pho3[J]. Journal of Experimental Botany, 2004, 55(400): 1221-1230.
[86] Lei M, Liu Y, Zhang Betal. Genetic and genomic evidence that sucrose is a global regulator of plant responses to phosphate starvation in Arabidopsis[J]. Plant Physiology, 2011, 156(3): 1116-1130.
[87] Muller R, Morant M, Jarmer Hetal. Genome-wide analysis of the Arabidopsis leaf transcriptome reveals interaction of phosphate and sugar metabolism[J]. Plant Physiology, 2007, 143(1): 156-171.
[88] Zhou K, Yamagishi M, Osaki Metal. Sugar signalling mediates cluster root formation and phosphorus starvation-induced gene expression in white lupin[J]. Journal of Experimental Botany, 2008, 59(10): 2749-2756.
[89] Zinn K E, Liu J, Allan D Letal. White lupin (Lupinusalbus) response to phosphorus stress: evidence for complex regulation of LaSAP1[J]. Plant and Soil, 2009, 322(1-2): 1-15.
[90] Lejay L, Gansel X, Cerezo Metal. Regulation of root ion transporters by photosynthesis: functional importance and relation with hexokinase[J]. Plant Cell, 2003, 15(9): 2218-2232.
[91] Lejay L, Wirth J, Pervent Metal. Oxidative pentose phosphate pathway-dependent sugar sensing as a mechanism for regulation of root ion transporters by photosynthesis[J]. Plant Physiology, 2008, 146(4): 2036-2053.
[92] Takei K, Sakakibara H, Taniguchi Metal. Nitrogen-dependent accumulation of cytokinins in root and the translocation to leaf: Implication of cytokinin species that induces gene expression of maize response regulator[J]. Plant and Cell Physiology, 2001, 42(1): 85-93.

[94] Sakakibara H, Takei K, Hirose N. Interactions between nitrogen and cytokinin in the regulation of metabolism and development[J]. Trends in Plant Science, 2006, 11(9): 440-448.
[95] Kiba T, Kudo T, Kojima Metal. Hormonal control of nitrogen acquisition: roles of auxin, abscisic acid, and cytokinin[J]. Journal of Experimental Botany, 2011, 62(4): 1399-1409.
[96] Rubio V, Bustos R, Irigoyen M Letal. Plant hormones and nutrient signaling[J]. Plant Molecular Biology, 2009, 69(4): 361-373.
[97] Krouk G, Ruffel S, Gutierrez R Aetal. A framework integrating plant growth with hormones and nutrients[J]. Trends in Plant Science, 2011, 16(4): 178-182.
[98] Werner T, Motyka V, Laucou Vetal. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity[J]. Plant and Cell, 2003, 15(11): 2532-2550.
[99] Higuchi M, Pischke M S, Mahonen A Petal. In planta functions of theArabidopsiscytokinin receptor family[J]. Proceedings of the National Academy of Science of the United States of America, 2004, 101(23): 8821-8826.
[100] Miyawaki K, Tarkowski P, Matsumoto-Kitano Metal. Roles of Arabidopsis ATP/ADP isopentenyltransperases and tRNA isopentenyltransferases in cytokinin biosynthesis[J]. Proceedings of the National Academy of Science of the United States of America, 2006, 103(44): 16598-16603.
[101] Laplaze L, Benkova E, Casimiro Ietal. Cytokinins act directly on lateral root founder cells to inhibit root initiation[J]. Plant Cell, 2007, 19(12): 3889-3900.
[102] Wang X, Yi K, Tao Yetal. Cytokinin represses phosphate-starvation response through increasing of intracellular phosphate level[J]. Plant Cell and Environment, 2006, 29(10): 1924-1935.
[103] Walch-Liu P, Ivanov I I, Filleur Setal. Nitrogen regulation of root branching[J]. Annals of Botany, 2006, 97(5): 875-881.
[104] Lopez-Bucio J, Hernandez-Abreu E, Sanchez-Calderon Letal. An auxin transport independent pathway is involved in phosphate stress-induced root architectural alterations in Arabidopsis. Identification of BIG as a mediator of auxin in pericycle cell activation[J]. Plant Physiology, 2005, 137(2): 681-691.
[105] Nacry P, Canivenc G, Muller Betal. A role for auxin redistribution in the responses of the root system architecture to phosphate starvation in Arabidopsis[J]. Plant Physiology, 2005, 138(4): 2061-2074.
[106] Chen W W, Yang J L, Qin Cetal. Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis[J]. Plant Physiology, 2010, 154(2): 810-819.
[107] Jin C W, He X X, Zheng S J. The iron-deficiency induced phenolics accumulation may involve in regulation of Fe (Ⅲ) chelate reductase in red clover[J]. Plant Signaling and Behavior, 2007, 2(5): 327-332.
[108] Wu T, Zhang H, Wang Yetal. Induction of root Fe (Ⅲ) reductase activity and proton extrusion by iron deficiency is mediated by auxin-based systemic signalling inMalusxiaojinensis[J]. Journal of Experimental Botany, 2012, 63(2): 859-870.
[109] Schmidt W, Tittel J, Schikira A. Role of hormones in the induction of iron deficiency responses in Arabidopsis roots[J]. Plant Physiology, 2000, 122(4): 1109-1118.
[110] Bacaicoa E, Mora V, Zamarreno A Metal. Auxin: a major player in the shoot-to-root regulation of root Fe-stress physiological responses to Fe deficiency in cucumber plants[J]. Plant Physiology and Biochemistry, 2011, 49(5): 545-556.
[111] Buhtz A, Springer F, Chappell Letal. Identification and characterization of small RNAs from the phloem ofBrassicanapus[J]. Plant Journal, 2008, 53(5): 739-749.
[112] Kehr J, Buhtz A. Long distance transport and movement of RNA through the phloem[J]. Journal of Experimental Botany, 2008, 59(1): 85-92.
[113] Jones-Rhoades M W, Bartel D P. Computational identification of plant microRNAs and their targets, including a stress-induced miRNA[J]. Molecular Cell, 2004, 14(6): 787-99.
[114] Chiou T J, Aung K, Lin S Ietal. Regulation of phosphate homeostasis by microRNA in Arabidopsis[J]. Plant Cell, 2006, 18(2): 412-421.
[115] Chiou T J. The role of microRNAs in sensing nutrient stress[J]. Plant Cell and Environment, 2007, 30(3): 323-332.
[116] Yamasaki H, Abdel-Ghany S E, Cohu C Metal. Regulation of copper homeostasis by micro-RNA in Arabidopsis[J]. Journal of Biological Chemistry, 2007, 282(22): 16369-16378.
[117] Buhtz A. Pieritz J, Springer Fetal. Phloem small RNAs, nutrient stress responses, and systemic mobility[J]. BMC Plant Biology, 2010, 10(1): 1-13.
[118] Kong W W, Yang Z M. Identification of iron-deficiency responsive microRNA genes and cis-elements in Arabidopsis[J]. Plant Physiology and Biochemistry, 2010, 48(2-3): 153-159.
[119] Vidal E A, Araus V, Lu Cetal. Nitrate-responsive miR393/AFB3 regulatory module controls root system architecture inArabidopsisthaliana[J]. Proceedings of the National Academy of Science of the United States of America, 2010, 107(9): 4477-4482.
[120] Fujii H, Chiou T J, Lin S Ietal. A miRNA involved in phosphate-starvation response in Arabidopsis[J]. Current Biology, 2005, 15(22): 2038-2043.
[121] Aung K, Lin S I, Wu C Cetal.Pho2, a phosphate overaccumlator, is caused by a nonsense mutation in a microRNA399 target gene. Plant Physiology, 2006, 141(3): 1000-1011.
[122] Bari R, Pant B D, Stitt Metal. PHO2, MicroRNA399,and PHR1define a phosphate signaling pathway in plants[J]. Plant Physiology, 2006, 141(3): 988-999.
[123] Pant B D, Buhtz A, Kehr Jetal. MicroRNA399 is a long-distance signal for the regulation of plant phosphate homeostasis[J]. Plant Journal, 2008, 53(5): 731-738.
[124] Wang X, Wang Y, Tian Jetal.Overexpressing AtPAP15 enhances phosphorus efficiency in soybean[J]. Plant Physiology, 2009, 151(1): 233-240.
[125] Branscheid A, Sieh D, Pant B Detal. Expression pattern suggests a role of miRNA399 in the regulation of the cellular response to local Pi increase during arbuscular mycorrhizal symbiosis[J]. Molecular Plant Microbe Interactions, 2010, 23(7): 915-926.
[126] Buhtz A, Springer F, Chappell Letal. Identification and characterization of small RNAs from the phloem ofBrassicanapus[J]. Plant Journal, 2008, 53(5): 739-749
[127] Lin S I, Chiang S F, Lin W Yetal. Regulatory network of microRNA399 andPHO2 by systemic signaling[J]. Plant Physiology, 2008, 147(2): 732-746.
[128] Liu J Q, Allan D L, Vance C P. Systemic signaling and local sensing of phosphate in common bean: cross-talk between photosynthate and microRNA399[J]. Molecular Plant, 2010, 3(2): 428-437.
[129] Li W, Wang Y, Okamoto Metal. Dissection of theAtNRT2.1:AtNRT2.2 inducible high-affinity nitrate transporter gene cluster[J]. Plant Physiology, 2007, 143(1): 425-433.
[130] Girin T, Lejay L, Wirth Jetal. Identification of a 150 bpcis-acting element of theAtNRT2.1 promoter involved in the regulation of gene expression by the N and C status of the plant[J]. Plant Cell and Environment, 2007, 30(11): 1366-1380.
