Performance of EDAB-HCl Acid Blended System as Fracturing Fluids in Oil Fields*
2014-03-25赵增迎吕国诚张以河LIANShengjiang连胜江田娜
(赵增迎)**(吕国诚)**(张以河) LIAN Shengjiang(连胜江)(田娜)
1School of Science, National Laboratory of Mineral Materials, China University of Geosciences (Beijing), Beijing 100083, China
2Research Institute of Petroleum Exploration and Development, PetroChina, Beijing 100083, China
Performance of EDAB-HCl Acid Blended System as Fracturing Fluids in Oil Fields*
ZHAO Zengying(赵增迎)1,**, LÜ Guocheng(吕国诚)1,**, ZHANG Yihe(张以河)1, LIAN Shengjiang(连胜江)2and TIAN Na(田娜)1
1School of Science, National Laboratory of Mineral Materials, China University of Geosciences (Beijing), Beijing 100083, China
2Research Institute of Petroleum Exploration and Development, PetroChina, Beijing 100083, China
Due to the high price and formation damage of the guargum fracturing fluid, many oilfields are more and more interested in surfactant based fracturing fluids. The rheological properties of erucicamide dimethyl amidopropyl betaine (EDAB)-HCl acid blended system and its suitability as fracturing fluid were investigated in this work. The effects of pH, concentration of EDAB, and temperature on the rheological properties of the blended system were studied. The results show that addition of EDAB improved the viscosity of the system from less than 10 mPa·s to about 400 mPa·s, which could retard the acid-rock reaction to about one half at 60 °C and one quarter at 90 °C comparing to straight HCl acid, suggesting that there is sufficient time for the blended fluid to react with formation rock when it is used as fracturing fluid in an oil field. Core flow tests demonstrated that the EDAB-acid blended fluid could divert itself from high permeability formation core to low permeability one, thus ensuring proper acid placement in the target reservoirs.
viscosity, surfactants, viscoelastic fluids, rheology
1 INTRODUCTION
Viscoelastic surfactant (VES) aqueous solutions have been studied for two decades [1-5]. Although there have been some studies on the viscoelastic characteristic of the aqueous solution of erucicamide dimethyl alkylamidopropyl betaine (EDAB) [6-8], the rheological properties of its HCl acid solution have not been systematically investigated, especially its feasibility as a fracturing fluid in an oil field. In aqueous solution, the surfactant molecules can form wormlike micelles and entangle to form transient network, which imparts viscoelastic properties to the solution [9-11]. Therefore, it can be used in fracturing fluids in oil fields. In the past five to ten years, the price of guar gel based fracturing fluids has risen from 500 USD to about 3500 USD per ton. Many researchers are looking for proper surfactant based fracturing fluid to replace the guar gel based one for the lower harm advantage of the former as another reason.
This work reported the rheological properties of the EDAB-HCl blended system. The results of core flow test and its reaction rates with formation of carbonate were also provided.
2 MATERIALS AND METHODS
2.1 Materials and instrumentation
The zwitterionic surfactant (EDAB) was prepared in our laboratory [12]. The surfactant has good solubility, foaming ability, thickening property and viscoelasticity in water. The EDAB-HCl blended system comprised of EDAB surfactant with mass content from 1% to 7% (similarly hereinafter), HCl with mass concentration from 10% to 20%, and corrosion inhibitor which is composed of 0.3% (by mass) aliphatic alcohol polyglycol ether and 0.3% (by mass) aromatic ketone amine derivative. The carbonate cores has about 70% (by mass) magnesium carbonate and 10% (by mass) calcium carbonate, and were finely ground to 150 μm (100 mush). The average carbonate mass content was 82.8%. The rheological properties were determined using the Rheostess-600 rheometer (Thermo Haake Inc., Germany) and CSL2-500 rheometer (TA Instruments Inc., USA).
2.2 Experimental procedures
The static and dynamic rheological experiments were performed on the Rheostess-600 rheometer (a cylinder rotor with radius of 14 mm and height of 42 mm, and cup radius of 15 mm) and CSL2-500 rheometer (a cone-and-plate rotor with diameter of 40 mm, cone angle of 2°), respectively. The temperature was controlled to ±0.1 °C. Finely ground formation carbonate (as described in Section 2.1) was added to the EDAB acid blended system, and the pH values were calculated by the hydrogen ion concentrations in the system which were determined by acid and alkali titration test. The acid-carbonate reaction rate was determined by measuring the mass difference of the carbonate cores before and after the reaction.
The schematic of the equipment was depicted in Fig. 1. The rotating speed was 500 r·min−1, which issimilar to the flow condition in oil field when the acidification displacement is 4 m3·min−1. The carbonate cores, with diameter of 2.5 cm and 5 cm in length, were collected from Daqing Oil Field in northeastern China. Their average carbonate mass content was 82.8% and the average permeability is 0.22 mD (1 mD=0.9869×10−15m2).

Figure 1 Schematic diagram of experimental equipment for acid-carbonate reaction 1—rotation speed sensor; 2—electric motor; 3—pressure sensor; 4—temperature sensor; 5—condensate; 6—main reactor; 7—core holder; 8—core; 9—reaction fluid; 10—valve; 11—pressure controlled acid flow direction; 12—high pressure N2; 13—secondary reactor; 14—HCl acid solution or the blended fluid
3 RESULTS AND DISCUSSION
3.1 Viscoelasticity performance
The viscoelastic performance of the blended system was evaluated by small amplitude oscillation because the system would become a gel after the acid-rock reaction. In a pure viscous system, storage modulus G′is equal to zero, whereas in a pure elastomer, the loss modulus G″ is zero [13]. In the EDAB-acid blended system, both G′ and G″ have numerical values. In general, they increase with the increasing oscillation frequency and the pH value of the system (Figs. 2 and 3). When the oscillation frequency is more than 10 rad·s−1, G″ has obvious attenuation at high pH. The dynamic rheological response at pH of 0.01 is presented in Fig. 4. The high frequency region is characterized by the fact that G′ is higher than G″, so the EDAB-HCl system behaves elastically; but at a low frequency region, G″ is higher than G′, so it behaves viscously [14-17]. Both moduli depend on frequency but follow different patterns. This region is characteristic of the occurrence of physical entanglements [18].
3.2 Viscosities of the blended fluid at different pH

Figure 2 The storage modulus G′ of the EDAB-acid blended system at different pH values [The base conditions: blended system contained 5% (by mass) of EDAB and 15% (by mass) of HCl, shear rate: 170 s−1, temperature: 20 °C. Similar conditions apply hereinafter if no special instruction]
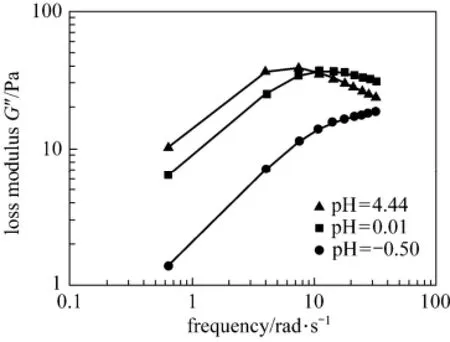
Figure 3 The loss modulus G″ of the blended system at different pH values

Figure 4 Rheology of the blended system when pH equals to 0.01
During the reaction between the EDAB-acid fluid and formation carbonate, the fluid viscosity increased from less than 10 mPa·s to about 400 mPa·s when the pH value changed from −1.0 to 9.0 (Fig. 5). The positively charged nitrogen groups on the EDAB surfactant cause the molecules to repel one another at low pH; the nitrogen-nitrogen repulsion is mitigated by negatively charged surfactant at higher pH. Therefore, the pH value is an important factor affecting the rheological performance of the system fluids. Surfactant molecules will form spherical micelles if their concentration is above critical micelle concentration (CMC) [19-21]. Besides, in the acid-rock reaction, when the pH value of the reaction fluid increases, and COOH groups in the surfactant molecule will release H−to become[22-25]. At the same time,carbonaterock releases Ca2+.Then, Ca2+connects to the COO−anions in the surfactant molecules under electrostatic attraction. Therefore, the surfactant molecules connect with each other by Ca2+in the system. In this case, the oleophilic radicals (the long carbon chain in the surfactant) align with each other making the molecules aggregate to even a network [26, 27]. Therefore, the viscosity of the blended system increases rapidly to a very high value compared to the viscosity before the reaction. The viscosity can be comparable with the viscosity of guar fracturing fluids widely used in oil fields at present. Moreover, the blended fluid system can greatly simplify the fracture operation and reduce the cost, for it avoids the addition of crosslinker and internal breaker in the guar fracturing fluids [28].
3.3 Viscosity of the blended system at different temperatures
Generally, the change of viscosity with temperature is attributed to the formation of surfactant micelles [29-34]. However, there is no a clear explanation about why the viscosity rises to a maximum, then falls as temperature is increased as shown in Fig. 6. Ahmed and Aramaki [35] explained that at lower temperature, the viscosity rises with temperature is attributed to mainly the increase in average micelle contour length due to curvature reduction with temperature. On the other hand, the following drop of the viscosity is due to the predominance of the junctions with rise in temperature [35]. These junctions are quite fluid, and can slide along the micellar contour. This motion provides a very fast stress relaxation mechanism, that is, the relaxation time is quite short. Therefore, the viscosity is also low.
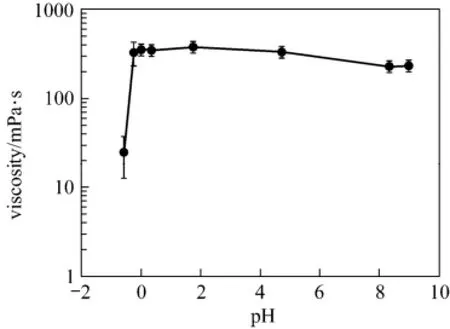
Figure 5 The viscosity of the blended system and pH (shear rate: 50 s−1)
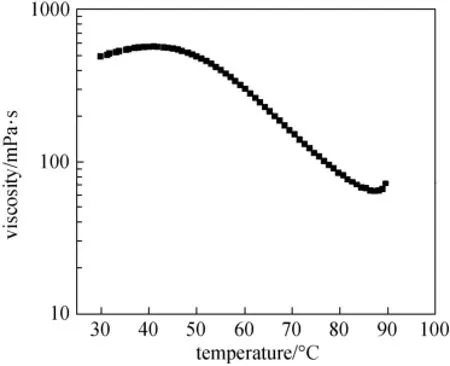
Figure 6 The viscosity of the blended system and temperature
3.4 Viscosity variation with EDAB concentration in the system
From Fig. 7, it seems that the optimal surfactant mass content in the blended system is 5% for practical applications.
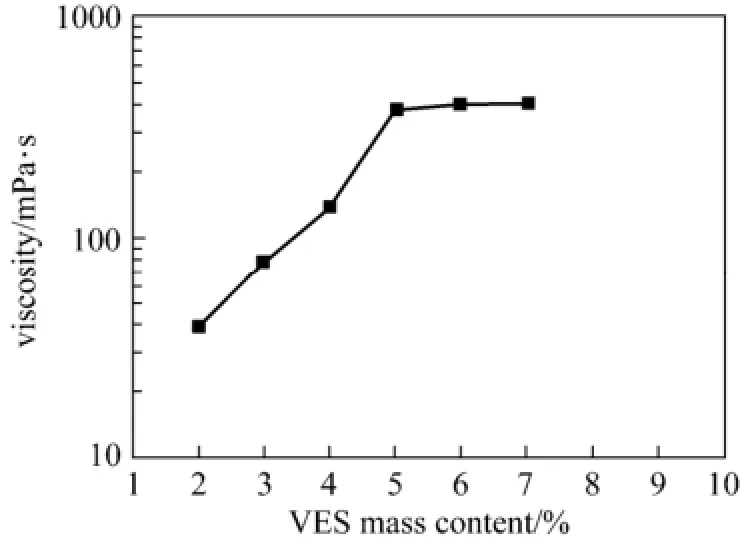
Figure 7 Viscosity with VES content
3.5 Shear resistant capability
After persistent shearing for over 30 min, the blended system can still retain a fairly high viscosity (Fig. 8), which indicates that the blended system has shear resistant capability and is suitable for use in oilfield acidification.
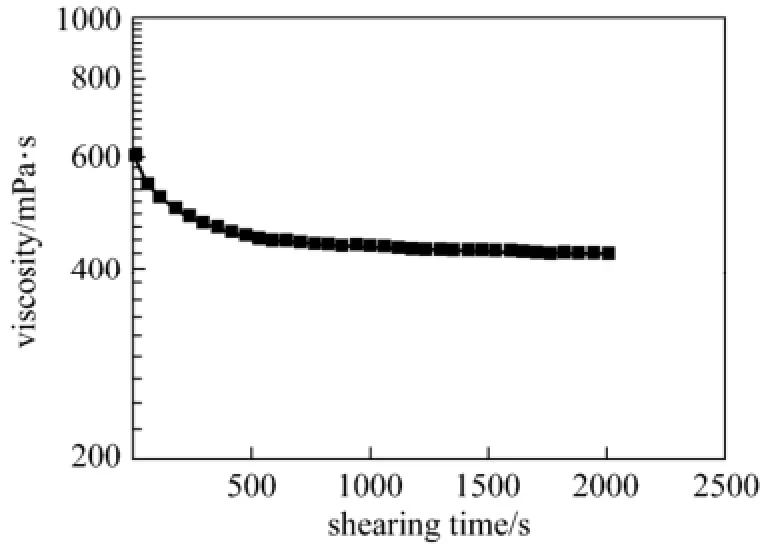
Figure 8 Shear resistance test

Figure 9 Acid-carbonate reaction rates
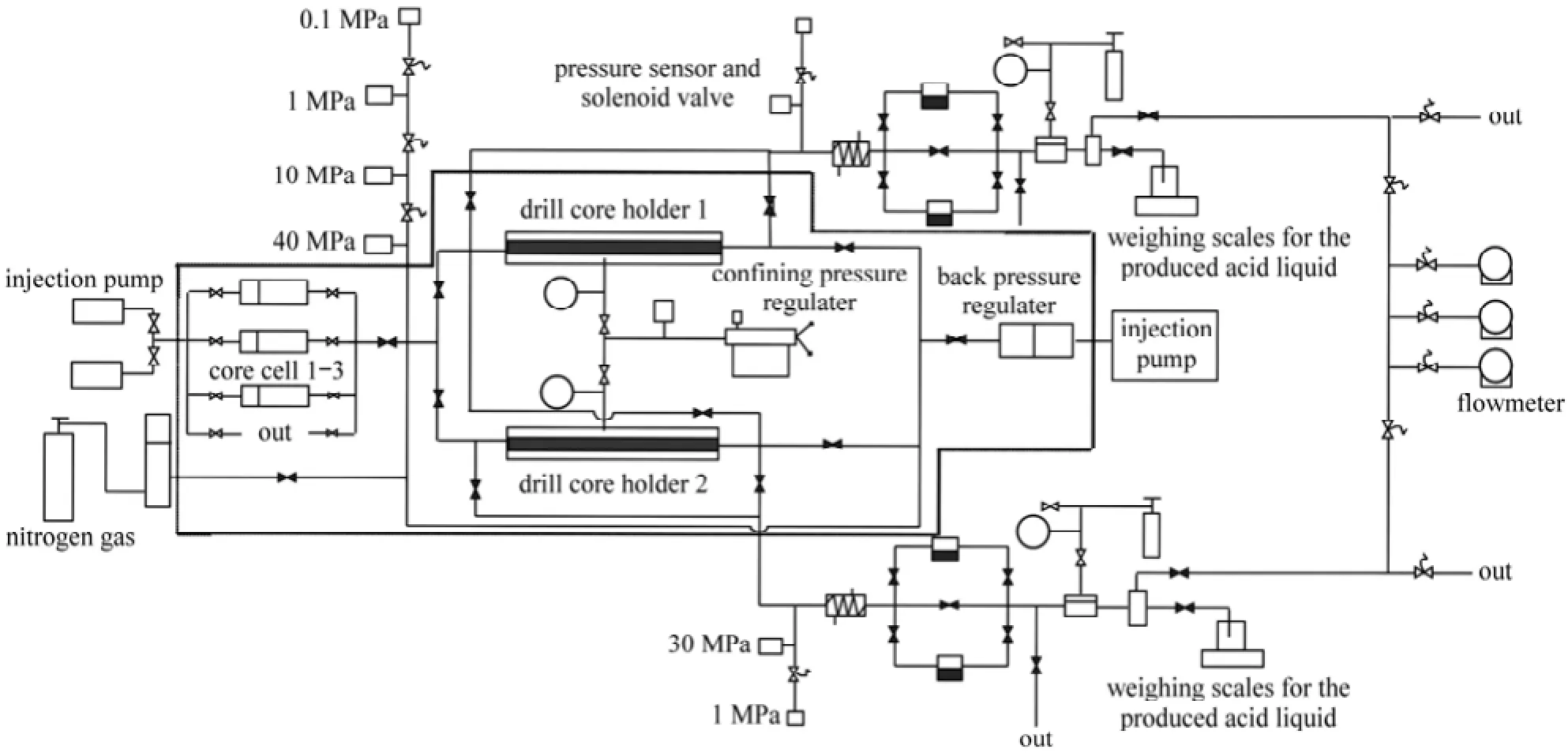
Figure 10 Schematic diagram for the multi-core flow apparatus
3.6 Reaction between the blended system and formation carbonate
Besides the rheological performance, the reaction rate between the EDAB-acid fluid and formation rock is also an important factor for the acidification process. The reaction rate can affect the shape and length of the flow channels in the formation etched by the injecting acid. The reaction between acid and formation carbonate is in a heterogeneous system, in which, gas is also produced. Fig. 9 shows that the viscosity of the system critically influences the reaction rate. This is because that the viscosity of the reaction fluid affects the diffusion rate of gases and ions when they move from the rock surface to the liquid phase [36]. Therefore, the addition of the surfactant can retard the acid-rock reaction when the viscosity increases during the reaction. The effect of surfactant addition is more effective than that of higher temperature. As a result, the longer acid-rock reaction time can ensure the blended acid fluid to etch longer and larger holes in the formation.
3.7 Multi-core flow tests
Figure 10 showed the multi-core flow apparatus and it also schematically illustrated the testing process. When 15% (by mass) HCl was injected into two carbonate cores placed side by side (Cores 1 and 2 in Table 1), the flat pressure profile indicated that no diversion took place (Fig. 11, Line a). It was because there was no obvious pressure increase in the curve. However, the obvious increased pressure was just the one required to divert the acid fluid from the high permeability core to low one. On the contrary, when the EDAB-acid blended fluid was injected into other two cores placed side by side (for example Cores 3 and 4 in Table 1), the pressure profile showed a rapid pressure increase as the fluid entered the cores. This was because the blended fluid would first enter the high permeability core, and react with the core, and then the fluid became viscous (referring to Section 3.2). The high viscousfluid blocked the high permeability core, and resulted in the rapid increase of pressure (Fig. 11, Line b). And then, the pressure forced the fluid flowed into the low permeability core. Therefore, the low permeability pores were also acidified, making the high permeability more uniformly distributed than the treatment by straight HCl fluid. Therefore, it can be deduced that the EDAB-acid blended fluid can divert itself from high permeability core to low one, which is just required for acidification and fracture in exploitation of oil.

Table 1 The permeability before and after the core flow test and acidification effect
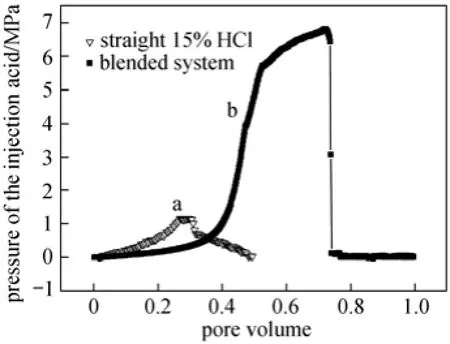
Figure 11 Multi-core flow tests of the pressure drop of the straight 15% (by mass) HCl and the surfactant blended system (Pore volume: the rate of the volume of acid entered the pore of the core to the porosity of the core)
4 CONCLUSIONS
The rheological properties and feasibility in oilfield acidification of the EDAB-HCl blended system were studied. The pH value of the blended system is the most important factor affecting the viscosity of the blended fluid. Small amplitude oscillation test demonstrated that the blended fluid is a type of viscoelastic fluid. The addition of EDAB can retarded the acid-rock reaction significantly. Core flow tests indicated that the blended fluid can divert itself from the high permeability reservoir core to low one. Therefore, the system is suitable for use in acidification and fracture for the exploitation of oil.
NOMENCLATURE
G′ storage modulus, Pa
G″ loss modulus, Pa
k0initial permeability, mD
krfinal permeability, mD
REFERENCES
1 Rami, A. R., Heinz, H., “The distinction of viscoelastic phases from entangled wormlike micelles”, Rheol. Acta., 45, 781-792 (2006).
2 Yoshida, T., Taribagil, R., Hillmyer, M.A., Lodge, T.P., “Viscoelastic synergy in aqueous mixtures of wormlike micelles and model amphiphilic triblock copolymers”, Macromolecules, 40, 1615-1623 (2007).
3 Sharma, S.C., Shrestha, L.K., Tsuchiya, K., Sakai, K., Sakai, H.,“Viscoelastic wormlike micelles of long polyoxyethylene chain phytosterol with lipophilic nonionic surfactant in aqueous solution”, J. Phys. Chem. B, 113, 3043-3050 (2009).
4 Couillet, I., Hughes, T., Maitland, G., Candau, F., Candau, S.J.,“Growth and scission energy of wormlike micelles formed by a cationic surfactant with long unsaturated tails”, Langmuir, 20, 9541-9550 (2004).
5 Bernhard, L., Chris, F., Mark, B., Matthew, M., Schlumberger, S.A., Kelly, H., “Diversion and cleanup studies of viscoelastic surfactant-based self-diverting Acid”, SPE (Society of Petroleum Engineers), 86504 (2004).
6 Sharma, S.C., Shrestha, R.G., Shrestha, L.K., Aramaki, K., “Viscoelastic wormlike micelles in mixed nonionic fluorocarbon surfactants and structural transition induced by oils”, J. Phys. Chem. B, 113, 1615-1622 (2009).
7 Moss, G.R., Rothstein, J.P., “Flow of wormlike micelle solutions through a periodic array of cylinders”, J. Non-Newt. Fluid Mech., 165, 1-3 (2010).
8 Kumar, R., Kalur, G.C., Ziserman, L., Dganit, D., Srinivasa, R.,“Wormlike micelles of a C22-tailed zwitterionic betaine surfactant: From viscoelastic solutions to elastic gels”, Langmuir, 23, 12849-12856 (2007).
9 Sharma, S.C., Shrestha, R.G., Shrestha, L.K., Aramaki, K.,“Rheological behavior of viscoelastic wormlike micelles in mixed sodium dodecyl trioxyethylene sulfate-monolaurin aqueous system”, Colloid Polym. Sci., 286, 1613-1619 (2008).
10 Huang, T.P., James, B.C., “Fluid-loss control improves performance of viscoelastic surfactant fluids”, SPE, 106227-MS (2008).
11 Chang, F., Qi, Q., Wayne, F., “A novel self-diverting-acid developed for matrix stimulation of carbonate reservoirs”, SPE, 65033 (2001).
12 Zhao, Z.Y., Lü, G.C., “Visco-elastic properties of VES diverting acid for carbonate reservoirs”, Chin. J. Chem. Eng., 18 (3), 511-514 (2010).
13 Hoffmann, H., “Viscoelastic surfactant solutions”, In: Structure and Flow in Surfactant Solutions, ACS Symposium Series, 578, Herb, C.A., Prudhomme, R.K., eds., American Chemical Society, Washington DC, 2-31 (1994).
14 Davies, T.S., Ketner, A.M., Raghavan, S.R., “Self-assembly of surfactant vesicles that transform into viscoelastic wormlike micelles upon heating”, J. Am. Chem. Soc., 128, 6669-6675 (2006).
15 Nasr-El-Din, H.A., Al-Habib, N.S., Al-Mumen, A.A., Jemmali, M., Samuel, M., “A new effective stimulation treatment for long horizontal wells drilled in carbonate reservoirs”, SPE, 86516 (2004).
16 Nasr-El-Din, H.A., Samuel, M., “Field application of emulsified acid-based system to stimulate deep, sour gas reservoirs in Saudi Arabia”, SPE, 71693 (2001).
17 Lungwitz, B., Fredd, C., Brady, M., Miller, M., Ali, S., Hughes, K.,“Diversion and cleanup studies of viscoelastic surfactant-based self-diverting acid”, SPE, 86504 (2004).
18 Ferry, J.D. Viscoelastic Properties of Polymers, 3rd edition., John Wiley & Sons Inc, New York, 56-59 (1980).
19 Scott, M., Qi, Q., Dan, V., “The successful use of polymer-free diverting agents for acid treatments in the Gulf of Mexico”, SPE, 73704 (2002).
20 Zeiler, C., Alleman, D., Qu, Q., “Use of viscoelastic surfactant-based diverting agents for acid stimulation”, SPE, 90062 (2004).
21 Chang, F., Acock, A., Geoghagan, A., “Experience in acid diversion in high permeability deep water formations using visco-elastic-surfactant”, SPE, 71691 (2001).
22 Danov, K.D., Kralchevska, S.D., Kralchevsky, P.A., “Mixed solutions of anionic and zwitterionic surfactant (betaine): Surface-tension isotherms, adsorption, and relaxation kinetics”, Langmuir, 20, 5445-5453 (2004).
23 Kato, K., Kondo, H., Morita, A., Esumi, K., Meguro, K., “Synthesis of polystyrene latex with amphoteric surfactant and its characterization”, Colloid Polym. Sci., 264, 737-742 (1986).
24 Weers, J.G., Rathman, J.F., Axe, F.U., Crichlow, C.A., “Effect of the intramolecular charge separation distance on the solution properties of betaines and sulfobetaines”, Langmuir, 7, 854-867 (1991).
25 Zana, R., “Dimeric and oligomeric surfactants behavior at interfaces and in aqueous solution: A review”, Adv. Colloid Interface Sci., 97, 205-253 (2002).
26 Imae, T., Ikeda, S., “Sphere-rod transition of micelles of tetradecyltrimethylammonium halides in aqueous sodium halide solutions and flexibility and entanglement of long rodlike micelles”, J. Phys. Chem., 90, 5216-5223 (1986).
27 Tse-Ve-Koon, K., Tremblay, N., Constantin, D., “Structure, thermodynamics and dynamics of the isotropic phase of spherical non-ionic surfactant micelles”, J. Colloid Interface Sci., 393, 161-173 (2013).
28 Yang, Y.Y., Xu, X.G., Yang, X.C., “Properties research on guar-crosslinked fracturing at different ph value”, Chem. Eng. Oil & Gas, 39 (5), 427-433 (2010).
29 Molchanov, V.S., Philippova, O.E., “Dominant role of wormlike micelles in temperature-responsive viscoelastic properties of their mixtures with polymeric chains”, J. Colloid Interface Sci., 394, 353-359 (2013).
30 Lu, Y.J., Fang, B., Fang, D.Y., “Formation and rheologic behavior of viscoelastic micelle fracturing fluids”, Oilfield Chem., 20 (4), 326-330 (2003). (in Chinese)
31 Shikata, T., Hirata, H., “Micelle formation of detergent molecules in aqueous media: Viscoelastic properties of aqueous cetyltrimethylammonium bromide solutions”, Langmuir, 3, 1081-1086 (1987).
32 Kern, F., Lemarechal, P., Candau, S.J., “Theological properties of semidilute and concentrated aqueous solutions of cetyltrimethylammonium bromide in the presence of potassium bromide”, Langmuir, 8, 437-440 (1992).
33 Pitkanen, I., Suuronen, J., Nurmi, J., “Partial molar volume, ionization, viscosity and structure of glycine betaine in aqueous solutions”, J. Solution Chem., 39, 1609-1626 (2010).
34 Ali, A., Makhlou, R., “Effect of organic salts on micellar growth and structure studied by rheology”, Colloid Polym. Sci., 277, 270-275 (1999).
35 Ahmed, T., Aramaki, K., “Temperature sensitivity of wormlike micelles in poly(oxyethylene) surfactant solution: Importance of hydrophilicgroup size”, J. Colloid Interface Sci., 336, 335-344 (2009).
36 Kasper, L., “Acidization. II The dissolution of dolomite in hydrochloric acid”, Chem. Eng. Sci., 30, 825-835 (1975).
Received 2012-03-20, accepted 2013-06-28.
* Supported by the Fundamental Research Funds for the Central Universities (2652013107), the Laboratory Open Funds of China University of Geosciences (Beijing).
** To whom correspondence should be addressed. E-mail: 1012278135@qq.com
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Effects of Solvent and Impurities on Crystal Morphology of Zinc Lactate Trihydrate*
- Optimization of Two-species Whole-cell Immobilization System Constructed with Marine-derived Fungi and Its BiologicalDegradation Ability*
- Numerical Simulation of Oxy-coal Combustion for a Swirl Burner with EDC Model*
- Kinetic and Thermodynamic Studies of Acid Scarlet 3R Adsorption onto Low-cost Adsorbent Developed from Sludge and Straw*
- Large-eddy Simulation of Ethanol Spray-Air Combustion and Its Experimental Validation*
- Synthesis of Sub-micrometer Lithium Iron Phosphate Particles for Lithium Ion Battery by Using Supercritical Hydrothermal Method
