A facile method to build a proton nanosensor with neutral to basic pH sensitive range∗
2014-03-07ZHONGRuiBo钟睿博LIUYuShuang刘雨双ZHANGPing张萍ZHAOGuoFen赵国芬andZHANGFeng张峰
ZHONG Rui-Bo(钟睿博),LIU Yu-Shuang(刘雨双),ZHANG Ping(张萍), ZHAO Guo-Fen(赵国芬),and ZHANG Feng(张峰),
1School of Life Sciences,Inner Mongolia Agricultural University,306 Zhaowuda Road,Hohhot 010018,China
A facile method to build a proton nanosensor with neutral to basic pH sensitive range∗
ZHONG Rui-Bo(钟睿博),1LIU Yu-Shuang(刘雨双),1ZHANG Ping(张萍),1ZHAO Guo-Fen(赵国芬),1and ZHANG Feng(张峰)1,†
1School of Life Sciences,Inner Mongolia Agricultural University,306 Zhaowuda Road,Hohhot 010018,China
Bio-nanosensors(Bio-NSs)have attracted much attention recently due to their unique properties.Among all of the bio-NSs,the intracellular proton sensor is significant for biomedicine studies and clinic diagnosis.Proton nanosensors(PNSs)with different pH sensitive ranges could satisfy different research requirements.Here we report a facile method to build a PNS with a neutral to basic pH sensitive range,in which the commercial pH indicator,fluoresceinamine(FA),was covalently coupled to the carboxylic-rich amphiphilic polymer(AP) coated gold nanoparticles(AuNPs).
Fluoresceinamine,Proton nanosensor,Fluorescence,Spectrometer,Amphiphilic polymer
I.INTRODUCTION
Nowadays,bio-nanosensors(bio-NSs)are opening new possibilitiessuitableforintracellularmeasurementsat submicron-sized dimensions.It is believed that building bio-NSs could provide great advances in our understanding of cellular function,thereby thoroughly revolutionizing cell biology[1].The ion channels imbedded in the cellular membrane control the transmembrane ion gradients and intercellularsignaling,sothationichomeostasisisparamountforliving and that any ionic perturbation could be a hallmark of disease. For example,tumor cells enjoy lower pH environments,while the multi-drug resistant cells like higher pH surroundings[2]. Hence,engineering proton nanosensors to sense intracellular pH will be helpful for both scientific research and clinical diagnosis.
There are lots of commercial H+probes available,such as BCECF,SNARF,Oregon Green and so on,which are popularly applied to biomedicine research and normally called pH indicators[3–6].In fact,biologists could directly use these commercial indicators for intracellular pH sensing.However,several disadvantages exist inherently with these organic molecules.First,these indicators are small molecules so cells cannot uptake them efficiently even though most of them are chemically engineered to be ester forms.Second,when entering cells,they are subject to be digested by intracellular enzymes.Third,the local spatial intensity of free indicators normally gives a bad signal/noise ratio which cannot satisfy the increasing requirements for both scientific research and clinical diagnoses.
To solve these three issues,a new generation of proton nanosensors(PNS)has been developed based on nanotechnology over the past decade. Firstly,CdTe-based quan-tum dots(QDs)as fluorescent nanocrystals can be directly used to sense pH,due to the additional passivation of the CdTe nanoparticle(NP)surface by a shell of cadmium thiolate complexes[7,8].In addition,lifetime-based pH sensing of CdSe/ZnS QDs was also reported[9].Secondly,the fluorescence resonance energy transfer(FRET)based NSs was built by covalently coupling organic pH sensitive dyes to QDs[10,11].Thirdly,two more dyes could be loaded into one single carrier for pH sensing,which is also promising to develop multiplexed ion sensing[12,13].
After learning the above advances of PNSs,it can be concluded there are several advantages to build PNSs via linking organic proton indicators to NPs.First,the cellular uptake rate of the NPs-based PNSs are more efficient than the free organic indicators[14].Second,when coupled to NPs,the organic indicators could successfully escape from intracellular enzymatic digestion due to surface-inhibition effects[15]. Third,compared with free organic indicators,the PNSs could provide a much higher signal/noise ratio at sub-cellular spatial resolution and have the potential to realize the multi-ionic detection.Here in this paper,we demonstrate a facile approach to build a PNS with an extended pH sensitive range just by conjugating a commercially available indicator to carboxylic-rich amphiphilic polymer(AP)coated gold NPs (AuNPs).
II.EXPERIMENTAL SECTION
A.Hydrophobic AuNPs Synthesis

Fig.1.(Color online)Schematic diagram of building a PNS.AP-AuNP-FA PNS(c)was built by covalently conjugating AP-AuNP(a)with FA(b)by EDC chemistry.
Dodencanethiol protected AuNPs were synthesized according to previously published protocols[16–19]with minor modifications.Briefly,2.17g tetraoctylammonium bromide was dissolved in 80mL toluene in a round flask.300mg tetrachloroauric acid was dissolved in 25mL Milli-Q water under sonication.Mixed these 2 solutions in a 500mL separation funnel and gently shook the mixture for~5min.Discarded the aqueous solution and transferred the left solution into a round flask stirred by a magnetic bar.Freshly dissolved 334mg sodium borohydride in 25mL Milli-Q water and added this solution dropwise into the above round flask within 1min.Continued stirring the reaction solution for at least 60min.Then transferred this solution back to a clean separation funnel and washed with 25mL HCl(10mM), NaOH(10mM)and Milli-Q water in sequence.And repeated this washing program at least 4 times until the aqueous phase became colorless.The final solution was transferred into a new round flask with overnight magnetic stirring in order to get a good size distribution by Oswald-Ripening.The resulting solution was then poured into 10mL 1-dodecanethiol and heated to 65◦C in a water bath for 2 hours with stirring.Then the solution was precipitated several times by either centrifugation or adding methanol in order to get rid of big aggregates and small impurities.Finally,all of the AuNPs were collected and redissolved in chloroform to a storing concentration of 6µM.
B.AP Synthesis
The AP was synthesized according to published protocols[20–22]. In practice,2.70g(15mmol)of dodecylamine powder was dissolved in 100mL THF.3.084g (20mmol monomer)poly(isobutylene-alt-maleicanhydride) powder was dissolved by the dodecylamine solution in a round flask under sonication.The solution was heated at 55–60◦C for 1 hour under stirring condition and during this time the solution volume was reduced by a vacuum to enhance the reaction and then left for overnight reaction under stirring. Finally,the THF was completely evaporated and the AP was redissolved in 40mL chloroform to a final monomer concentration of 0.5M.
C.Polymer Coating
The polymer coating procedure was completed according to previous reports[18–22].In this work we mixed the AP solution with hydrophobic NPs by a molar ratio of 100–200 polymer monomers per nm2of the NP’s effective surface area (The core diameter of the hydrophobic NPs was determined by the TEM analysis.The effective diameter(deff)includes the organic molecules which were considered to contribute a layer thickness of 1nm around the inorganic core.Then the surface area per NP(Aeff)was estimated by using the calculation equation of Aeff=4π(deff/2)2).After mixing,the solvent was slowly evaporated under reduced pressure until the sample was completely dried.The remaining solid film in the flask was redissolved in a SB12(sodium borate,50mM, pH 12)buffer under vigorous stirring until the solution turned clear.
D.Purification
In this work,we used ultrafiltration by ultrafilters(membrane:100kDa Mw cut off PES,Sartorius Stedim)and size exclusion chromatography(SEC)with a self-packed sephacryl S-300 HR column(GE Healthcare)connected with a high performance liquid chromatography(HPLC)system (Agilent 1260)equipped with an autosampler,a fraction collector,a UV-vis absorption,a fluorescence detector,and an extra refractive index detector(RID).The flow phase is a SB9(sodium borate,50mM,pH 9)buffer,and we kept the running flow rate at 0.5mL/min.
E.Size and Zeta-potential measurement
Dynamic light scattering(DLS)equipment(Malvern, ZS90)was used to measure both size and zeta-potential of nanoparticles before and after linking with FA.
III.RESULTS AND DISCUSSION
A.Chemically Engineering PNS
To build a PNS,we designed a facile chemical synthesis route as shown in Fig.1.The main idea is to use AP coated NPs(AP-NPs)coupled with commercial pH indicators with several considerations:AP coating is a general approach for all hydrophobic NPs with different material properties because the coating employs the hydrophobic interaction between AP and NPs.In details,NPs synthesized in nonpolar solvents show much more robust and better quality than those synthesized in water solution due to the higher temperature annealing most of the surface defects of the NPs. Thinking of the simplicity of synthesis,we selected dodecanethiol protected AuNPs for a typical kind of NP.With the previously published coating methods[11],the hydrophobic AuNPs were firstly coated by AP and then followed by EDC chemistry to covalently conjugate FA to AP.Because of the huge difference of size,the PNS can be well purified by SEC to remove the excess of FA molecules,and this can be also checked by gel electrophoresis(GE,data not shown).
B.Characterization of PNS
From the design and synthesis strategy of PNS,we can see that the only impurities that need removing are the excess AP,AP-AuNP,AP-FA and FA itself.Normally,amphiphilic molecules like to self-assemble into micelles in buffers,so does AP.However,because the AP micelles and AP-AuNPs have same surface chemistry,both these two impurities won’t influence the pH sensitivity of PNS,and in fact,AP micelles can be easily removed by ultracentrifugation or GE rather than SEC due to the similar size.Hence,the only issue is FA which can be readily removed by SEC in turn because of the huge difference in size(Fig.2(a)).By DLS,the size of purified AP-AuNPs were measured before and after linking with FA by EDC chemistry.The results show that after conjugating with FA,AP-AuNPs have no significant change in size(Fig.2(b)and 2(c),both are 12nm in diameter),indicating the small FA molecules won’t contribute too much to the PNS’s size.The zeta potentials were also measured to -30mV and-25mV for AP-AuNPs before and after linking with FA,respectively.This proved the successful covalent conjugation by EDC because every FA will consume one carboxylic group of AP to form a peptide bond,since the rise of zeta-potential reflects the reduction of carboxylic groups of AP.
C. pH Sensitivity Test
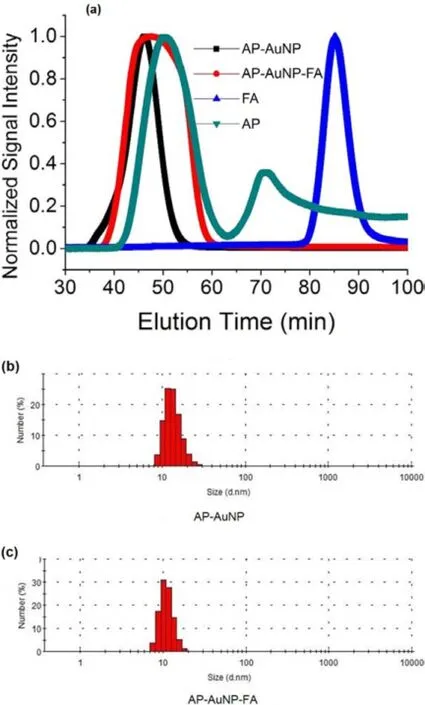
Fig.2.(Color online)Size and Zeta-potential characterization of PNS.(a)SEC results of AP-AuNP,AP-AuNP-FA,FA and AP.The Y axis was normalized to[0,1]for a convenient comparison.(b) Size distribution of AP-AuNP by DLS,12nm.(c)Size distribution of AP-AuNP-FA by DLS,12nm.
Both free FA and the PNS were tested for pH sensitivity by using a static fluorescence spectrometer.From Fig.3 we can see the free FA molecules show pH dependent fluorescence, and the maximum fluorescent intensity is at 509nm.In addition,we found the pH sensitivity of FA is not regular below pH 5,especially pH 3 and pH 4(Fig.3(a)and 3(b)),which is ascribed to the interference of the primary amino group(the pKaof amino group is 9.4)on FA(the carboxylic fluorescein doesn’t show the same spectra,data no shown).In contrast, the newly built PNS shows better pH dependent fluorescence spectra(Fig.3(c),and from pH 2 to pH 10 all of fluorescent intensities(maximum is little shifted to 512nm,Fig.3(d))are regularly increased as pH rises,further indicating the effect of the primary amino group on disturbing the pH sensitivity of the FA indicator.From Fig.3 it can be figured out that both the free indicator and the newly built NS show a positive correlation between pH and fluorescent intensity.However,after plotting the maximum fluorescent intensities against the pH, we can see a distinctly optimizing effect after conjugating the free indicator to AP-AuNPs.For the free indicator,the positive correlation is only found from pH 5 to pH 8,while for the PNS,the positive correlation can be extended from pH 5 to pH 10,which is due to the Debye-screening effect as discussed in our previous papers[23].More importantly,the pH sensitivity of newly built PNS is obviously better than the free indicator itself,which can be read out from the gradients of the plotted sigmoid curves.

Fig.3.(Color online)pH dependent test of free and conjugated forms of FA.(a)Fluorescence spectra of free FA in different pH buffers. (b)Plot of fl uorescent intensity at 509nm of FA against pH with error bar(average of 5 independent measurements)and its corresponding Boltzmann sigmoid fitting.(c)Fluorescence spectra of PNS in different pH buffers.(d)Plot of fl uorescent intensity at 512nm of PNS against pH with error bar(average of 5 independent measurements)and its corresponding Boltzmann sigmoid fitting.
IV.CONCLUSION
ByconjugatingacommercialpHindicatorFAtoAPcoated AuNPs,we report how to build a new PNS with not only better pH sensitivity(a lower gradient),but also an extended pH sensitive range(from pH 5 to pH 10).To our best knowledge, this could be the first PNS which can sense more than 5 pH units.We attribute this changing of pH sensitivity to a combination of the conjugated amino group of free indicator and the carboxylic-rich AP coated AuNPs,which is promising to be applied to other existing molecules probes.We also hope to give inspirations to the novel engineering of NPs-based NS.
[1]Vo-Dinh T,Griffin G D,Alarie J P,et al.J Nanopart Res,2000, 2:17–27.
[2]Zhang F,Ali Z,Amin F,et al.ChemPhysChem,2010,11:730–735.
[3]Owen C S,Carango P,Grammer S,et al.J Fluoresc,1992,2: 75–80.
[4]Hunter R C and Beveridge T J.Appl Environ Microb,2005, 71:2501–2510.
[5]Delmotte C and Delmas A.Bioorg Med Chem Lett,1999,9: 2989–2994.
[6]Lin H J,Szmacinski H,Lakowicz J R.Anal Biochem,1999, 269:162–167.
[7]Gao M,Kirstein S,M¨ohwald H,et al.J Phys Chem B,1998, 102:8360–8363.
[8]Susha A S,Mu˜noz Javier A,Parak W J,et al.Colloid Surface A,2006,281:40–43.
[9]Jin T,Sasaki A,Kinjo M,et al.Chem Commun,2010,46: 2408–2410.
[10]Snee P T,Somers R C,Nair G,et al.J Am Chem Soc,2006, 128:13320–13321.
[11]Zhang F,Lees E,Amin F,et al.Small,2011,7:3113–3127.
[12]Doussineau T,Smaihi M,Mohr G J.Adv Funct Mater,2009, 19:117–122.
[13]Doussineau T,Trupp S,Mohr G J.J Colloid Interf Sci,2009, 339:266–270.
[14]Chithrani B D,Ghazan A A,Chan C W.Nano Lett,2006,6: 662–668.
[15]Maccormack T J,Clark R J,Dang M K,et al.Nanotoxicology, 2012,6:514–525.
[16]Brust M,Walker M,Bethell D,et al.Chem Commun,1994, 801–802.
[17]Fink J,Kiely C J,Bethell D,et al.Chem Mater,1998,10:922–926.
[18]Pellegrino T,Manna L,Kudera S,et al.Nano Lett,2004,4: 703–707.
[19]Sperling R A,Pellegrino T,Li J K,et al.Adv Funct Mater, 2006,16:943–948.
[20]Fern´andez-Arg¨uelles M T,Yakovlev A,Sperling R A,et al. Nano Lett,2007,7:2613–2617.
[21]Lin C A J,Sperling R A,Li J K,et al.Small,2008,4:334–341.
[22]Yakovlev A V,Zhang F,Zulqurnain A,et al.Langmuir,2009, 25:3232–3239.
[23]Riedinger A,Zhang F,Dommershausen F,et al.Small,2010, 6:2590–2597.
(Received May 3,2014;accepted in revised form June 18,2014;published online July 5,2014)
10.13538/j.1001-8042/nst.25.040503
∗Supported by grants from the National Natural Science Foundation of China(Nos.21171086and81160213),andInnerMongoliaGrasslandTalent(No.108-108038),and Inner Mongolia Autonomous Region Natural Science Foundation(No.2013MS1121)and the Inner Mongolia Agricultural University(Nos.211-109003 and 211-206038)
†Corresponding author,fengzhang1978@hotmail.com
猜你喜欢
杂志排行
Nuclear Science and Techniques的其它文章
- Simulation studies on two-frequency RF gun
- Tidal effects on hydrostatic leveling system used in high precision alignment of particle accelerator∗
- External micro-PIXE analysis of Nd3+accumulation in Euglena gracilis∗
- Mass thickness measurements for dual-component samples utilizing equivalent energy of X-rays∗
- Synthesis of N,N′-dimethyl-N,N′-didecyl-3-oxa-diglycolamide for extraction of lanthanides∗
- A K-CELL injection system for SH-PermEBIT∗
