BTATz-Pb复合物对双基和RDX-改性双基推进剂的热行为、非等温动力学及燃烧性能的影响
2013-10-18任莹辉赵凤起张鲜波马海霞徐抗震王伯周仪建华宋纪蓉胡荣祖
李 文 任莹辉,,* 赵凤起 张鲜波 马海霞 徐抗震 王伯周 仪建华 宋纪蓉,3 胡荣祖
(1西北大学化工学院,陕西省物理无机化学重点实验室,西安 710069;2西安近代化学研究所,燃烧与爆炸技术重点实验室,西安 710065;3故宫博物院文保科技部,北京 100009)
1 Introduction
3,6-Bis(1H-1,2,3,4-tetrazol-5-yl-amino)-1,2,4,5-tetrazine(BTATz),a novel energetic material,was synthesized firstly by Hiskey et al.1at Los Alamos National Laboratory.It was one of the high-nitrogen energetic compounds with an excellent performance,such as high nitrogen content of 79.02%,density of 1.76 g·cm-3,enthalpy of formation of 883 kJ·mol-1,and moderate mechanical and thermal sensitivity.2-7Our research group has been involved in a systematic experimental study about the synthesis and purification,6the burning rate measurement,8the quantum chemistry study,5thermal behavior and kinetic investigation of the thermal decomposition processes,7even as a substitute of hexogen(RDX)in the composite modified double base(CMDB)propellant formulation.9Many researches focusing on the BTATz showed that it had a prospect function as a primary component in the high burning rate propellant for the booster rocket motor and the kinetic energy ammunition,and it also could be used in the minimum signature propellant for the smokeless ammunition.8,10-11
Lead salts,12-15oxides16or nano-oxides17were usually added to the solid propellant as the combustion catalyst.Although the lead compounds could bring about the heavy metal pollution to the surrounding and human health,it was well known that they could improve the solid propellant ballistic properties,i.e.,they could increase burning rate and decrease the pressure exponent of the burning rate.So they are extensively used as one of the key ballistic modifiers in solid propellant formulations.18Therefore,the study on them had not any significance unless the excellent substitute was developed.In this work,the lead complex of BTATz(LCBTATz)was synthesized and its structure was also characterized.The thermal behaviors,nonisothermal decomposition reaction kinetics,thermal safety,and combustion properties of double-base(DB)propellant containing LCBTATz or composite modified double-base(CMDB)propellant containing catalyst were investigated.
2 Experimental
Caution!Although none of the compounds described herein have exploded or detonated in the course of this research,these materials should be handled with extreme care using the best safety practices.
2.1 Equipment and conditions
The thermogravimetry-derivative thermogravimetry(TGDTG)and differential scanning calorimetry(DSC)curves under the condition of flowing nitrogen gas(purity,99.999%;atmospheric pressure)were obtained by using a TA2950 thermal analyzer(TACo.,USA)and a 204HP differential scanning calorimeter(Netzsch Co.,Germany).The conditions of TG-DTG were:sample mass,about 1 mg;N2flowing rate,40 cm3·min-1;heating rate(β),10 K·min-1.The conditions of DSC analyses were:sample mass,about 1 mg;N2flowing rate,50 cm3·min-1;heating rate,5,10,15,and 20 K·min-1;furnace pressures,0.1 MPa;reference sample,α-Al2O3;type of crucible,aluminum pan with apierced lid.The specific heat capacity(Cp,J·g-1·K-1)was determined with continuous Cpmode on a Micro-DSC III mircocalorimeter(Setaram Co.,France).Heating rate,0.15 K·min-1;sample mass,about 100 mg;atmosphere,N2;reference sample,calcined α-Al2O3.The burning rates of the samples were measured in a strand burner filled with nitrogen at different pressures,and the samples prepared were the Φ 5 mm×100 mm cylinder strand coated with polyvinyl formal.19
2.2 Materials
The lead complex of BTATz used in the propellant was carried out as follows:BTATz(provided by Xi′an Modern Chemistry Research Institute,purity,99.9%,measured by high performance liquid chromatography)5and Pb(CH3COO)2·3H2O(AR grade)with mole ratio of 1:2 were dissolved into DMSO and deionized water,respectively.The mixture was stirred for 1.5 h at 70°C.After that,a precipitate was filtered,washed with water repeatedly and dried at 80°C.Finally,the orange powder was obtained.Anal.Calc.for C12H16N28O5Pb(%):C 17.20,H 1.81,N 47.32,Pb 23.63.Found:C 17.2,H 1.9,N 46.7,Pb 24.7.IR(KBr,v/cm-1):3017,2863,1624,1506,1438,1323,1042,780,674.
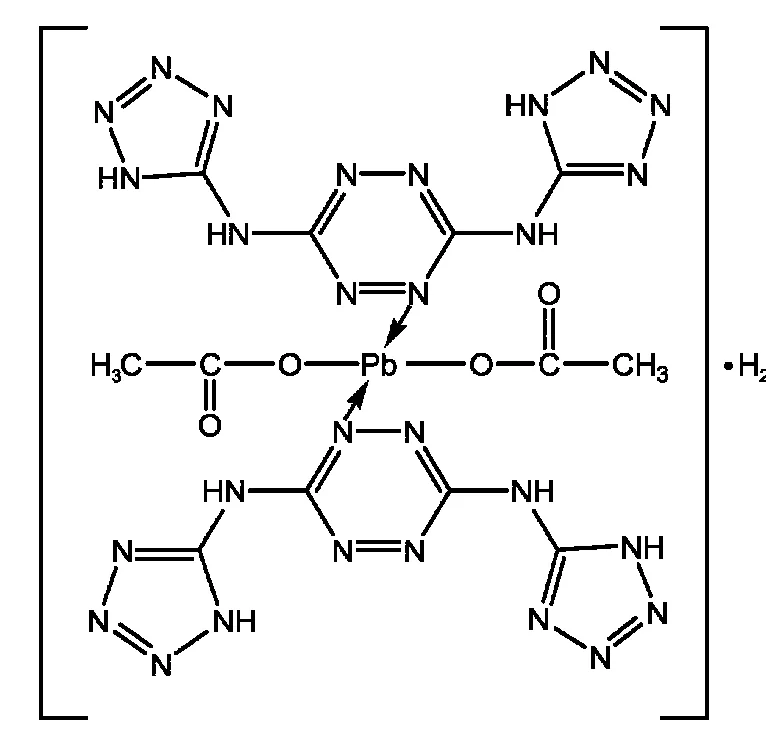
Scheme 1 Structure of the title complex
The chemical structure of the title complex is shown in Scheme 1.
The sample of DB001 used in the experiment was a DB propellant containing LCBTATz composed of 59%(mass fraction)nitrocellulose(NC),30%nitroglycerin(NG),8.5%diethyl phthalate(DEP),and other additives,etc.The strand sample composed of 500 g ingredients and 12.5 g LCBTATz,LCBTATz as ballistic modifier was prepared by a solventless DB propellant extrusion technique,including slurry mixing,rolling,and extruding.The control DB propellant without ballistic modifier(No.DB002)was also prepared for the comparison with the sample DB001.
The RDX-CMDB propellant sample of CMDB100 used in the experiment was composite modified double base propellant containing LCBTATz,composed of 38%(mass fraction)nitrocellulose(NC),28%nitroglycerin(NG),2.5%LCBTATz,26%hexogen(RDX),5%N-nitro-dihydroxyethylamine-dinitrate(DINA)and other auxiliary.The strand sample composed of 500 g ingredients and 12.5 g LCBTATz,LCBTATz as ballistic modifier was prepared by a solventless CMDB propellant extrusion technique,including slurry mixing,rolling,and extruding.The control CMDB propellant without ballistic modifier(No.CMDB101)was also prepared for the comparison with the sample CMDB100.20-23
3 Results and discussion
3.1 Thermal behaviors
The TG-DTG curves of DB001 and CMDB100 propellants at the heat rate of 10 K·min-1are shown in Figs.1 and 2,and the DSC curves at the heating rate of 5,10,15,20 K·min-1are shown in Figs.3 and 4,respectively.
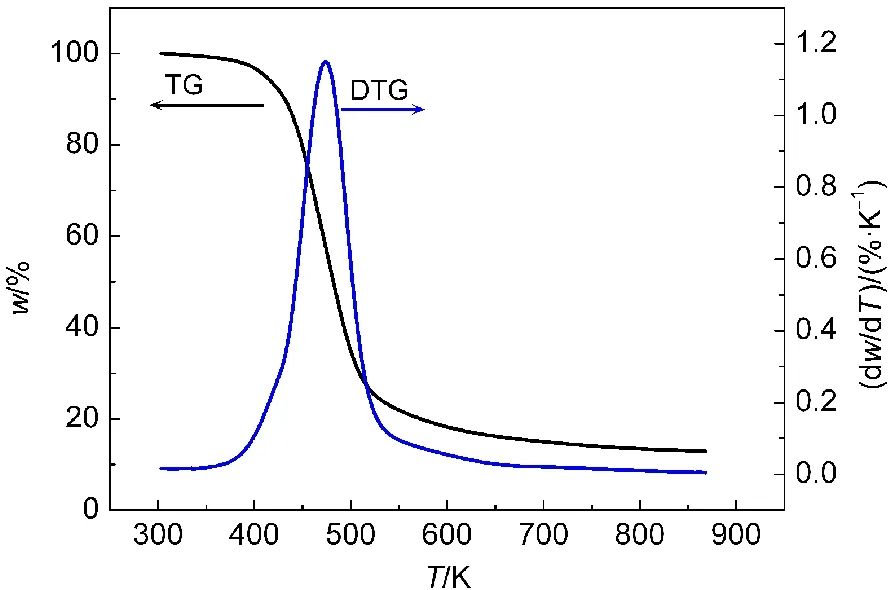
Fig.1 TG-DTG curves for propellant DB001
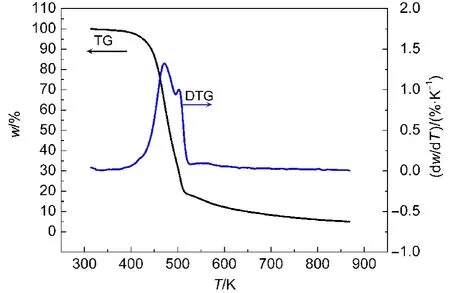
Fig.2 TG-DTG curve for propellant CMDB100
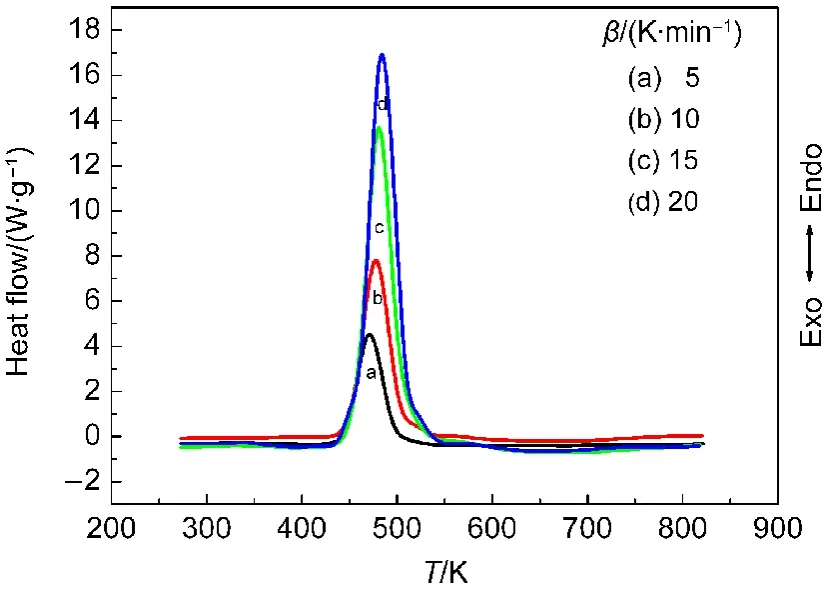
Fig.3 DSC curves for propellant DB001
From Fig.1,it can be found that there is only one mass-loss stage in TG curve,corresponding to one invisible exothermic peak in DSC curve.For TG curve,there is a mass loss of about 77%between 380 and 540 K,which is close to the mass(80%)of NG and NC,and it likely attributes to the volatilization and decomposition of NC and decomposition of NG.24
There are two mass loss stages in Fig.2,corresponding to the two continuous exothermic peaks in the DSC curve.The first begins at 390 K and stops at 495 K,witnessing about 61%mass loss,which is close to the total mass(54%)of the nitroglycerine and nitrocellulose mixture in the RDX-CMDB propellant.It can be attributed to the volatilization and decomposition of the NG/NC mixture,corresponding to the invisible peak in the DSC curve(main exothermic stage,Fig.4).The second follows the decomposition of NG/NC process,and stops at 540 K,with 20%mass loss,which belongs to the RDX decom-position in the RDX-CMDB propellant.Because the decomposition of RDX occurs in succession,the decomposition heats of the two processes overlap each other part.
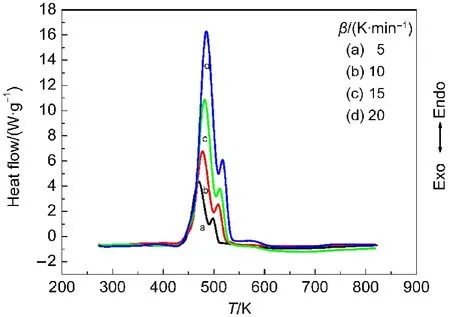
Fig.4 DSC curves for propellant CMDB001
3.2 Calculation of nonisothermal reaction kinetics
To explore the reaction mechanisms of the main exothermic decomposition processes of the propellants DB001 and CMDB100 and obtain the corresponding kinetic parameters[apparent activation energy(Ea/(kJ·mol-1)),pre-exponential constant(A/s-1)and the most probable kinetic model functions],the DSC curves at the heating rate of 5,10,15,and 20 K·min-1were dealt with mathematic means,and the basic data and temperature data corresponding to the conversion degrees(α)were found and shown in Tables 1 and 2,respectively.
Five integral methods(General integral,MacCallum-Tanner,Šatava-Šesták,Agrawal,Flynn-Wall-Ozawa)and one differential method(Kissinger)were employed.25-27While the values of Eaobtained by Ozawa′s method from the isoconversional DSC curves at the heating rate of 5,10,15,and 20 K·min-1,the Ea-α curves were shown in Fig.5.It shows that the activation energy changed slightly in the section of 0.20-0.84(α)for DB001 and 0.22-0.80(α)for CMDB100,which were properly selected to calculate the nonisothermal reaction kinetics.
Forty-one types of kinetic model functions and the basic data(Table 2)for the propellant were put into the integral and dif-ferential equations for calculation.The values of Ea,lg A,and the linear correlation coefficient(r)were calculated on computer with the linear least-squares method,and the most probable mechanism function was selected by the better value of r.25-27The results of satisfying the conditions at the same time were the final results as listed in Table 3,and the relevant functions were the reaction mechanism function of the decomposition process.
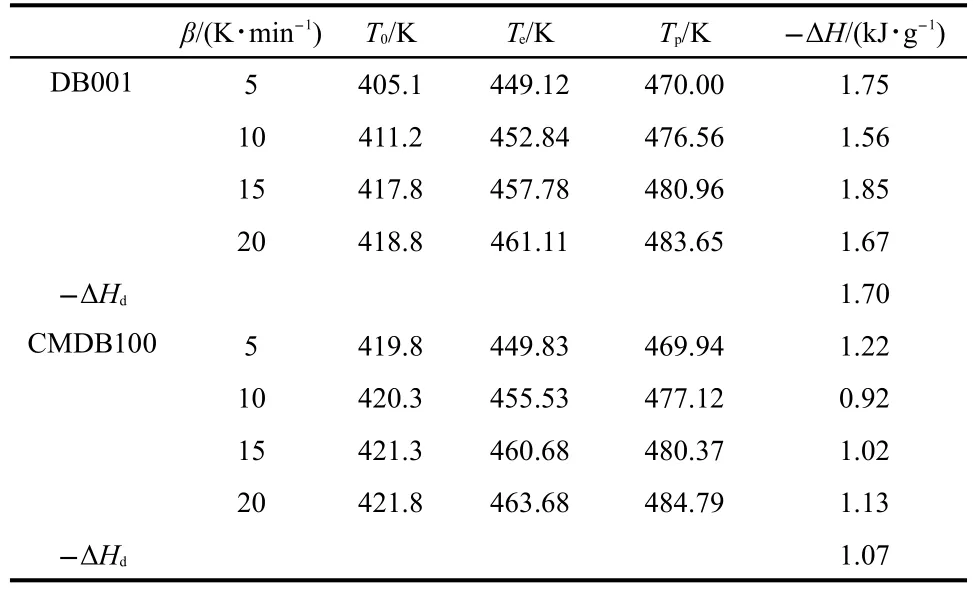
Table 1 Basic data for the main exothermic decomposition processes of the propellants DB001 and CMDB100
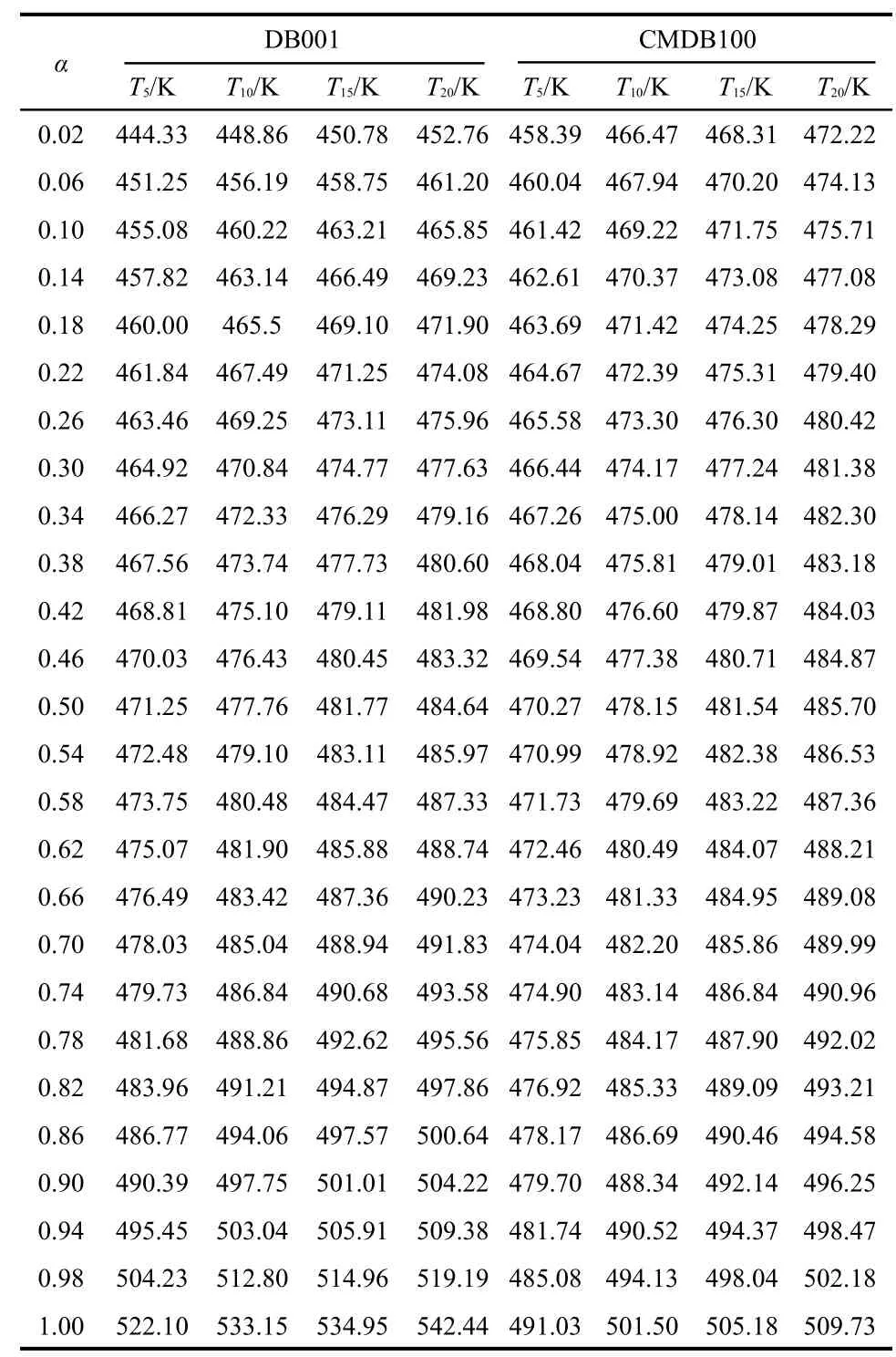
Table 2 Temperature data for the main exothermic decomposition processes of the propellants DB001 and CMDB100

Fig.5 E a- α curves for the propellants of DB001 and CMBD100 E ais the apparent activation energy of kinetic model functions.
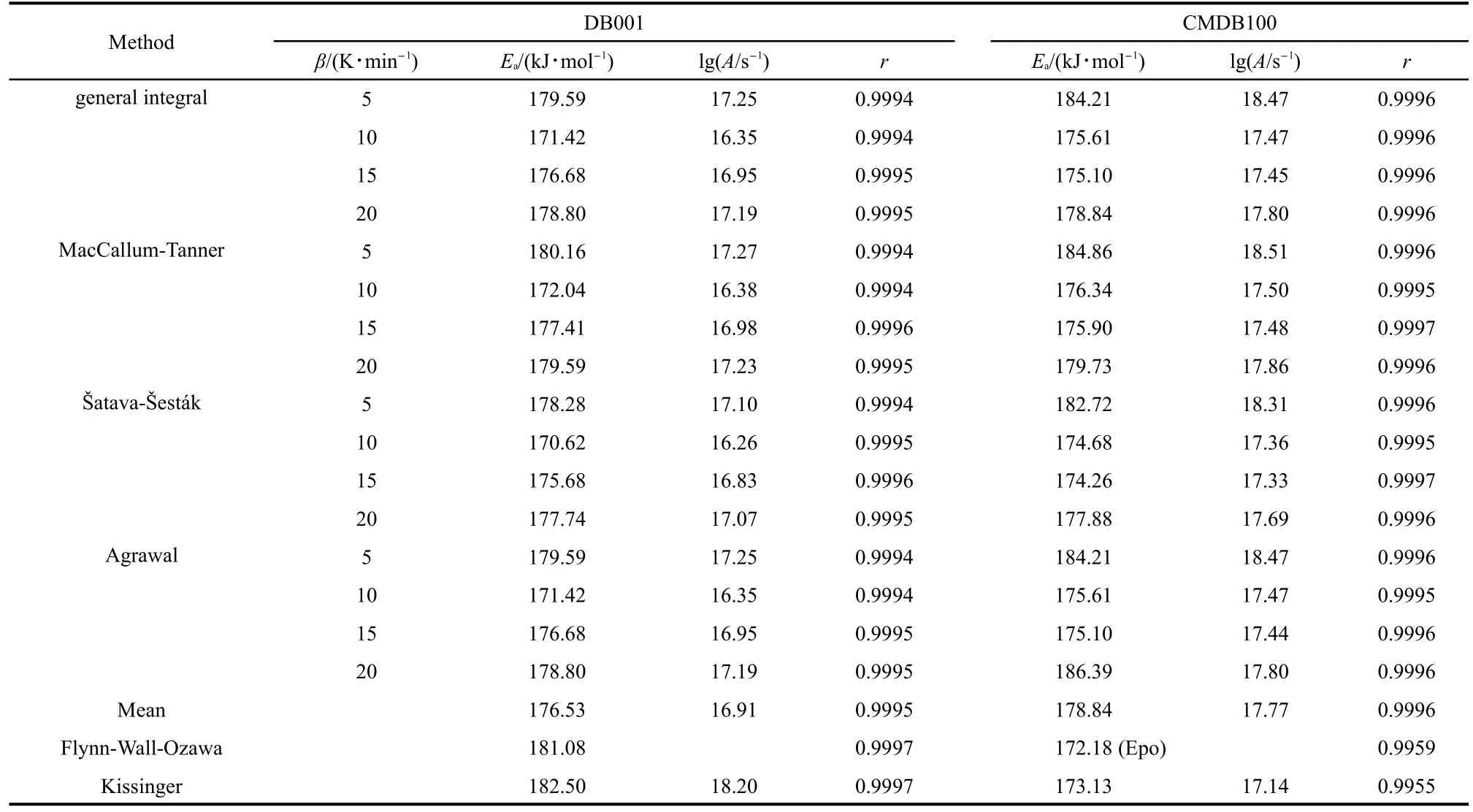
Table 3 Kinetic parameters for the main exothermic decomposition process of the propellants DB001 and CMDB100
From Table 3,one can see that the values of Eaand lg A obtained from thenonisothermal DSC curves are approximately in agreement with the values calculated by Kissinger′s method and Ozawa′s method,and the mechanism function number is determinated.The decomposition reaction mechanism functions of two propellants are listed in Table 4.The substituting f(α)expression,and the values of Eaand A into Eq.(1),the corresponding kinetic equations[Eqs.(2)and(3)]of the decomposition reaction of the propellants are obtained and shown in Table 4.
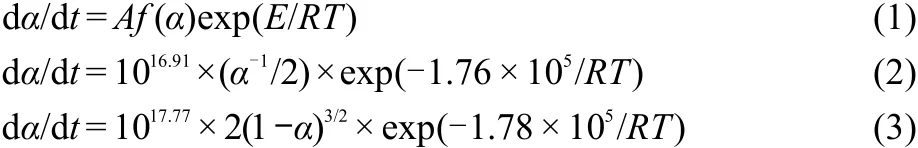
3.3 Thermal safety studies
3.3.1 Self-accelerating decomposition temperature
The values(Te0and Tp0)of the onset temperature(Te)and peak temperature(Tp)corresponding to β→0 obtained by Eq.(4),25-27are listed in Table 5.
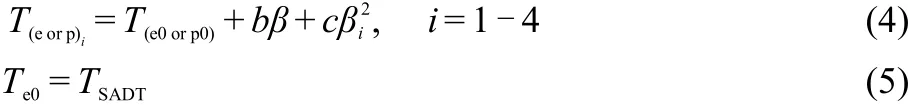
where b and c are coefficients,TSADTis the self-accelerating de-composition temperature.
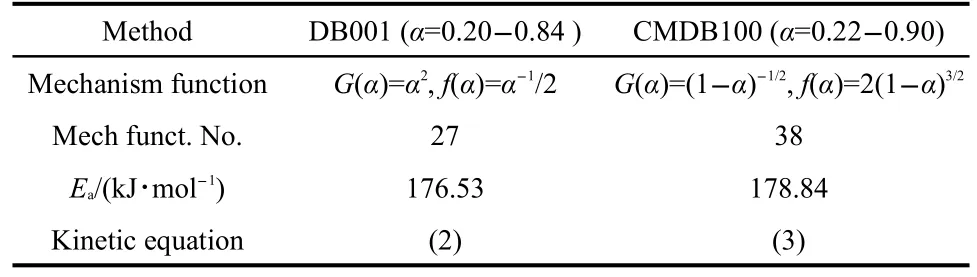
Table 4 Mechanism functions,apparent activation energies,and kinetic equations for the propellants DB001 and CMDB100
3.3.2 Thermal ignition temperature(TTITT)and critical temperatures of thermal explosion(Tb)
The corresponding critical temperatures of thermal explosion(Tbeand Tbp)obtained from Eq.(6),28are also listed in Table 5.The high values of Tbfor DB001 and CMDB100 propellants show that the transition from thermal decomposition to thermal explosion is not easy to take place.
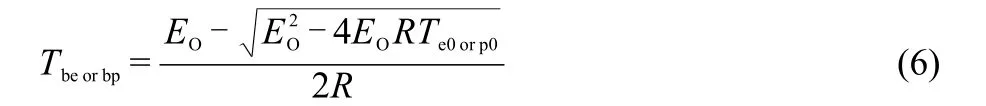
where R is the gas constant,EOis the value of Eaby Ozawa′s method.
3.3.3 Adiabatic time-to-explosion
The adiabatic time-to-explosion(tTIad)of energetic materials is the time of decomposition transiting to explosion under the adiabatic conditions and is an important parameter for assessing their thermal stability and safety.
Substituting the following data into Smith equations[Eqs.(7)-(9)],29,30the values of tTIadfor the propellants are acquired and also listed in Table 5.
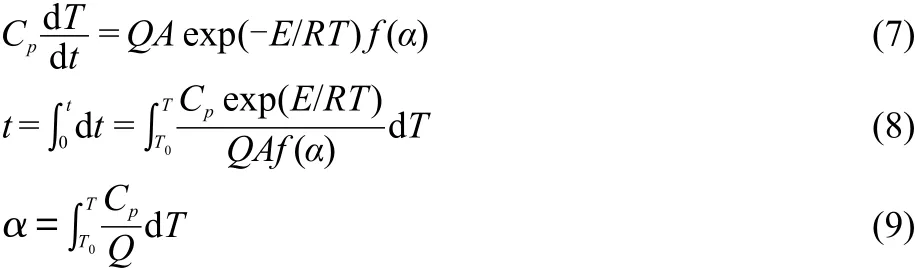
For the propellant DB001:Cp=-20.95+0.195 T-5.69×10-4T2+5.61×10-7T3(J·g-1·K-1),Q=-ΔHd=1703.5 J·g-1,A=AK=1018.20s-1,E=EK=182500 J·mol-1,R=8.314 J·mol-1·K-1,mechanism f(α)=α-1/2,the integral lower limit T1=Teo=444.5 K,the upper limit T2=Tb=471.8 K.EKand AKare calculated by Kissinger method.

Table 5 Derivative parameters for DB001 and CMDB100
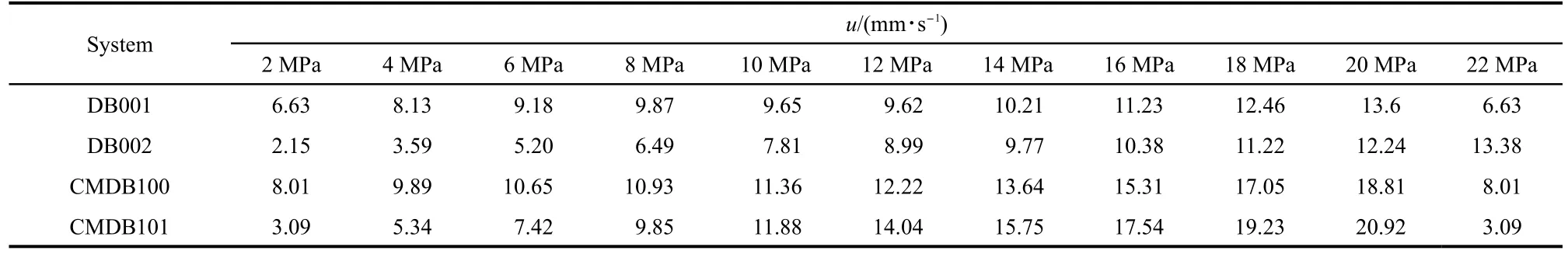
Table 6 Burning rates(u)of DB001,DB002,CMDB100,and CMDB101 at different pressures
For the propellant CMDB100:the specific heat capacity,Cp=2.60-1.16×10-2T+2.49×10-5T2(J·g-1·K-1),Q=-ΔHd=1072.5 J·g-1,A=AK=1017.14s-1,E=EK=173130 J·mol-1,R=8.314 J·mol-1·K-1,mechanism f(α)=2(1-α)3/2,the integral lower limit T1=Teo=442.4 K,the upper limit T2=Tb=464.1 K.
3.3.4 Thermodynamic parameters of activation reaction
The entropy of activation(ΔS≠),enthalpy of activation(ΔH≠),and free energy of activation(ΔG≠)of the decomposition reaction,corresponding to T=Tp0,Ea=EK,and A=AK,are obtained from Eqs.(10)-(12),25,26respectively,and also listed in Table 5.The positive values of ΔG≠indicate that the exothermic decomposition reaction of the propellants must proceed under the heat condition.

where kBis the Boltzmann constant,and h is the Plank constant.
From Table 5,the calculated values of TSADT,TTITT,and Tbfor the propellant DB001 and CMDB100 show that they have the high thermal safety from adiabatic decomposition to explosion.
3.4 Combustion property
For the sake of the possible application of LCBTATz in DB propellant and RDX-CMDB propellant,the burning rates[u/(mm·s-1)]of DB002(mass fractions of NC/NG/DEP/auxiliary:59%/30%/8.5%/2.5%)and DB001 were measured under different pressures(p).
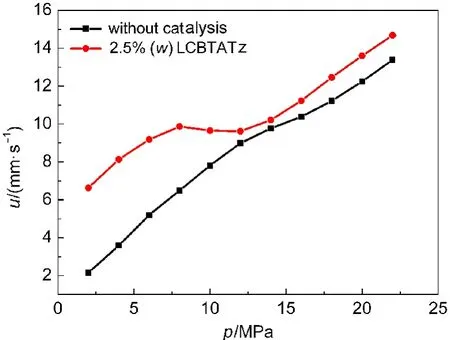
Fig.6 Burning rate curves of DB001
Also,the CMDB101(mass fractions of NC/NG/RDX/auxiliary:38%/28%/26%/8%)and CMDB100 were measured under different pressures,respectively.The data are listed in Table 6 and the curves of burning rates are shown in Figs.6 and 7.It can be seen that with the help of the catalysis of LCBTATz,the burning rate of the DB propellant and RDX-CMDB propellant are improved observably.The burning rate of the DB propellant can be improved by 208.4%at 2 MPa and“super burning effect”is occurred at 2-8 MPa,and burning rate of the CMDB propellant can be improved by 159.2%at 2 MPa and the burning rate is increased remarkably at 2-8 MPa.
In order to evaluate the effects of the ballistic modifier on the combustion property of the propellants,the pressure exponent(n)of the burning rate was calculated,and the mean value of the catalysis efficiency(Zˉ)was compared before and after LCBTATz was added into the propellant formulation.The values of n and Zˉwere obtained by Eqs.(13)and(14),31,32and the relationship between Z and test pressure were shown in Figs.8 and 9.

where,I:without catalysis DB propellant,II:2.5%LCBTATz DB propellant,III:without catalysis RDX-CMDB propellant,IV:2.5%LCBTATZ RDX-CMDB propellant
For DB001:
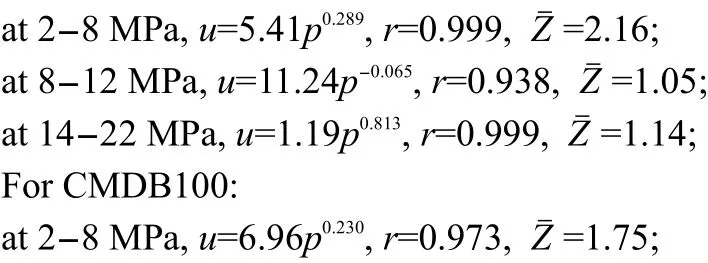
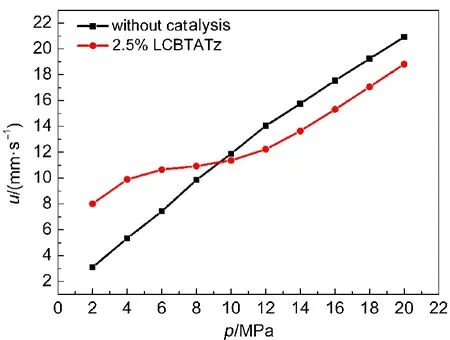
Fig.7 Burning rate curves of CMDB100
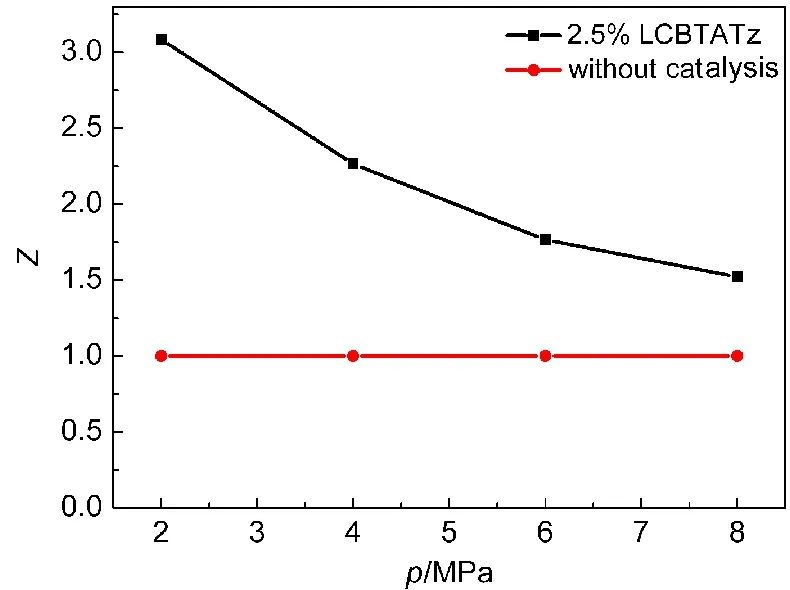
Fig.8 Relationship of catalytic efficiency and text pressure at 2-8 MPa in DB001
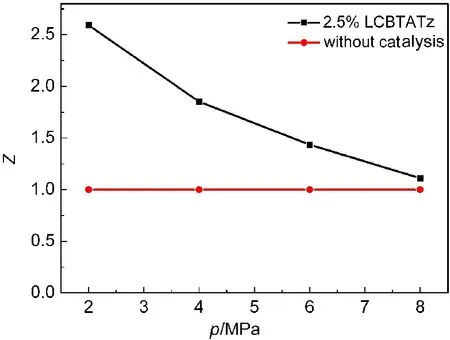
Fig.9 Relationship of catalytic efficiency and text pressure at 2-8 MPa in CMDB100
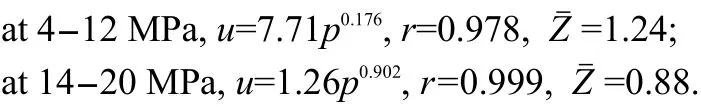
Fig.8 shows that the value of Z is higher than 2 at 2-8 MPa,and that the pressure exponent of DB100 propellant is reduced by 64%from 0.805 to 0.289 at 2-8 MPa.It is reduced to-0.065 at 8-12 MPa,which is in the range of“mesa effect”.From Fig.9,it can be seen that the value of Z is higher than 1.7 and the pressure exponent of CMDB propellant is reduced to 0.178 at 2-8 MPa,within the range of which“plateau combustion”occurs.
As one can see,LCBTATz as an auxiliary ballistic energetic modifier can be effective to increase the burning rate and reduce the pressure exponent of propellants.Especially,super burning effect occurs at low pressure and the“mesa effect”occurs at 8-12 MPa in the DB propellant.The pressure exponent of CMDB100 is reduced to 0.178,and“plateau combustion”occurs at 4-12 MPa.
4 Conclusions
The lead complex based BTATz(LCBTATz)was prepared,characterized,and investigated.It was used as a catalyst for DB001 and CMDB100.The reaction kinetic equations of the main exothermal decomposition processes of DB001 and CMDB100 are dα/d t=1016.91×(α-1/2)×exp(-1.76×105/RT)and dα/d t=1017.77×2(1-α)3/2×exp(-1.78×105/RT),respectively.The The results of evaluating the thermal safety for the propellants were obtained as:(1)for DB001,TSADT=444.50 K,TTITT=453.96 K,Tb=471.84 K;when EK=182500 J·mol-1,and AK=1018.20s-1,tTlad=39.36 s,ΔS=99.87 J·mol-1·K-1;ΔH=178.66 kJ·mol-1,ΔG=132.56 kJ·mol-1;(2)For CMDB100,TSADT=442.38 K,TTITT=452.89 K,Tb=464.13 K;when EK=162100 J·mol-1,and AK=1015.9s-1,tTlad=21.3 s,ΔS=79.72 J·mol-1·K-1,ΔH=169.36 kJ·mol-1,ΔG=133.18 kJ·mol-1.With the help of the ballistic modifier in the DB propellant formulation,the burning rate can be improved by 208.4%at 2 MPa and 126.5%at 4 MPa,and the pressure exponent can be reduced to-0.06 at 8-12 MPa.Also,in the CMDB propellant formulation,the burning rate can be improved by 159.2%at 2 MPa,the pressure exponent can be reduced to 0.18 at 4-12 MPa.The results show that LCBTATz has obvious catalysis in increasing the rate and excellent ability to reduce pressure index in the combustion of double-base propellants.Compared with other general lead compounds,or nanocompounds,LCBTATz can either enhance the burning rate or lower pressure index in DB propellants,which is a kind of high efficient combustion catalyst at the rang of medium and low pressure.33-38
(1)Chavez,D.;Hiskey,M.;Darrenl,D.N.Propellants,Explosives Pyrotech nics 2004,29,209.
(2)Hickey,M.A.;Chavez,D.E.;Naud,D.Preparation of 3,3′-Azobis(6-amino-1,2,4,5-tetrazine).US Patent,6342589,2002.
(3)Hickey,M.A.;Chavez,D.E.;Naud,D.3,6-bis(1 H-1,2,3,4-tetrazol-5-ylamino)-1,2,4,5-tetrazine or Salt Thereof.US Patent,6657059,2003.
(4)Yue,S.T.;Yang,S.Q.Chin.J.Energy Mater.2004,12,155.[岳守体,阳世清.含能材料,2004,12,155.]
(5)Wang,B.Z.;Lai,W.P.;Liu,Q.;Lian,P.;Xue,Y.Q.Chin.J.Org.Ch em.2008,28,422.[王伯周,来蔚鹏,刘 愆,廉 鹏,薛永强.有机化学,2008,28,422.]
(6)Saikia,A.;Sivabalan,R.;Polke,B.G.;Gore,G.M.;Singh,A.;Rao,A.S.;Sikder,A.K.J.Hazard.Mater.2009,170,306.doi:10.1016/j.jhazmat.2009.04.095
(7)Zhang,X.G.;Zhu,H.;Yang,S.Q.;Zhang,W.;Zhao,F.Q.;Liu,Z.R.;Pan,Q.Ch in.J.P rop.Technol.2007,8,322.[张兴高,朱 慧,阳世清,张 炜,赵凤起,刘子如,潘 清.推进技术,2007,8,322.]
(8)Son,S.F.;Berghout,H.L.;Bolme,C.A.;Chavez,D.E.;Naud,D.;Hiskey,M.A.Proceedings of the Combustion Institute 2000,28,919.doi:10.1016/S0082-0784(00)80298-2
(9)Yi,J.H.;Zhao,F.Q.;Wang,B.Z.;Liu,Q.;Zhou,C.;Hu,R.Z.;Ren,Y.H.;Xu,S.Y.;Xu,K.Z.;Ren,X.N.J.Hazard.Mater.2010,181,432.doi:10.1016/j.jhazmat.2010.05.029
(10)Hickey,M.A.;Chavez,D.E.;Naud,D.Low-Smoke Pyrotechnic Compositions.US Patent,6312537,2001.
(11)Li,S.W.;Zhao,F.Q.;Yuan,C.;Luo,Y.;Gao,Y.Chin.J.Solid R ocket Tech.2002,25,36. [李上文,赵凤起,袁 潮,罗 阳,高 茵.固体火箭技术,2002,25,36.]
(12)Fan,X.Z.;Li,J.Z.;Zhang,L.Y.;Wang,B.Z.;Liu,X.G.Chin.J.Energ.Mater.2007,15,316.[樊学忠,李吉祯,张腊莹,王伯周,刘小刚.含能材料,2007,15,316.]
(13)Zhao,F.Q.;Chen,P.;Li,S.W.;Wang,B.C.;Du,H.;Deng,M.Z.Acta A rmamentarii.2004,25,30.[赵凤起,陈 沛,李上文,王百成,杜 恒,邓敏智.兵工学报,2004,25,30.]
(14)Deng,M.Z.;Du,H.;Zhao,F.Q.;Luo,Y.;Yuan,C.J.Solid Rocket.Tech nol.2003,26,53.[邓敏智,杜 恒,赵凤起,罗阳,袁 潮.固体火箭技术,2003,26,53.]
(15)Singh,G.;Felix,P.J.Hazard.Mater.2002,A90,1.
(16)Denisyuk,A.P.;Demidova,L.A.Comb ustion,E xplosion,and S hock Waves 2004,40,311.doi:10.1023/B:CESW.0000028944.73795.0e
(17)Zhu,C.G.;Wang,F.W.;Chu,D.B.Chin.J.Inorg.Chem.2007,23,335.[朱传高,王凤武,褚道葆.无机化学学报,2007,23,335.]
(18)Dong,X.F.;Li,Y.;Xiong,X.F.;Cao,R.L.Chin.J.Expl.Prop.2011,34,69.[董秀芳,李 煜,熊贤峰,曹瑞林.火炸药学报,2011,34,69.]
(19)Zhao,F.Q.;Gao,H.X.;Luo,Y.;Hu,R.Z.;Pei,C.;Gao,S.L.;Yang,X.W.;Shi,Q.Z.J.Therm.Anal.Cal.2006,85,791.doi:10.1007/s10973-005-7455-4
(20)Yi,J.H.;Zhao,F.Q.;Xu,S.Y.;Gao,H.X.;Hu,R.Z.Chem.Res.Chin.Univ.2008,24,1.doi:10.1016/S1005-9040(08)60001-X
(21)Yi,J.H.;Zhao,F.Q.;Xu,S.Y.;Hang,L.Y.;Gao,H.X.;Hu,R.Z.J.Hazard.Mater.2009,165,853.doi:10.1016/j.jhazmat.2008.10.107
(22)Yi,J.H.;Zhao,F.Q.;Xu,S.Y.;Gao,H.X.;Hu,R.Z.;Hao,H.X.;Pei,Q.;Gao,Y.Acta Phys.-Chim.Sin.2007,23,1316.[仪建华,赵凤起,徐司雨,高红旭,胡荣祖,郝海霞,裴 庆,高 茵.物理化学学报,2007,23,1316.]doi:10.1016/S1872-1508(07)60065-5
(23)Yi,J.H.;Zhao,F.Q.;Xu,S.Y.;Zhang,L.Y.;Ren,X.N.;Gao,H.X.;Hu,R.Z.J.Therm.Anal.Cal.2009,95,381.doi:10.1007/s10973-008-9241-6
(24)Yi,J.H.;Zhao,F.Q.;Ren,Y.H.;Wang,B.Z.;Zhou,C.;Ren,X.N.;Xu,S.Y.;Hao,H.X.;Hu,R.Z.J.Therm.Anal.Cal.2011,104,1029.doi:10.1007/s10973-010-1258-y
(25)Hu,R.Z.;Gao,S.L.;Zhao,F.Q.;Shi,Q.Z.;Zhang,T.L.;Zhang,J.J.Th ermal Analysis Kinetics,2nd ed.;Science Press:Beijing,2008;pp 322-334.[胡荣祖,高胜利,赵凤起,史启桢,张同来,张建军.热分析动力学.北京:科学出版社,2008:322-334.]
(26)Zhao,F.Q.;Hu,R.Z.;Gao,H.X.;Ma,H.X.T hermochemical P roperties,Nonisothermal Decomposition R eaction Kinetics and Quantum Chemical Investigation of 2,6-Diamino-3,5-dinitropyrazine-1-oxide(LLM-105),New Developments in Hazardous Materials Research;Nova Science Publishers Inc.:New York,2006;Chapter 4.
(27)Ma,H.X.;Song,J.R.;Zhao,F.Q.;Hu,R.Z.;Xiao,H.M.J.Phys.Chem.A 2007,111,8642.doi:10.1021/jp073092o
(28)Zhang,T.L.;Hu,R.Z.;Xie,Y.;Li,F.P.Thermochim.Acta 1994,244,171.doi:10.1016/0040-6031(94)80216-5
(30)Hu,R.Z.;Zhang,H.;Xia,Z.M.;Guo,P.J.;Gao,S.L.;Shi,Q.Z.;Lu,G.E.;Jiang,J.Y.Chin.J.Energ.Mater.2003,11,130.[胡荣祖,张 海,夏志明,郭鹏江,高胜利,史启祯,路桂娥,江劲勇.含能材料,2003,11,130.]
(31)Chen,P.;Zhao,F.Q.;Luo,Y.;Hu,R.Z.;Gao,S.L.;Zheng,Y.M.;Deng,M.Z.;Gao,Y.Chinese Journal of Ch emistry 2004,22,1056.
(32)Yi,J.H.;Zhao,F.Q.;Hong,W.L.;Xu,S.Y.;Hu,R.Z.;Chen,Z.Q.;Zhang,L.Y.J.Hazard.Mater.2009,11,021.
(33)Li,Z.L.;Ma,Z.L.;Xiao,Z.L.;Zhang,X.Z.Ch in.J.E nerg.Mater.2006,14,95.[李志良,马忠亮,肖忠良,张续柱.含能材料,2006,14,95.]
(34)Wang,H.;Zhao,F.Q.;Gao,H.X.;Li,S.W.;Hao,H.X.Chin.J.Energ.Mater.2006,14,45.[王 晗,赵凤起,高红旭,李上文,郝海霞.含能材料,2006,14,45.]
(35)Hong,W.L.;Liu,J.H.;Zhao,F.Q.;Li,Y.M.;Luo,Z.K.Acta Chim.S in.2005,63,249.[洪伟良,刘剑洪,赵凤起,李玉梅,罗仲宽.化学学报,2005,63,249.]
(36)Hong,L.W.;Li,L.L.;Zhao,F.Q.;Tian,D.Y.;Liu,J.H.;Zhang,P.X.J.Solid Rocket.Technol.2007,30,135. [洪良伟,李琳琳,赵凤起,田德余,刘剑洪,张陪新.固体火箭,2007,30,135.]
(37)Hong,L.W.;Zhao,F.Q.;Liu,J.H.;Tian,D.Y.;Luo,Z.K.;Chen,P.;Luo,Y.Chin.J.Inorg.Ch em.2004,20,996.[洪良伟,赵凤起,刘剑洪,田德余,罗仲宽,陈 沛,罗 阳.无机化学学报,2004,20,996.]
(38)Yi,J.H.;Zhao,F.Q.;Gao,H.X.;Xu,S.Y.;Wang,M.C.;Hu,R.Z.J.Hazard.Mater.2008,153,261.doi:10.1016/j.jhazmat.2007.08.064
