樟脑酸根及双咪唑基配体构筑的镉ギ配位聚合物的合成、晶体结构及荧光性质
2013-10-17刘光祥
刘光祥
(南京晓庄学院生物化工与环境工程学院,南京 211171)
0 Introduction
Metal-organic coordination polymers(MOCPs)on the basis ofself-assembly ofmetalions and multifunctional ligands have been rapidly developed because of their fascinating structural diversities and potential applications in the fields of catalysis,gas absorption,chirality,luminescence,nonlinear optics,and magnetism[1-3].Designing of coordination polymers with specified properties remains an intriguing challenge to synthetic chemists,in terms of choosing both organic ligands as well as metal ions[4-5].A vast literature has been reported in the judicious selection of organic ligands in attaining the desirable topologies[6].MOCPs,based on rigid carboxylate linkers,have been widely studied and highly explored[7].In contrast to the rigid ligands,the rational design of coordination polymers based on flexible ligands are quite interesting in terms of the self-assembly process and structureproperty relationship[8].Because of the conformational freedom of the flexible ligands,it results in formation of both discrete macrocylces and infinite polymeric structures;conversion between these two types of structures (discrete and polymer)could also be achieved by the ring-opening isomerism[9].Cao and his group studied and reviewed the coordination polymers based on flexible ligands[10]The conformational freedom of flexible ligands offers the possibility to construct unpredictable and interesting coordination networks with useful properties.The final structures,based on flexible ligands,are subjected to several factors,such as synthetic conditions (temperature,pH,pressure,and solvents), coordination geometry of metal ions,geometricaldisposition ofdonor sites,template molecules,and so on[11-13].Thus,an investigation of the correlation between the subtle conformation of a flexible ligand and the topology of the coordination network formed is a challenging task for inorganic chemists.
On the other hand,among the N-donor bridging ligands,bis(imidazole)ligands,as an important family of flexible N-donor ligands,have attracted great interest[14].In our previous work[15],we have reported a series of fascinating archetypal structures based on the 1,4-bis(imidazol-1-ylmethyl)benzene(bix)or 4,4-bis(imidazol-1-ylmethyl)biphenyl (bimb) ligands.On careful inspection of the reported cases,we found that the flexible nature of the alkyl(-CH2-)spacer allows the ligands to bend and rotate freely so as to conform to the coordination geometries of central metal atoms.1,3,5-Tris (imidazol-1-ylmethyl)-2,4,6-trimethylbenzene)(titmb)is a flexible tri(imidazole)ligand that was concerned in recentyearsasa resultoftheir pluridentated and excellent coordinated ability[16].Building on the above consideration,we select camphor acid (H2CAM)as the main ligand,in the presence of neutral titmb as auxiliary ligand and Cd(NO3)2·4H2O to construct a new three-dimensional coordination polymer of{[Cd2(titmb)(CAM)2]·2H2O}n(1).Herein,we report the synthesis, structural characterization and fluorescent properties.
1 Experimental
1.1 Materials and general methods
All reagents for syntheses and analyses were purchased from commercial sources and used as received without further purification.The titmb ligand was synthesized in accordance with the procedure reported in the literature[17].Elemental analyses(C,H and N)were performed on a Vario EL III elemental analyzer.The IR spectrum as a KBr disk was recorded on a Nicolet Avatar 360 FTIR spectrometer.The luminescence spectra for the powdered solid samples were measured at room temperature on a Hitachi F-4500 fluorescence spectrophotometer with a xenon arc lamp as the light source.In the measurements of emission and excitation spectra the pass width is 5 nm.All the measurements were carried out under the same experimental conditions.Thermal gravimetric analyses (TGA)were performed on a Netzsch STA-409PC instrument in flowing N2with a heating rate of 10℃·min-1.
1.2 Synthesis of{[Cd2(titmb)(CAM)2]·2H2O}n(1)
A mixture containing Cd(NO3)2·4H2O (61.6 mg,0.2 mmol),H2CAM (20.0 mg,0.1 mmol),titmb(36.0 mg,0.1 mmol)and LiOH·H2O (8.4 mg,0.2 mmol)in 15 ml deionized water was sealed in a 25 mL Teflon lined stainless steel container and heated at 140℃for 3 d.Colorless pillar crystals of 1 were collected by filtration and washed with water and ethanol several timeswith a yield of52%.Anal.Calcd.for C41H56N6O10Cd2(%):C,48.39;H,5.55;N,8.26.Anal.Found (%):C,48.42;H,5.58;N,8.24.IR spectrum:3 463,3 049,2 927,1 607,1 561,1 533,1 519,1 441,1 382,1 281,1 132,1 090,1 029,952,827,822,769,725,651 and 543 cm-1.
1.3 X-ray crystallography
A colorless block single crystal of 1 with 0.28 mm×0.24 mm×0.22 mm was carefully selected under a polarizing microscope and glued at the tip of a thin glass fiber with cyanoacrylate (super glue)adhesive.Single crystal structure determination by X-ray diffraction was performed on a Bruker Smart Apex II CCD diffractometer equipped with a normal focus,3 kW sealed tube X-ray source (Mo Kα radiation,λ=0.071 073 nm)operating at 50 kV and 30 mA.Data processing was accomplished with the SAINT processing program[18].Empirical absorption correction was applied.The structure was solved by the direct method using SHELXS-97[19]and refined by full-matrix least squares on F2using SHELXL-97[20].All of the nonhydrogen atomswererefined anisotropically.The hydrogen atoms were added according to the theoretical model.The crystal parameters date collection and refinement results for complex 1 is shown in Table 1.The selectedbonddistancesandanglesarelistedinTable2.
CCDC:899224.

Table 1 Crystal data and structure refinement for complex 1

Table 2 Selected bond lengths(nm)and angles(°)
2 Results and discussion
2.1 Crystal structure
Single-crystal X-ray structural analysis shows that complex 1 crystallizes in the monoclinic system with P1 space group.The asymmetric unit of 1 contains Cd1 and Cd2 cations,both lying in general positions,two crystallographically independent CAM2-dianions,one titmb ligand and two free lattice water molecules.As shown in Fig.1,both Cd1 and Cd2 ions are sixcoordinated, displaying distorted octahedral geometries.Cd1 ion is coordinated by five carboxylate oxygen atoms from three different CAM2-dianions and one nitrogen atom from one titmb ligand.Cd2 ion is coordinated by four carboxylate oxygen atoms from two different CAM2-dianions,and two nitrogen atoms from two individual titmb ligands.The Cd-O distances range from 0.218 9(4)to 0.244 6(4)nm,and the Cd-N distance is the range of 0.218 1(5)~0.223 7(5)nm.The O(N)-Cd-O(N)bond angles range from 50.95(14)to 161.36(15),which are similar to those observed in the reported complexes[21].It is interesting to note that the two types of CAM2-dianions show different coordination modes.One bridges two Cdギ atoms with both two carboxylate groups in bidentate chelating coordination modes,while the other connects three Cdギatoms with one of two carboxylate groups in a bidentate chelating coordination mode and the other one in a bidentate bridging coordination mode.As such,CAM2-dianions bridge Cd(II)ions to form a 1D[Cd(CAM)]ndouble chain with[Cd4(CAM)4]loops(Fig.2).Interestingly,these 1D[Cd(CAM)]ndouble-chains are further linked by titmb ligands with cis,trans,trans conformation to generate a 3D framework(Fig.3).In addition, intermolecular hydrogen-bonding interactions exist among the lattice water molecules and carboxylate oxygen atoms,which further stabilized the structure of 1.
Better insight into such elegant frameworks can be achieved using the topology method:first,based on the considerations of the their connectivity,Cd2,the dimer Cd2and titmb ligand are viewed to be four-,six-,and three-connected nodes;second,Cd-O/N coordination bonds,CAM ligands are simplified to be linear connectors;then the combination ofnodes and connectors suggests the(3,4,6)-connected frameworks for the present case.The topological notation for such(3,4,6)-connected frameworks is(4.64.8)2(42.64.89)(62.8)2net(Fig.4).In the literature[22-24],the(3,4)-,(3,6)-and(4,6)-connected frameworks are often encountered,but we are not aware of a precedent characterized by such topology like that found in 1.
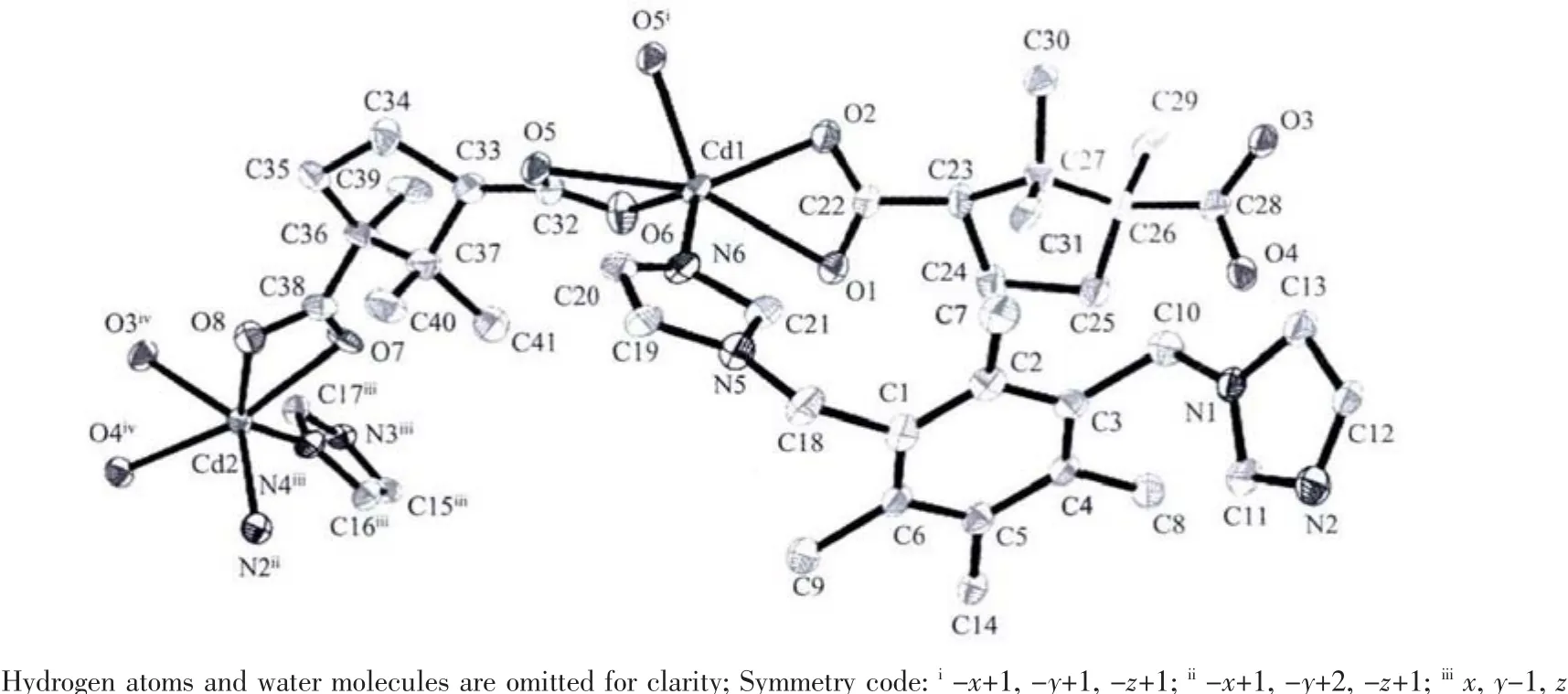
Fig.1 Asymmetric unit and coordination environment of Cdギ in complex 1

Fig.2 1D double-chain structure with rhombic voids in complex 1

Fig.3 View of a 3D framework extended by the titmb ligands in 1

Fig.4 Topological illustration for the trinodal(3,4,6)-connected(4.64.8)2(42.64.89)(62.8)2net of 1
2.2 IR spectra
The IR spectrum of 1 shows characteristic bands of carboxylate groups at 1607 and 1561 cm-1for the antisymmetric stretching and at1382 cm-1for symmetric stretching.The separations (Δν)between νasym(CO2)and νsym(CO2)bands indicate the presence of bidentate chelating and bridging coordination modes[25].Single-crystal X-ray diffractions show that carboxylate groups coordinate to the metal atoms in 1 in an absolutely same fashion.The absence of the characteristic bands at around 1 700 cm-1in 1 attributed to the protonated carboxylate group indicates that the complete deprotonation of H2CAM upon reaction with Cd ion.The IR spectra of 1 exhibit a characteristic peak of imidazole at 1 519 cm-1.
2.3 Thermogravimetric analyses
Thermal gravimetric analyses(TGA)were carried out to examine the thermal stability of 1.The samples were heated up in flowing N2with a heating rate of 10 ℃·min-1.Complex 1 has two steps of weight losses.The first weight loss of 3.60%in the range of 20~105 ℃ is consistent with the removal of two free lattice water molecules (Calcd.3.54%).The second step from 280 to 530℃can be attributed to the release of the organic ligands.The remaining weight is assigned to CdO(Obsd.25.01%,Calcd.25.23%).
2.4 Luminescent properties
Luminescent complexes are currently of great interestbecause oftheirvariousapplicationsin chemical sensors, photochemistry, and electroluminescent display[26-27]. The luminescent properties of Zn and Cd carboxylate compounds have been investigated[28].The photoluminescent spectra of 1 and free neutral titmb ligand were measured at room temperature.Asillustrated in Fig.5,the intense emission band at 447 nm (λex=370 nm)for 1 is observed.The free titmb ligand exhibits fluorescent emission bands at 423 nm (λex=330 nm).In order to understand the nature of the emission band,the photoluminescence properties of the H2CAM ligand were analyzed.A weak emission(λmax=561 nm)could be observed.In comparison to the free ligands,the emission maximums of 1 have changed.This may be caused by a change in the HOMO and LUMO energy levels of deprotonated CAM2-dianions and neutral ligands coordinating to metal centers,a charge-transfer transition between ligands and metal centers,and a joint contribution of the intraligand transitions or charge-transfer transitions between the coordinated ligands and the metal centers[29-30].These observations indicate that they may be excellent candidates for potential photoactive materials.

Fig.5 Solid-state emission spectra of 1 in the solid state at room temperature
[1]Kitagawa S,Matsuda R.Coord.Chem.Rev.,2007,251:2490-2509
[2]Alexandrov E V,Blatov V A,Kochetkov A V,et al.CrystEngComm,2011,13:3947-3958
[3]Férey G.Chem.Soc.Rev.,2008,37:191-214
[4]Yoon J W,Jhung S H,Hwang Y K,et al.Adv.Mater.,2007,19:1830-1834
[5]Bar A K,Chakrabarty R,Chi K W,et al.Dalton Trans.,2009:3222-3229
[6]Ma B Q,Mulfort K L,Hupp J T.Inorg.Chem.,2005,44:4912-4914
[7]Yaghi O M,O′Keeffe M,Ockwig N,et al.Nature,2003,423:705-714
[8]Dai F,Dou J,He H,et al.Inorg.Chem.,2010,49:4117-4124
[9]Su C Y,Goforth A M,Smith M D,et al.Inorg.Chem.,2003,42:5685-5692
[10]Liu T F,Lu J,Cao R.CrystEngComm,2010,12:660-670
[11]Tanake D,Kitagawa S.Chem.Mater.,2008,20:922-931
[12]Carlucci L,Ciani G,Proserpio D M,et al.Chem.Commun.,2000:1319-1320
[13]Tong X L,Wang D Z,Hu T L,et al.Cryst.Growth Des.,2009,9:2280-2286
[14]Dong B X,Peng J,Gomez-Garcia C J,et al.Inorg.Chem.,2007,46:5933-5941
[15]Liu G X,Zhu K,Xu H M,et al.CrystEngComm,2010,12:1175-1185
[16]Liu H K,Huang X H,Lu T H,et al.Dalton Trans.,2008:3178-3188
[17]Zhang W L,Liu Y Y,Ma J F,et al.Polyhedron,2008,27:3351-3358
[18]SAINT,Version 6.02a;Bruker AXS Inc.:Madison,W1,2002.
[19]Sheldrick G M.SHELXS-97,Program for Crystal Structure Solution,University of Göttingen,Germany,1997.
[20]Sheldrick G M.SHELXL-97,Program for the Refinement of Crystal Structure,University of Göttingen,Germany,1997.
[21]Wang L,You W,Huang W,et al.Inorg.Chem.,2009,48:4295-4305
[22]Yao R X,Hao Z M,Guo C H,et al.CrystEngComm,2010,12:4416-4423
[23]SongX,ZouY,LiuX,etal.NewJ.Chem.,2010,34:2396-2399
[24]Zhang L,Li Z J,Qin Y Y,et al.Chinese J.Struct.Chem.,2008,27:915-918
[25]Nakamoto K.Infrared and Raman Spectra of Inorganic and CoordinatedCompounds.5thEd.NewYork:Wiley&Sons,1997.
[26]Wu Q G,Esteghamatian M,Hu N X,et al.Chem.Mater.,2000,12:79-83
[27]McGarrah J E,Kim Y J,Hissler M,et al.Inorg.Chem.,2001,40:4510-4511
[28]Zheng S L,Yang J H,Yu X L,et al.Inorg.Chem.,2004,43:830-838
[29]Li S L,Lan Y Q,Ma J F,et al.Cryst.Growth Des.,2008,8:675-684
[30]Shi X,Zhu G S,Fang Q R,et al.Eur.J.Inorg.Chem.,2004:185-191
