lmproving Photocatalytic Performance for Hydrogen Generation over Co-Doped Znln2S4under Visible Light
2013-07-25YUANWenHuiLIUXiaoChenLILi
YUAN Wen-Hui LIU Xiao-Chen LI Li
(1School of Chemistry and Chemical Engineering,South China University of Technology,Guangzhou 510640,P.R.China;2College of Environmental Science and Engineering,South China University of Technology,Guangzhou 510640,P.R.China)
1 lntroduction
Photocatalytic hydrogen production from water splitting utilizing solar energy has drawn increasing attention due to its possibility to solve serious problems of energy crisis and environmental pollution.Many effective photocatalysts have been reported,including NaTaO3,1La2Ti2O7,2and K2La2Ti3O10.3However,these photocatalysts can only take advantage of ultraviolet irradiation,which occupies only 5%of the solar energy.Therefore,exploring novel visible-light-driven photocatalysts is quite desired in current photocatalysis research.Recently,various types of visible-light-driven photocatalysts have been reported for hydrogen generation.4-8However,the number of photocatalysts working in the visible light region is limited,and a higher efficiency photocatalyst is needed to be developed.
Compared to other photocatalysts,many kinds of metal sulfides have narrow band gaps that correspond to visible light ab-sorption,implying that they are good candidates for the photoproduction of hydrogen from water.However,binary sulphide photocatalysts such as CdS are known for their instability in the photocatalytic reaction,9and the photocatalytic efficiency is still low.Recently,several multicomponent sulfides have been reported to show high photocatalytic efficiency for hydrogen evolution under visible-light irradiation,10-12informing that multicomponent sulfides may be a new class of efficient visible-light-driven photocatalysts.
Ternary sulfides ZnIn2S4,which belongs to the family of AB2X4semiconductor,has attracted wide interest because of its potential applications in different fields such as charge storage,13thermoelectricity,14photoconduction15and so on.Leiet al.16synthesized ZnIn2S4by hydrothermal method and firstly treated ZnIn2S4as an efficient visible-light-driven photocatalyst for hydrogen evolution in 2003.Guo's group17-19has synthesized ZnIn2S4microspheresviahydrothermal/solvothermal processes and explored their visible-light-driven photocatalytic hydrogen production performance.The results showed that ZnIn2S4turned to be a good candidate for photocatalytic hydrogen production from water under visible light irradiation.It is well known that the doping metal is often indispensable for achieving efficient hydrogen evolution.Luet al.20reported that photocatalytic activity can be effectively improved by doping ZnO with Co2+.
In the present study,we synthesized a series of ZnIn2S4doped with different amounts of Coviaa solvothermal method.Herein,Co-doped ZnIn2S4showed significant improvement of photocatalytic activity compared to pure ZnIn2S4.The effects of Co2+doping on the crystal structure,morphology,optical property,and photocatalytic activity of ZnIn2S4products were discussed in detail.The possible mechanism related to the photocatalytic process was proposed.
2 Experimental
2.1 Chemicals
ZnCl2(AR,≥98.0%,Shanghai Shun Qiang Chemical Reagent Co.Ltd.,China);In(NO3)3·4.5H2O(AR,≥99.5%,Sinopharm Chemical reagent Co.Ltd.,China);CH3CSNH2(AR,≥99.0%,Tianjin Damao Chemical Reagent Factory,China);CoCl2·6H2O(AR,≥99.0%),Na2SO3(AR,≥97.0%),(Tianjin Kemiou Chemical Reagent Co.Ltd.,China);C2H5OH(AR,≥99.7%,Nanjing Chemical Reagent Co.Ltd.,China);Na2S·9H2O(AR,≥99.0%,Guangzhou Chemical Reagent Factory,China).
2.2 Preparation of photocatalysts
All chemicals are analytical grade and used without further purification.The doped ZnIn2S4products were prepared by a solvothermal synthetic method.In a typical procedure,the stoichiometric amounts of ZnCl2(2 mmol),In(NO3)3·4.5H2O(4 mmol),a double excess of thioacetamide,and calculated amount of CoCl2·6H2O were dissolved in 50 mL of absolute ethanol.The mixed solution was then transferred into an autoclave and sealed.The autoclave was maintained at 160°C for 6 h and then cooled down to room temperature naturally.A yellow precipitate was obtained,which was then filtered and washed with absolute ethanol and distilled water for several times.The final product was obtained after dried in a vacuum oven at 60°C for 4 h.
2.3 Characterization
Phase structure of prepared photocatalysts was confirmed by X-ray diffraction(XRD)on a Bruker D8 Advance powder diffractometer(Germany)using CuKαradiation operating at 40 kV and 40 mA.The morphology of ZnIn2S4products was characterized by scanning electron microscopy(SEM,1530 VP,LEO,Germany).The X-ray photoelectron spectroscopy(XPS)measurement was conducted on an Axis Ultra DLD photoelectron spectrometer(Kratos,Britain)using AlKα(1486.6 eV)radiation.The diffuse reflection spectroscopy of the samples was determined by a Hitachi U-3010 UV-Vis-near-IR spectrophotometer(Japan)with BaSO4as the reference.
2.4 Evaluation of photocatalytic activity
Photocatalytic hydrogen evolution reaction was performed in a closed gas-circulating system.The powder of photocatalyst(0.2 g)was dispersed by a magnetic stirrer in an aqueous solution(300 mL)containing Na2SO3(0.25 mol·L-1)and Na2S(0.35 mol·L-1)as electron donors in the cell.A 500 W Xe lamp was used as the light source.Nitrogen was purged through the cell before the reaction to remove oxygen.The temperature for all photocatalytic reactions was kept at(25.0±0.5)°C.The evolved amounts of H2were analyzed by a gas chromatography(thermal conductivity detector(TCD),molecular sieve 0.5 nm column andAr carrier).
In the experiment under visible light,1 mol·L-1NaNO2solution was introduced as the internal circulation condensate agent to remove light with wavelengths shorter than 400 nm.The UV-Vis spectrum of the NaNO2solution showed that it could absorb light effectively with wavelengths below 400 nm and thus act as a cut off filter.
3 Results and discussion
3.1 Structure characterization
Fig.1A gives out the XRD patterns of ZnIn2S4samples with various Co2+doping prepared by solvothermal method.All of these ZnIn2S4samples have almost the same XRD pattern in which all the characteristic peaks can be indexed as hexagonal ZnIn2S4(ICSD-JCPDS card No.01-072-0773,a=0.385 nm,c=2.468 nm).No other impurities such as ZnS,In2S3,oxides or organic compounds related to reactants were detected,indicating that the phase of ZnIn2S4has a high purity.It is noted that the position of peak(006)is shifted slightly to lower angle with increasing Co doping compared to pure ZnIn2S4,as shown in Fig.1B,which results in the increase ofd(006)space.This means that Co2+is incorporated into the lattice of ZnIn2S4,because the ionic radius of Co2+(0.072 nm)is similar to radius of Zn2+(0.074 nm).Furthermore,Co2+may occupy the Zn2+site,since charge compensation was very easy in this case.0.3%(w)doping may be the upper limit of Co2+doping for its substitution for Zn2+.Higher doping with concentrations more than 0.3%(w)can not obviously affect the position of ZnIn2S4diffraction peaks anymore.

Fig.1 (A)XRD patterns of ZnIn2S4samples with various Co2+doping concentrations;(B)the enlarged diffraction peak at the position of(006)in(A)
3.2 Morphology
The morphology of as-synthesized ZnIn2S4samples was characterized by SEM,which is shown in Fig.2.Under the solvothermal synthetic condition,the ZnIn2S4crystallites self-organize into the microsphere morphology,with an average diameter of about 1-4µm,and have a marigold-like spherical superstructure which is made up of numerous nanosheets,but the microspheres tend to aggregate together.This growth tendency of lamellar structures can be explained by the layered feature of hexagonal ZnIn2S4.21It can be found that different photocatalysts have the similar morphology,when doping amount ranges from 0.0%to 0.7%.When the amount of Co-doping is 1.0%,the shape of microspheres for Co-doped ZnIn2S4could be destructed partially and even thoroughly.This means that the higher amount of Co doping will hinder the assembly of ZnIn2S4microspheres.It is found that the amount of Co doping has an important influence on the morphology of ZnIn2S4.
3.3 Compositional analysis
To investigate the surface compositions and chemical state,the ZnIn2S4sample was also characterized by XPS,as shown in Fig.3.The binding energy in the XPS analysis was corrected for specimen charging by referencing carbon 1sto 284.6 eV.The peaks around 443.3 and 450.8 eV correspond to the binding energy of In 3d5/2and In 3d3/2of ZnIn2S4(Fig.3b),which is in agreement with the value for In3+.22Zn 2pshows two peaks at 1019.9 and 1043.2 eV,which is consistent with a valence of Zn2+(Fig.3c).22The S 2ppeak of ZnIn2S4at 160.0 eV(Fig.3d)can be assigned to S2-.17The Co 2pcore(Fig.3e)splits into 2p3/2(781.0 eV)and 2p1/2(795.3 eV)peaks which confirms that Co is present as Co2+.20It should be mentioned that,owing to the low concentration and high dispersion of Co2+ions,the XPS data of sample show a high noise.All these results indicate that the chemical states of the sample are In3+,Zn2+,S2-,and Co2+.The molar ratio is observed to be 1:2.16:4.09(Zn:In:S)which is very closely matching with the theoretical one.C and O in the sample may come from the reference and adsorbed gaseous molecules,respectively.23

Fig.2 SEM images of ZnIn2S4prepared with various Co2+doping concentrations
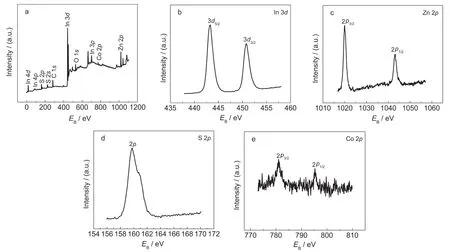
Fig.3 XPS spectra of 0.3%(w)Co doped ZnIn2S4
3.4 Optical properties
Fig.4 shows diffuse reflectance spectra of various Co-doped ZnIn2S4samples.It can be seen that the absorption edges shift to the lower energy region with the increase in the concentration of Co,corresponding to longer wavelength of the spectra in the visible region.There is an almost monotonous enhancement of absorption in visible light region(>500 nm)with the increment of Co concentration.Additionally,Co2+is a colored ion,which acts as a chromophore and can easily absorb light in the visible region.24Co-doped ZnIn2S4may enhance the light absorption and lead to the high light harvesting efficiency in the visible range.Furthermore,the absorption band in the visible light region 650-800 nm can be ascribed tod→dtransition of Co2+.Such kind of transition cannot be used for photocatalytic reactions.20

Fig.4 UV-Vis spectra of ZnIn2S4with various Co2+doping concentrations
3.5 Evaluation of photocatalytic activity

Fig.5 Hydrogen evolution over ZnIn2S4photocatalysts with various Co2+doping concentrations
Photocatalytic H2production with various Co2+doping was evaluated.As shown in Fig.5,all the samples are active and stable for hydrogen productionviawater splitting.With increasing concentration of doped Co,the photocatalyst shows higher photocatalytic activity.ZnIn2S4with 0.3%(w)Co doping displays the highest activity and the hydrogen production rate can reach as high as 200.5 μmol·h-1.These observations indicate that Co2+doping can improve the photocatalytic activity of the ZnIn2S4photocatalyst.From the XRD results,we can observe that substitution of Zn2+by Co2+results in the increase ofd(006)space,which promotes the photogenerated charge sepa-ration in ZnIn2S4photocatalysts.18It was also found that the upper limit of Co2+doping is 0.3%(w),which is in line with hydrogen production results,showing that 0.3%(w)Co2+doping exhibits the highest hydrogen production.As the concentration of Co doping was above 0.3%(w),the photocatalytic activity was decreased,though the visible-light absorption band grew further.Such a similar dependence of photocatalytic H2evolution upon the amount of dopant has been observed for several other photocatalysts.20,25,26These observations indicate that the photocatalytic activites are dependent upon not only the visible-light absorption but also some other factors.One of the reasons for the decrease in photocatalytic activity may be due to the excess of Co doping.Higher Co2+doping can hardly dope into the ZnIn2S4lattice and might just stay at its surface.25The excessive Co may result in the increase of the induced surface defects where the recombination of photogenerated electrons and holes take place,leading to the decreased activity.Another possible inactivation factor for the ZnIn2S4doped with excessive Co is that the microspheres could be destructed gradually with the amount of Co increasing further,as revealed by SEM images.However,the forming of microspheres would facilitate photocatalytic hydrogen production performance of photocatalysts.27
3.6 Mechanism
Based on the experimental results,the possible reaction mechanism can be discussed as follows(shown in Fig.6).The incorporation of Co extends photoresponse region.Such red shift in the absorption edge can be attributed to the formation of localized state dopant energy levels of Co in the band gap of ZnIn2S4.A negative and a positive correction,respectively,to the conduction band(CB)and the valence band(VB)edges result in the band gap narrowing,28which leads to the high light harvesting efficiency.For the ZnIn2S4sample,the photogenerated electrons(e-)can easily transfer from the VB of ZnIn2S4to the localized state dopant energy level.It is reasonable to deduce that there exist strong electronic interactions between Co and ZnIn2S4.For the localized state dopant energy levels of Co with split impurity band states,theelevels of Co are fully occupied,and thet2levels are unoccupied.Consequently,the electrons(e-)inelevels can be excited tot2levels by absorption of visible light.20The incorporation of Co should be beneficial for the effective separation and transport of photogenerated electron-hole pairs in ZnIn2S4and inhibit their recombination,resulting in a superior visible light photocatalytic activity.However,at a high dopant concentration,one charge carrier may be trapped more than once and may recombine with the charge carrier generated by next photo.So the net result is that the dopant again becomes recombination center for photogenerated e-/h+pairs.29This certifies the existence of an optimal Co concentration for highest activity for the doped photocatalyst.

Fig.6 Schematic illustration of the mechanism of photocatalytic hydrogen evolution over Co-doped ZnIn2S4
4 Conclusions
Co doped ZnIn2S4microsphere with flower-like nanoscale petals were synthesizedviaa solvothermal synthesis method.Co2+doping results in the increasing ofd(006)space,which can promote the photogenerated charge separation.Additionally,the light absorption can be enhanced by doping Co2+into crystalline photocatalysts.The incorporation of Co results in the band gap narrowing,which leads to the high light harvesting efficiency.There is an optimal Co doping content of 0.3%(w),which displays the highest activity,with the rate of hydrogen evolution to be 200.5 μmol·h-1.The excessive Co doping works as recombination sites between photogenerated electrons and holes,leading to the decreased activity.
(1)Kato,H.;Asakura,K.;Kudo,A.J.Am.Chem.Soc.2003,125(10),3082.doi:10.1021/ja027751g
(2)Kim,H.G.;Hwang,D.W.;Bae,S.W.;Jung,J.H.;Lee,J.S.Catal.Lett.2003,91(3-4),193.
(3)Chen,W.;Dong,X.F.;Chen,Z.S.;Chen,S.Z.;Lin,W.M.Acta Phys.-Chim.Sin.2009,25(6),1107.[陈 威,董新法,陈之善,陈胜洲,林维明.物理化学学报,2009,25(6),1107.]doi:10.3866/PKU.WHXB20090624
(4)Huang,L.H.;Chan,Q.Z.;Zhang,B.;Wu,X.J.;Gao,P.;Jiao,Z.B.;Liu,Y.L.Chin.J.Catal.2011,32(11-12),1822.doi:10.1016/S1872-2067(10)60286-0
(5) Zou,Z.;Ye,J.;Arakawa,H.;Sayama,K.Nature2001,414(6864),625.doi:10.1038/414625a
(6)Kim,H.G.;Hwang,D.W.;Lee,J.S.J.Am.Chem.Soc.2004,126(29),8912.doi:10.1021/ja049676a
(7) Maeda,K.;Teramura,K.;Lu,D.L.;Takata,T.;Saito,N.;Inoue,Y.;Domen,K.Nature2006,440(7082),295.doi:10.1038/440295a
(8)Ritterskamp,P.;Kuklya,A.;Wustkamp,M.A.;Kerpen,K.;Weidenthaler,C.;Demuth,M.Angew.Chem.Int.Edit.2007,46(41),7770.
(9) Chaudhari,N.S.;Bhirud,A.P.;Sonawane,R.S.;Nikam,L.K.;Warule,S.S.;Rane,V.H.;Kale,B.B.Green Chem.2011,13(9),2500.doi:10.1039/c1gc15515f
(10) Tsuji,I.;Kato,H.;Kobayashi,H.;Kudo,A.J.Phys.Chem.B2005,109(15),7323.doi:10.1021/jp044722e
(11) Tsuji,I.;Kato,H.;Kobayashi,H.;Kudo,A.J.Am.Chem.Soc.2004,126(41),13406.doi:10.1021/ja048296m
(12) Tsuji,I.;Kato,H.;Kudo,A.Chem.Mater.2006,18(7),1969.doi:10.1021/cm0527017
(13) Romeo,N.;Dallaturca,A.;Braglia,R.;Sberveglieri,G.Appl.Phys.Lett.1973,22(1),21.doi:10.1063/1.1654457
(14) Castro,S.L.;Bailey,S.G.;Raffaelle,R.P.;Banger,K.K.;Hepp,A.F.Chem.Mater.2003,15(16),3142.doi:10.1021/cm034161o
(15)Seo,W.S.;Otsuka,R.;Okuno,H.;Ohta,M.;Koumoto,K.J.Mater.Res.1999,14(11),4176.doi:10.1557/JMR.1999.0565
(16) Lei,Z.;You,W.;Liu,M.;Zhou,G.;Takata,T.;Hara,M.;Domen,K.;Li,C.Chem.Commun.2003,2142.
(17) Shen,S.H.;Zhao,L.;Guo,L.J.J.Phys.Chem.Solids2008,69(10),2426.doi:10.1016/j.jpcs.2008.04.035
(18) Shen,S.H.;Zhao,L.;Guo,L.J.Int.J.Hydrog.Energy2008,33(17),4501.doi:10.1016/j.ijhydene.2008.05.043
(19) Shen,S.H.;Zhao,L.;Guo,L.J.Mater.Res.Bull.2009,44(1),100.doi:10.1016/j.materresbull.2008.03.027
(20) Lu,Y.C.;Lin,Y.H.;Wang,D.J.;Wang,L.L.;Xie,T.F.;Jiang,T.F.Nano Res.2011,4(11),1144.doi:10.1007/s12274-011-0163-4
(21) Bai,X.F.;Li,J.S.Mater.Res.Bull.2011,46(7),1028.doi:10.1016/j.materresbull.2011.03.012
(22) Peng,S.J.;Zhu,P.N.;Thavasi,V.;Mhaisalkar,S.G.;Ramakrishna,S.Nanoscale2011,3,2602.doi:10.1039/c0nr00955e
(23) Cai,W.;Zhao,Y.S.;Hu,J.;Zhong,J.S.;Xiang,W.D.J.Mater.Sci.Technol.2011,27(6),559.doi:10.1016/S1005-0302(11)60108-4
(24) Dubey,N.;Labhsetwar,K.;Devotta,S.;Rayalu,S.Catal.Today2007,129(3-4),428.doi:10.1016/j.cattod.2006.09.041
(25) Jing,D.W.;Liu,M.C.;Guo,L.J.Catal.Lett.2010,140(3-4),167.doi:10.1007/s10562-010-0442-9
(26) Shen,S.H.;Zhao,L.;Guo,L.J.J.Phys.Chem.C2008,112(41),16148.doi:10.1021/jp804525q
(27)Zhang,X.;Jing,D.;Liu,M.;Guo,L.J.Catal.Commun.2008,9(8),1720.doi:10.1016/j.catcom.2008.01.032
(28) Wang,B.Q.;Xia,C.H.;Iqbal,J.;Tang,N.J.;Sun,Z.R.;Lv,Y.;Wu,L.Solid State Sci.2009,11(8),1419.doi:10.1016/j.solidstatesciences.2009.04.024
(29) Jing,D.W.;Zhang,Y.;Guo,L.J.Chem.Phys.Lett.2005,415(1-3),74.doi:10.1016/j.cplett.2005.08.080
猜你喜欢
杂志排行
物理化学学报的其它文章
- Origin of the cis-Effect:a Density Functional Theory Study of Doubly Substituted Ethylenes
- Thermodynamic Properties of Nd(Gly)2Cl3·3H2O and Pr(Ala)3Cl3·3H2O
- Vapor-Liquid Equilibrium Data for Carbon Dioxide+Dimethyl Carbonate Binary System
- Core-Shell Nanospheres(HP-Fe2O3@TiO2)with Hierarchical Porous Structures and Photocatalytic Properties
- 中国化学会第四届全国热分析动力学与热动力学学术会议(第一轮通知)
- 第十一届东方胶化杯全国胶体化学研究生优秀成果奖评选通知
