Hepatoprotective effects of cathepsin B inhibitor on acute hepatic failure induced by lipopolysaccharide/D-galactosamine in mice
2013-05-22
Harbin, China
Hepatoprotective effects of cathepsin B inhibitor on acute hepatic failure induced by lipopolysaccharide/D-galactosamine in mice
Bing-Zhu Yan, Li-Yan Chen, Lan Kang, Xiao-Ren Wang, Man-Ru Bi, Wei Wang and Bao-Shan Yang
Harbin, China
BACKGROUND:Increasing evidence suggests that the inactivation of cathepsin B attenuates hepatocyte apoptosis and liver damage. This study aimed to investigate the protective effects of a cathepsin B inhibitor (CA-074me) on lipopolysaccharide (LPS)/D-galactosamine (D-GalN)-induced acute hepatic failure (AHF) in mice.
METHODS:Mice were intraperitoneally injected with a combination of LPS/D-GalN to induce AHF with or without CA-074me pretreatment. The cumulative survival rates were calculated 48 hours after the induction of AHF. As well as changes in biochemical indicators and liver histology, hepatocyte apoptosis was assessed using a TUNEL method. Serum tumor necrosis factor-α (TNF-α) production, caspase-3, caspase-8, and caspase-9 activity was evaluated. Cytosolic cytochrome c and Bcl-2 expression were measured by Western blotting.
RESULTS:The marked elevation in serum aminotransferase activity and prothrombin time found in LPS/D-GalN-treated mice was signif i cantly improved by pretreatment with CA-074me. The eff i cacy of CA-074me was also conf i rmed by histological analysis and TUNEL assay. The survival rate signif i cantly improved in LPS/D-GalN-induced mice given CA-074me compared with untreated mice. LPS/D-GalN-induced caspase-3 and caspase-9 activation was remarkably suppressed by CA-074me. However, the increased levels of serum TNF-α and elevated caspase-8 activity in AHF mice were not signif i cantly reduced by CA-074me. Moreover, CA-074me sharply reduced the increased expression of cytosolic cytochrome c and markedly augmented Bcl-2 expression.
CONCLUSION:These results suggest that CA-074me has a protective effect in acute hepatic failure induced by LPS/D-GalN. (Hepatobiliary Pancreat Dis Int 2013;12:80-86)
acute hepatic failure;cathepsin B inhibitor; hepatocyte apoptosis; caspases; cytochrome c
Introduction
The clinical syndrome of acute hepatic failure (AHF) is life-threatening and complex, including hepatic encephalopathy, severe coagulopathy, jaundice, and hydroperitoneum[1,2]The mortality rate of AHF is extremely high without liver transplantation, and there is still a lack of satisfactory therapeutic approaches.[3,4]Although recent advances in the treatment of AHF have been widely reported,[5]the precise mechanisms involved in recovery remain to be fully elucidated. Therefore, prevention and management of AHF is still a major clinical challenge in the fi eld of liver disease.
Lipopolysaccharide (LPS) in combination with D-galactosamine (D-GalN)-induced liver injury is a well-known experimental model used to develop AHF as a result of massive hepatocyte death.[6]It is generally accepted that the model mimics clinical liver dysfunction and is useful for evaluating the eff i ciency of treatment.[7]A previous study[8]showed that the progression of acute liver injury is cloesely associated with the overproduction of proinf l ammatory cytokines such as tumor necrosis (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6).
Cathepsin B, a lysosomal cysteine protease, is thought to play a pivotal role in apoptosis.[9]Another study[10]reported that cathepsin B is released from lysosomes to the cytosol in response to TNF-α and appears to be dueto the activation of caspase-8. Furthermore, another research group reported that cathepsin B is involved in TNF-α-mediated hepatocyte apoptosis by promoting the release of cytochrome c from mitochondria.[11,12]CA-074me (N-[L-trans-propylcarbamoyloxirane-2-carbonyl]-L-isoleucyl-L-prolinemethylester), which is a highly specif i c cathepsin B inhibitor,[13]is considered to have a protective effect on acute pancreatitis and reduce its severity.[14]
However, no research has been performed on the protective capacity of this cathepsin B inhibitor in hepatic failure. In this study, we focused on exploring the possible mechanisms responsible for the protection by CA-074me against AHF.
Methods
Animals
Male Kunming mice weighing 20-22 g obtained from the Laboratory Animal Center of Harbin Medical University were used. All experiments were carried out according to the guidelines of Harbin Medical University for the care and use of laboratory animals. The mice were maintained under controlled conditions (22±1 ℃, 55% humidity and 12 hours day/night rhythm). All animals were allowed for standard laboratory food and water.
Reagents
The following reagents and kits were used: LPS, D-GalN and CA-074me (Sigma, USA); TUNEL reagent kit (Zhongshan Biotechnical Ltd., Beijing, China); DAB kits (Wuhan Boster Biological Technology Co., Ltd., Wuhan, China); alanine aminotransferase (ALT), aspartate aminotransferase (AST) and prothrombin time (PT) detection kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China); TNF-α ELISA kit (BD Biosciences, San Diego, CA., USA); cytosol fractionation kit (Biovision, CA., USA); caspase-3, caspase-8 and caspase-9 colorimetric assay kits (Bio Vision, CA., USA); and goat anti-mouse cytochrome c mAb and Bcl-2 (Santa Cruz Biotechnology Inc., Santa Cruz, USA).
Experimental groups
For induction of AHF, the mice were intraperitoneally injected with LPS (100 µg/kg) and D-GalN (800 mg/kg). All animals were randomly divided into three groups with 15 mice/group: control group: mice were given physiological saline; LPS/D-GalN group: mice were given only LPS/D-GalN; LPS/D-GalN+CA-074me group: mice were intraperitoneally administered CA-074me (10 mg/kg) 30 minutes before LPS/D-GalN. CA-074me alone did not cause liver damage (data not shown). The survival rate was monitored for 48 hours after LPS/D-GalN administration. Another 75 mice were also grouped as described above. Some of them were sacrif i ced at 1.5 or 6 hours after LPS/D-GalN and serum was collected for measurement of TNF-α. The remaining mice were sacrif i ced by decapitation at 6 hours after LPS/D-GalN administration, and biochemical and histological analyses of blood and liver samples were performed.
Blood biochemistry assay
Blood samples were collected at 6 hours after LPS/ D-GalN injection. After centrifugation for 10 minutes at 3000×g, all serum samples were hemolysis-free and kept at -70 ℃ for determination of biochemical parameters. The serum levels of ALT, AST and PT were measured with an automatic analyzer (AU2700, Olympus, Japan) and a Sysmex 1500 automatic coagulometer.
Histological evaluation
For light microscopy, liver specimens were processed by standard histological techniques (f i xed in 10% formalin, embedded in paraff i n and stained with hematoxylin and eosin). The degree of histological changes including necrosis, hemorrhage and inf l ammation were classif i ed on a severity scale of - to +++ (-: no change; ±: slight change; +: mild change; ++: moderate change; +++: strong change), according to previous descriptions.[15]
TUNEL assay
The extent of apoptosis was determined by terminal deoxynucleotidy1-transferase (TdT)-mediated deoxyuridine triphosphate-digoxigenin (dUTP) nickend labeling (TUNEL), according to the manufacturer's instructions. Hepatocyte apoptosis was quantif i ed by counting the number of TUNEL-positive cells (number of apoptotic nuclei/total number of nuclei ×100). The positive cells were identif i ed in randomly-selected highpower fi elds (×400).
Measurement of serum TNF-α Levels
Serum TNF-α levels were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit, following the manufacturer's instructions.
Evaluation of caspase-3, caspase-8 and caspase-9 activity
Caspase protease activity was measured usingin vitrofl uorogenic peptide substrates, referred to previous studies.[16]Brief l y, a liver sample was homogenized in lysis buffer. After centrifugation for 15 minutes at40 000×g, the supernatant was incubated with substrate peptide (DEVD-AFC for caspase-3, IETD-AFC for caspase-8 and LEHD-AFC for caspase-9). The change in fl uorescence (excitation at 400 nm and emission at 490 nm) was monitored after 120-minute incubation.
Cytochrome c and Bcl-2 determination by Western blotting
The cytosolic proteins of liver samples were prepared using cytoplasmic extraction reagents according to the manufacturer's instructions. Brief l y, equivalent aliquots of protein extract were separated on 12% SDSPAGE gel. After electrophoretic separation, proteins were transferred to polyvinylidene fl uoride (PVDF) membranes, followed by incubation with primary antibodies against cytochrome c, Bcl-2 and β-actin and treatment with secondary antibodies. The image density of specif i c bands was quantif i ed and scanned using a bio-imaging analyzer.
Statistical analysis
Data were expressed as mean±standard deviation, and differences between the groups were calculated by Student'sttest or by ANOVA where appropriate. Survival curves were drawn using the Kaplan-Meier method and analyzed by the log-rank test.Pvalues ≤0.05 were considered statistically signif i cant.
Results
Effects of CA-074me on mortality of LPS/D-GalN-induced mice
All mice in the control group survived. After the induction of AHF, severe hepatic failure rapidly developed and the mice began to die at 8 hours. The mortality of mice not given CA-074me was 53.3% (8/15) at 24 hours and reached 100% (15/15) at 48 hours. However, mice treated with CA-074me had markedly better survival, the mortality being 13.3% (2/15) at 24 hours and 33.3% (5/15) at 48 hours (the Kaplan-Meier method,P<0.01) (Fig. 1).
Effects of CA-074me on serum aminotransferase levels and PT of LPS/D-GalN-induced mice
To identify the acute hepatic injury induced by LPS/D-GalN, the serum levels of ALT and AST were measured. The results showed that they were signif i cantly elevated at 6 hours after LPS/D-GalN treatment. In the mice pretreated with CA-074me, the ALT and AST activities were remarkably decreased by 59.23% and 49.27%, respectively, compared to the LPS/ D-GalN group (P<0.05, Fig. 2A). A marked change in blood coagulation was found as indicated by prolonged PT in LPS/D-GalN mice. Administration of CA-074me shortened PT compared with LPS/D-GalN mice (P<0.05, Fig. 2B).
Histopathological analysis
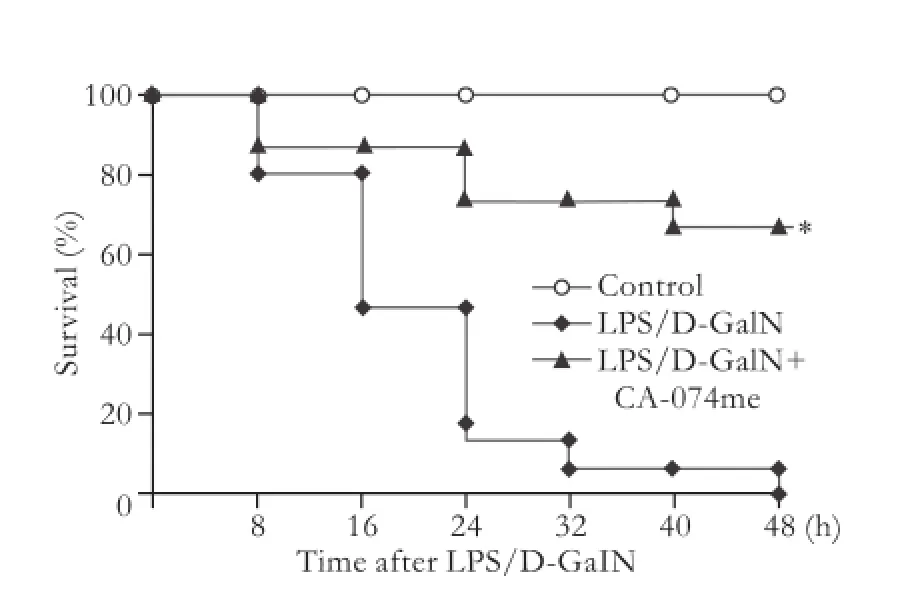
Fig. 1.Effects of CA-074me on the survival rate of LPS/D-GalN-induced mice. *:P<0.01, compared with LPS/D-GalN.
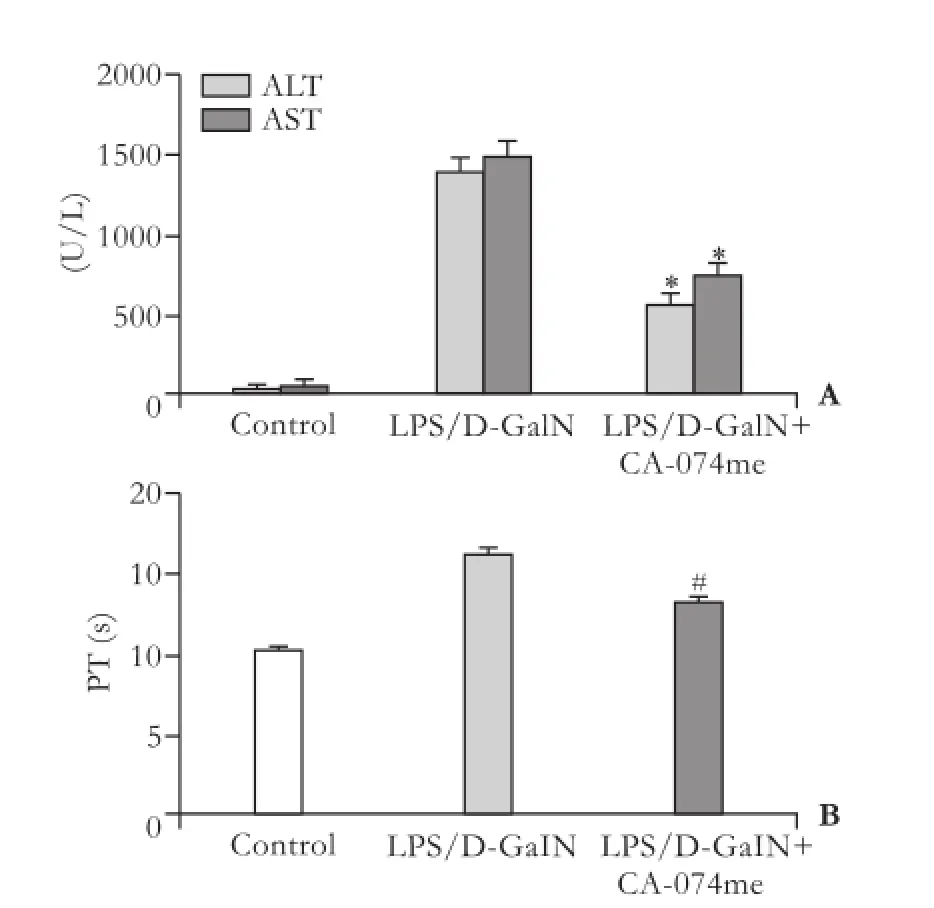
Fig. 2.Effects of CA-074me on the elevation of serum aminotransferases (A) and TP (B) in mice after LPS/D-GalN administration. *, #:P<0.05, compared with LPS/D-GalN.
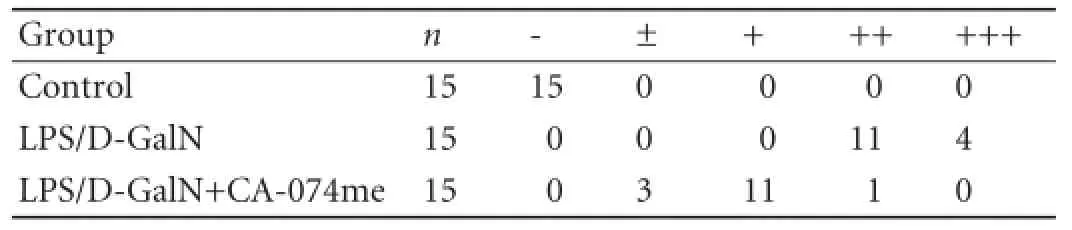
Table 1.Pathological grading of hematoxylin-eosin staining in mice after LPS/D-GalN administration

Fig. 3.Histological fi ndings in mouse liver. Typical images from the different groups (original magnif i cation ×400).A:Control;B:LPS/ D-GalN;C:LPS/D-GalN+CA-074me.
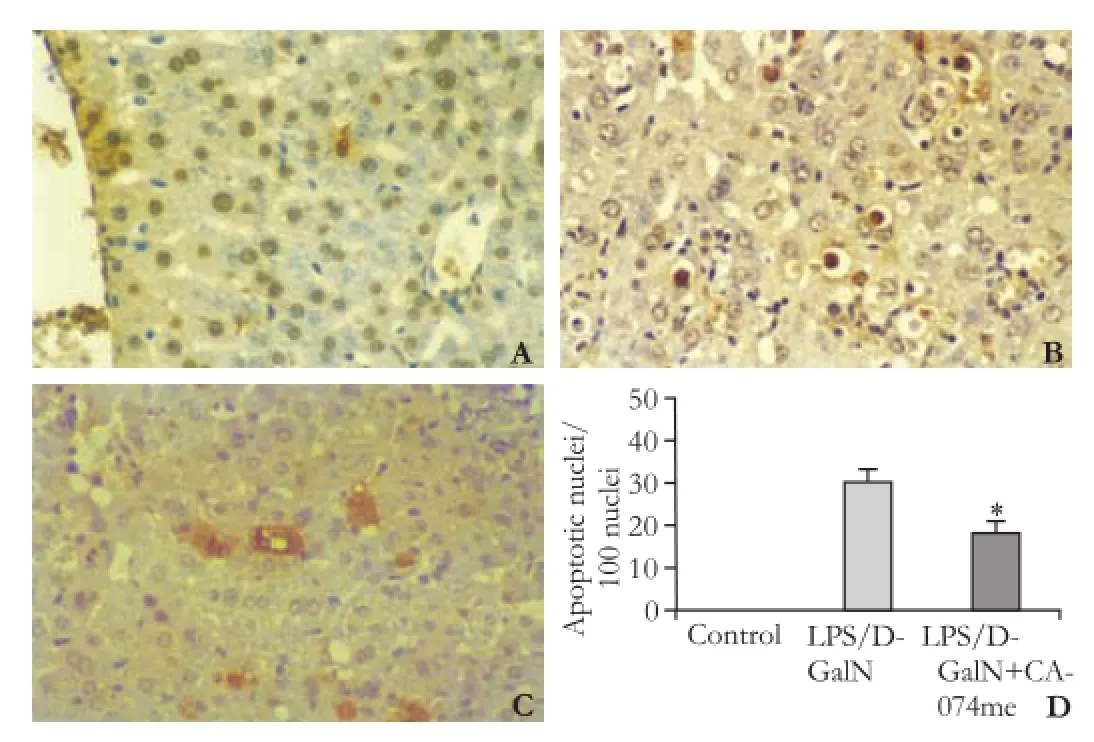
Fig. 4.Counts of apoptotic (TUNEL-positive) hepatocytes in the liver of mice after LPS/D-GalN administration (original magnification ×400).A:Control;B:LPS/D-GalN;C:LPS/ D-GalN+CA-074me;D:TUNEL-positive hepatocytes. Numbers are apoptotic cells in randomly selected high-power fields. *:P<0.01, compared with LPS/D-GalN.
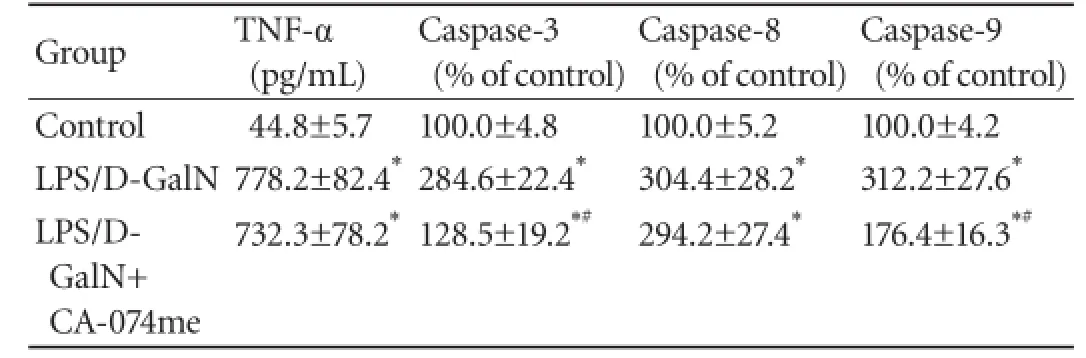
Table 2.Effects of CA-074me on serum TNF-α levels, caspase-3, caspase-8 and caspase-9 activity in mice after LPS/D-GalN administration
The severity of the pathological changes induced by LPS/D-GalN exposure were scored (Table 1). The histological analysis of the liver in the control group revealed normal lobular architecture and cell structure. However, administration of LPS/D-GalN resulted in extensive hepatocyte damage, including inf l ammatory cell inf i ltration, massive necrosis, cellular vacuolar degeneration and hemorrhage. The mice given CA-074me showed clear improvement indicated by a signif i cant reduction in the amount of necrosis (Fig. 3).
CA-074me inhibits hepatocyte apoptosis in mice with LPS/D-GalN-induced AHF
Hepatocyte apoptosis plays a central role in the hepatic injury induced by LPS/D-GalN, which was conf i rmed by the TUNEL assay (Fig. 4). A large number of apoptotic hepatocytes were observed in the liver after administration of LPS/D-GalN. However, only a few apoptotic hepatocytes were found in the liver of mice pretreated with CA-074me compared with the LPS/ D-GalN group (P<0.01).
Effects of CA-074me on LPS/D-GalN-induced TNF-α production
As TNF-α is a critical mediator of the hepatic injury caused by LPS/D-GalN, the effect of CA-074me on TNF-α was assayed by ELISA. Our result showed that the serum levels of TNF-α peaked at 1.5 hours after LPS/ D-GalN administration in comparison with the control group (P<0.01). But administration of CA-074me did not notably decrease this elevation compared with the LPS/D-GalN group (Table 2). A similar observation was made at 6 hours after LPS/D-GalN treatment (415.4± 31.2 vs 398.2±29.4 pg/mL,P>0.05).
Effects of CA-074me on LPS/D-GalN-induced caspase-3, caspase-8 and caspase-9 activity
The activity of caspases 3, 8 and 9 in the cytosol fraction at 6 hours after LPS/D-GalN administration was rapidly increased in comparison with the control group (P<0.01) (Table 2). The elevated activity of caspases 3 and 9 was sharply attenuated by pretreatment with CA-074me (P<0.01). However, CA-074me had no depressive effect on the augmentation of caspase-8 activity induced by LPS/D-GalN injection (P>0.05).
Effects of CA-074me on LPS/D-GalN-induced cytosolic cytochrome c and Bcl-2
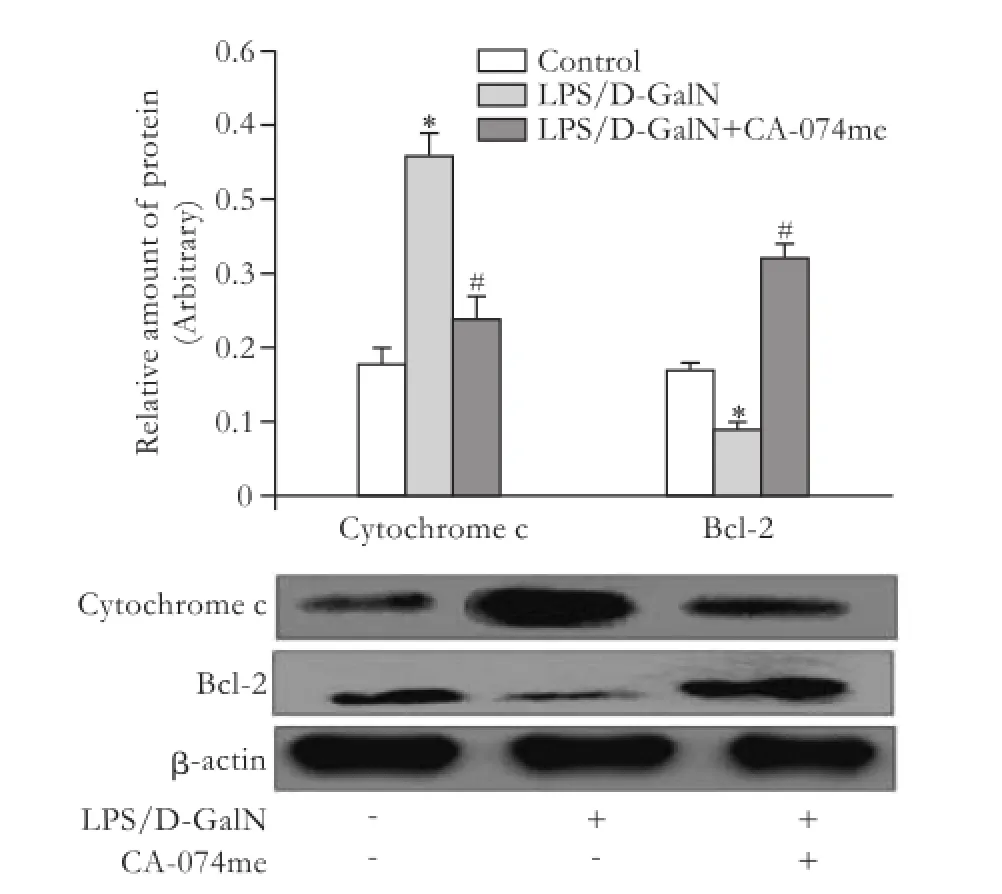
Fig. 5.Effects of CA-074me on cytosolic cytochrome c and Bcl-2 protein expression by Western blotting analysis in liver after LPS/ D-GalN administration. *:P<0.01, compared with control; #:P<0.01, compared with LPS/D-GalN.
After injection of LPS/D-GalN, the cytosolic level of cytochrome c protein increased remarkably compared with the control group. Administration of CA-074me sharply reduced its expression compared with the LPS/D-GalN group. While Bcl-2 protein showed little expression in the LPS/D-GalN group, pretreatment with CA-074me augmented it (Fig. 5).
Discussion
Cathepsin B has been shown to act as the main executor of apoptosis[17]or necrosis.[18]The inactivation of cathepsin B attenuates hepatocyte apoptosis and improves liver damage in several liver disease models.[19,20]Our earlier study[21]showed that cathepsin B plays an essential role in the pathogenesis of AHF, and that LPS/D-GalN-induced elevation of cathepsin B is signif i cantly suppressed by pretreatment with CA-074me.
Exposure to LPS and D-GalN was done to characterize the development of AHF, which is similar to clinical AHF.[22]In this experiment, the death of mice treated with LPS/D-GalN began at 8 hours, and reached 100% by 48 hours. The survival rate of mice was clearly improved by pretreatment with CA-074me. It is generally accepted that serum ALT and AST activity and PT are important indicators, which ref l ect the extent of liver damage in AHF[23,24]Our study showed that pretreatment with CA-074me ameliorated liver function by lowering the serum aminotransferases and reversing PT.
Massive hepatocyte death by apoptosis and necrosis is a key feature of the development of AHF.[25,26]There is growing evidence that anti-apoptosis treatment is likely to be therapeutic in AHF.[27,28]In the present study, the classical histological injuries of inf l ammation, necrosis and apoptosis of hepatocytes induced by LPS/D-GalN were massive. However, the histological grading revealed that the aggravation of hepatic injury was ameliorated by CA-074me. According to the TUNEL assay, CA-074me greatly reduced the hepatocyte apoptosis induced by LPS/D-GalN.
It is well documented that TNF-α is an important pathological factor in mediating hepatic injury, and is involved in the development of AHF induced by administration of LPS/D-GalN.[29]Therefore, the regulation of TNF-α production may be a target for the prevention and treatment of AHF. To explore this possibility, we assessed the effects of CA-074me on the production of TNF-α. As expected, LPS signif i cantly promoted the release of TNF-α in GalN-sensitized mice. However, the up-regulation of the TNF-α level was not signif i cantly reduced by CA-074me pretreatment. Therefore, CA-074me-mediated protection against LPS/D- GalN-treated liver injury is independent of the inhibition of TNF-α production.
A family of cysteine-aspartyl proteases called caspases plays an essential part in the induction and execution of apoptosis.[30]There are two major apoptotic pathways: the extrinsic pathway triggered by the activation of caspase-8, and the intrinsic pathway triggered by the activation of caspase-9.[31]In addition, caspase-3 is a predominant downstream effector activated by caspases 8 and 9.[32]Massive activation of caspases is a crucial process in the induction of apoptosis involved in the pathogenesis of acute hepatic failure.[33]To further conf i rm the specif i c anti-apoptotic mechanisms of CA-074me, caspase activity (caspases 3, 8 and 9) was measured. The activity of all three caspases signif i cantly increased after administration of LPS/D-GalN. Pretreatment with CA-074me sharply reduced the elevated activity of caspases 3 and 9, but not caspase 8.
In the process of apoptosis, the caspase cascade is mainly associated with the release of cytochrome c from mitochondria, and caspases 3 and 9.[31]It is established that the Bcl-2 family, including pro-apoptotic and antiapoptotic proteins, takes part in the control of apoptosis, and the overexpression of Bcl-2 is anti-apoptotic.[34]So, the levels of cytosolic cytochrome c and Bcl-2 were determined by Western blotting analysis in this study. Our results showed that the levels of the anti-apoptotic Bcl-2 protein markedly decreased, while the levels of cytosolic cytochrome c strikingly increased after administration of LPS/D-GalN. Pretreatment with CA-074me inhibited cytochrome c release and augmentedBcl-2 expression. The possible mechanisms underlying the protective effect CA-074me are correlated with the inhibition of apoptosis.
In conclusion, the administration of cathepsin B inhibitor (CA-074me) can increase survival and attenuate hepatic injury. Our fi ndings show that the amelioration is mainly attributable to the inhibition of apoptosis, which is mediated by its blockade of the LPS/D-GalN-induced mitochondrial apoptosis pathway. Therefore, CA-074me may offer an alternative therapy for the prevention of AHF.
Contributors:YBZ and YBS designed the study. YBZ wrote the manuscript. YBZ, CLY, KL, WXR, BMR and WW performed most of the experiments. All authors contributed to the interpretation of the study and to further drafts. YBS is the guarantor.
Funding:None.
Ethical approval:The study was approved by the Ethics Committee of Harbin Medical University.
Competing interest:No benef i ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Lee WM, Squires RH Jr, Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure: Summary of a workshop. Hepatology 2008; 47:1401-1415.
2 Palmes D, Skawran S, Spiegel HU. Acute liver failure: from bench to bedside. Transplant Proc 2005;37:1628-1631.
3 Stravitz RT, Kramer DJ. Management of acute liver failure. Nat Rev Gastroenterol Hepatol 2009;6:542-553.
4 Van Thiel DH, Brems J, Nadir A, Idilman R, Colantoni A, Holt D, et al. Liver transplantation for fulminant hepatic failure. J Gastroenterol 2002;37:78-81.
5 Ben-Ari Z, Zilbermints V, Pappo O, Avlas O, Sharon E, Greif F, et al. Erythropoietin increases survival and attenuates fulminant hepatic failure injury induced by D-galactosamine/ lipopolysaccharide in mice. Transplantation 2011;92:18-24.
6 Rahman TM, Hodgson HJ. Animal models of acute hepatic failure. Int J Exp Pathol 2000;81:145-157.
7 Wang F, Wen T, Chen XY, Wu H. Protective effects of pirfenidone on D-galactosamine and lipopolysaccharideinduced acute hepatotoxicity in rats. Inf l amm Res 2008;57: 183-188.
8 Sekiyama KD, Yoshiba M, Thomson AW. Circulating proinf l ammatory cytokines (IL-1 beta, TNF-alpha, and IL-6) and IL-1 receptor antagonist (IL-1Ra) in fulminant hepatic failure and acute hepatitis. Clin Exp Immunol 1994;98:71-77.
9 Chwieralski CE, Welte T, Bühling F. Cathepsin-regulated apoptosis. Apoptosis 2006;11:143-149.
10 Roberts LR, Adjei PN, Gores GJ. Cathepsins as effector proteases in hepatocyte apoptosis. Cell Biochem Biophys 1999;30:71-88.
11 Guicciardi ME, Deussing J, Miyoshi H, Bronk SF, Svingen PA, Peters C, et al. Cathepsin B contributes to TNF-alphamediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest 2000;106:1127-1137.
12 Guicciardi ME, Miyoshi H, Bronk SF, Gores GJ. Cathepsin B knockout mice are resistant to tumor necrosis factor-alphamediated hepatocyte apoptosis and liver injury: implications for therapeutic applications. Am J Pathol 2001;159:2045-2054.
13 Murata M, Miyashita S, Yokoo C, Tamai M, Hanada K, Hatayama K, et al. Novel epoxysuccinyl peptides. Selective inhibitors of cathepsin B, in vitro. FEBS Lett 1991;280:307-310.
14 Van Acker GJ, Saluja AK, Bhagat L, Singh VP, Song AM, Steer ML. Cathepsin B inhibition prevents trypsinogen activation and reduces pancreatitis severity. Am J Physiol Gastrointest Liver Physiol 2002;283:G794-800.
15 Fukuda T, Mogami A, Tanaka H, Yoshikawa T, Hisadome M, Komatsu H. Y-40138, a multiple cytokine production modulator, protects against D-galactosamine and lipopolysaccharide-induced hepatitis. Life Sci 2006;79:822-827.
16 Morin D, Pires F, Plin C, Tillement JP. Role of the permeability transition pore in cytochrome C release from mitochondria during ischemia-reperfusion in rat liver. Biochem Pharmacol 2004;68:2065-2073.
17 Foghsgaard L, Wissing D, Mauch D, Lademann U, Bastholm L, Boes M, et al. Cathepsin B acts as a dominant execution protease in tumor cell apoptosis induced by tumor necrosis factor. J Cell Biol 2001;153:999-1010.
18 Hentze H, Lin XY, Choi MS, Porter AG. Critical role for cathepsin B in mediating caspase-1-dependent interleukin-18 maturation and caspase-1-independent necrosis triggered by the microbial toxin nigericin. Cell Death Differ 2003;10:956-968.
19 Baskin-Bey ES, Canbay A, Bronk SF, Werneburg N, Guicciardi ME, Nyberg SL, et al. Cathepsin B inactivation attenuates hepatocyte apoptosis and liver damage in steatotic livers after cold ischemia-warm reperfusion injury. Am J Physiol Gastrointest Liver Physiol 2005;288:G396-402.
20 Ben-Ari Z, Mor E, Azarov D, Sulkes J, Tor R, Cheporko Y, et al. Cathepsin B inactivation attenuates the apoptotic injury induced by ischemia/reperfusion of mouse liver. Apoptosis 2005;10:1261-1269.
21 Yan BZ, Wang W, Chen LY, Bi MR, Lu YJ, Li BX, et al. Role of cathepsin B-mediated apoptosis in fulminant hepatic failure in mice. World J Gastroenterol 2009;15:1231-1236.
22 Nakama T, Hirono S, Moriuchi A, Hasuike S, Nagata K, Hori T, et al. Etoposide prevents apoptosis in mouse liver with D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure resulting in reduction of lethality. Hepatology 2001;33:1441-1450.
23 Molander DW, Wroblewski F, Ladue JS. Serum glutamic oxalacetic transaminase as an index of hepatocellular integrity. J Lab Clin Med 1955;46:831-839.
24 Batra Y, Acharya SK. Acute liver failure: prognostic markers. Indian J Gastroenterol 2003;22:S66-68.
25 Liu LM, Zhang JX, Luo J, Guo HX, Deng H, Chen JY, et al. A role of cell apoptosis in lipopolysaccharide (LPS)-induced nonlethal liver injury in D-galactosamine (D-GalN)-sensitized rats. Dig Dis Sci 2008;53:1316-1324.
26 Patel T, Gores GJ. Apoptosis and hepatobiliary disease. Hepatology 1995;21:1725-1741.
27 Lian LH, Wu YL, Wan Y, Li X, Xie WX, Nan JX. Antiapoptotic activity of gentiopicroside in D-galactosamine/ lipopolysaccharide-induced murine fulminant hepatic failure. Chem Biol Interact 2010;188:127-133.
28 Lin BR, Yu CJ, Chen WC, Lee HS, Chang HM, Lee YC, etal. Green tea extract supplement reduces D-galactosamineinduced acute liver injury by inhibition of apoptotic and proinf l ammatory signaling. J Biomed Sci 2009;16:35.
29 Leist M, Gantner F, Jilg S, Wendel A. Activation of the 55 kDa TNF receptor is necessary and suff i cient for TNF-induced liver failure, hepatocyte apoptosis, and nitrite release. J Immunol 1995;154:1307-1316.
30 Fan TJ, Han LH, Cong RS, Liang J. Caspase family proteases and apoptosis. Acta Biochim Biophys Sin (Shanghai) 2005; 37:719-727.
31 Green DR, Reed JC. Mitochondria and apoptosis. Science 1998;281:1309-1312.
32 Harrington HA, Ho KL, Ghosh S, Tung KC. Construction and analysis of a modular model of caspase activation in apoptosis. Theor Biol Med Model 2008;5:26.
33 Leifeld L, Nattermann J, Fielenbach M, Schmitz V, Sauerbruch T, Spengler U. Intrahepatic activation of caspases in human fulminant hepatic failure. Liver Int 2006;26:872-879.
34 Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 1997;275:1132-1136.
Received November 25, 2011
Accepted after revision July 28, 2012
AuthorAff i liations:Department of Infectious Diseases, Second Clinical Hospital, Harbin Medical University, Harbin 150086, China (Yan BZ, Chen LY, Kang L, Wang XR, Bi MR, Wang W and Yang BS)
Bao-Shan Yang, Professor, Department of Infectious Diseases, Second Clinical Hospital, Harbin Medical University, Harbin 150086, China (Tel: 86-451-86297420; Fax: 86-451-86605330; Email: baoshanyang@126.com)
© 2013, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(13)60010-7
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Melanoma in the ampulla of Vater
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Salvage living-donor liver transplantation to previously hepatectomized hepatocellular carcinoma patients: is it a reasonable strategy?
- Clinical operational tolerance in liver transplantation: state-of-the-art perspective and future prospects
- Radiologic-histological correlation of hepatocellular carcinoma treated via pre-liver transplant locoregional therapies
- Outcomes of side-to-side conversion hepaticojejunostomy for biliary anastomotic stricture after right-liver living donor liver transplantation
