Fluorescence Discrimination and Determination Technique for Phytoplankton Composition by Coif2 Wavelet Packet
2013-04-17DUANYaliSURongguoSHIXiaoyongWANGXiulinZHUChenjianandSUNYan
DUAN Yali,SU Rongguo,SHI XiaoyongWANG XiulinZHU Chenjianand SUN Yan
1) College of Chemistry and Chemical Engineering,Ocean University of China,Qingdao 266100,P. R.China
2) Key Laboratory of Marine Chemistry Theory and Technology,Ministry of Education,Ocean University of China,Qingdao 266100,P. R.China
3) Taiyuan University of Technology,Taiyuan 030024,P. R.China
1 Introduction
Harmful algal blooms pose a high threat that requires efforts to reduce or eliminate their negative impacts and consequences (Boeschet al.,1996).In recent years,the frequency and intensity of red tides have increased in coastal areas of China (data from marine environment quality bulletin of China),which leads to untimely restrictions on commercial and recreational shellfish harvesting and deleterious effects on tourism and public health.So,mitigating of some harmful effects from these algal blooms is imperative.Describing and modeling the variability of phytoplankton community structure is a major goal and a base for monitoring harmful algal blooms (HABs) in biological oceanography.
Until now,oceanographers have developed many methods for discrimination of red tide algae species in the ocean.Traditional microscopic method is based on morphology of phytoplankton,and image technology(Pech-Pachecoet al.,1998) is combined with computer technology.Spectral absorption (Johnsenet al.,1994;Millieet al.,1997),spectral fluorescence (Yentschet al.,1985; Seppaelaeet al.,1998) and HPLC analysis(Mackeyet al.,1996) are all based on measurements of phytoplankton pigment composition,and they can provide key information about phytoplankton community structure.Molecular probe method (Walshet al.,1998) is based on DNA sequences of phytoplankton,but it is on the lab scale still.Among these methods,fluorometric analysis has drawn much attention for its higher sensitivity,lower detection levels,and no interference from carotenoid.Here,an approach is presented based on the measurement of the fluorescence excitation-emission spectrum of chlorophyll (Chl) and phycobiliproteins (PBs)with several wavelengths.It yields the differentiated assessment of algae population distributions effectively and fleetly.
Fluorescence information of the photosystem (PS II) is widely used as a tool for describing the Chl contents of algae in aquatic system.In the early seventies,some researches have been made to distinguish different groups of phytoplankton using their fluorescence propertiesin vivo(Yentschet al.,1979; Yentschet al.,1985).Seppaelae & Balodel (1998) used the fluorescence intensity of main accessory pigment to detect changes in the phytoplankton community.Beutler (2002) used light-emitting diodes by means of a five-point excitation spectrum (450,525,570,590,610 nm) and stable norm spectra to differentiate between four algal groups (Chlorophyceae,Cyanophyceae,Cryptophyceae,and the brown group).The distinction between only four spectral groups is not high enough in some important ecological situations,especially in China seas where Bacillario- phyceae and Dinophyceae are ubiquitous.In this work,the fluorescence excitation-emission spectra can give ‘Fingerprint’ information of the algae that can be used to differentiate‘spectral groups’in vivo,especially for discriminating diatom and dinoflagellate.In previous studies,Wavelet analysis (Zhanget al.,2009),Fourth-derivative and Gauss analysis (Lu,2007),and Support vector machine analysis (Huet al.,2008) were utilized to establish the fluorescence discrimination technique.Those results (data not given) indicate the feasibility of the approach based on the differences of the fluorescent characteristics.Here,a novel fluorescence discrimination model for the estimation of algae composition by means of coif2- wavelet packet transform is developed.
2 Materials and Methods
2.1 Algae Culture in Lab
Twenty-four red tide algae were selected from Marine Pollution Eco-chemistry Key laboratory of Ocean University of China (Table 1).All of them were maintained in af/2 (with or without silicate as appropriate) medium at given temperatures (20℃,25℃) for 12 d.The cultures were occasionally given a couple of shakes,under continuous illumination of cool-white fluorescent tubes at 4000,7000,12000,20000lux with a 12h light/12h dark cycle.A total of 276 mixtures were obtained with these cultures in their exponential phase and the dominant species accounted for 75% of the total according to Chlaconcentration that measured by Algae Analyzer (BBE Moldaenke,Germany) (Beutleret al.,2002).
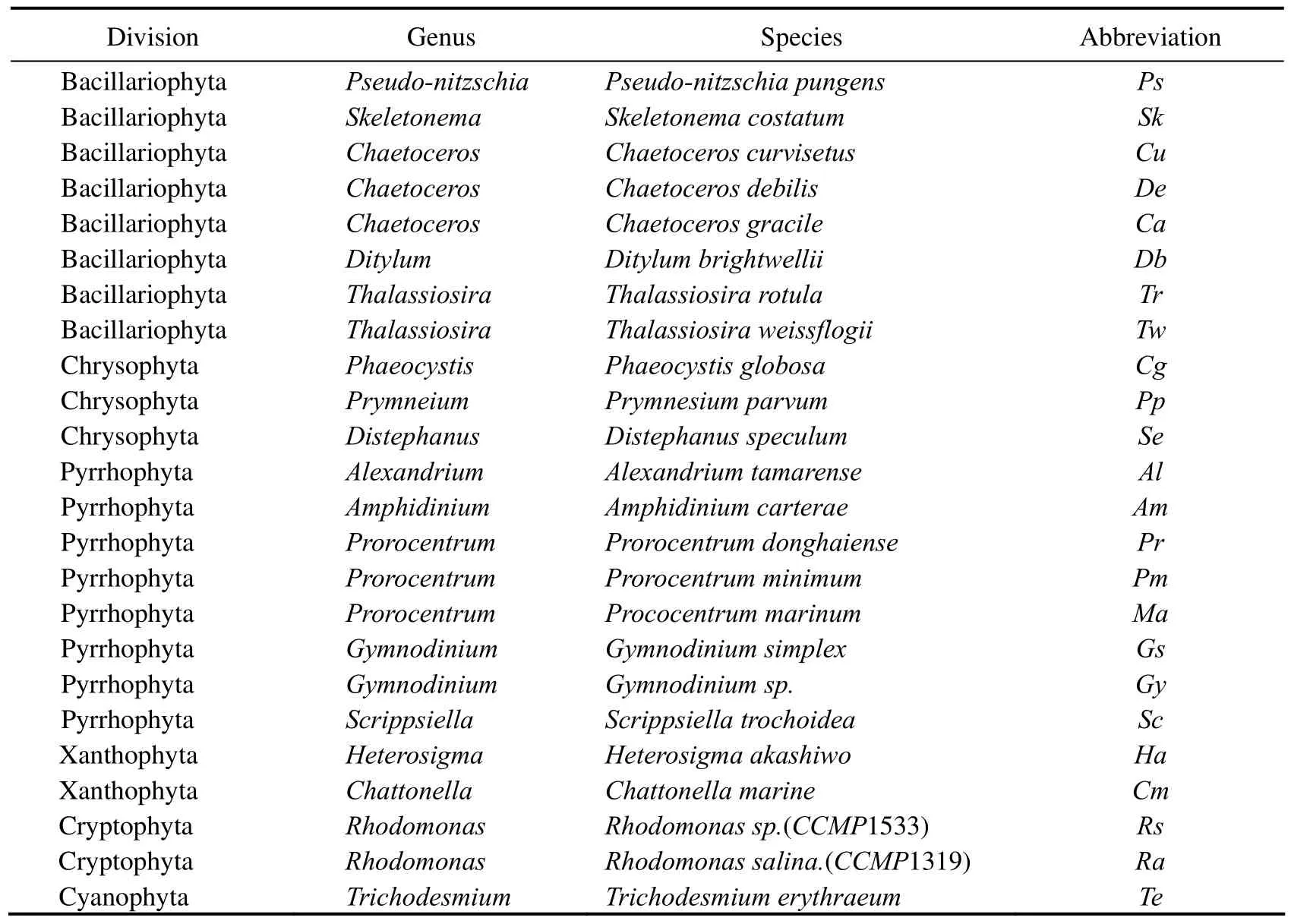
Table 1 Twenty-four phytoplankton species used in this study
2.2 Simulative Mixtures
Special procedure was employed to construct simulative mixtures.The concept of the simulations was that a series of new-construction spectra were constructed with individual spectra of any two species belonging to different divisions,with the dominant species accounting for 60%,70%,80%,90% of the total biomass.The other simulative mixtures were constructed with individual spectra of any two species belonging to different genera with the dominant species accounting for 60%,70%,80%,90%,100% of the total biomass,respectively.
2.3 Field Samples
Twelve water samples were collrrected from the mesocosm experiment conducted at Maidao station in Qingdao.A microscope (OLYMPUS,Japan) was used to identify and count the algal cells.And the proposed fluorescence discrimination technique was applied to analyze the samples.
Another twelve field samples were collected from Jiaozhou Bay (120.17˚E,36.0˚N) on 21 August 2007(Fig.1).Water samples,each being 1-5 L,were taken at the surface and concentrated following the standard method (GB 12763.6-91.,1991) to 50-100 mL before the fluorescence measurements.Chlaconcentrations were determined by the BBE Algae Analyzer.For phytoplankton cell counting,the water samples were first fixed with Lugol’s iodine solution.

Fig.1 sampling stations in Jiaozhou Bay.
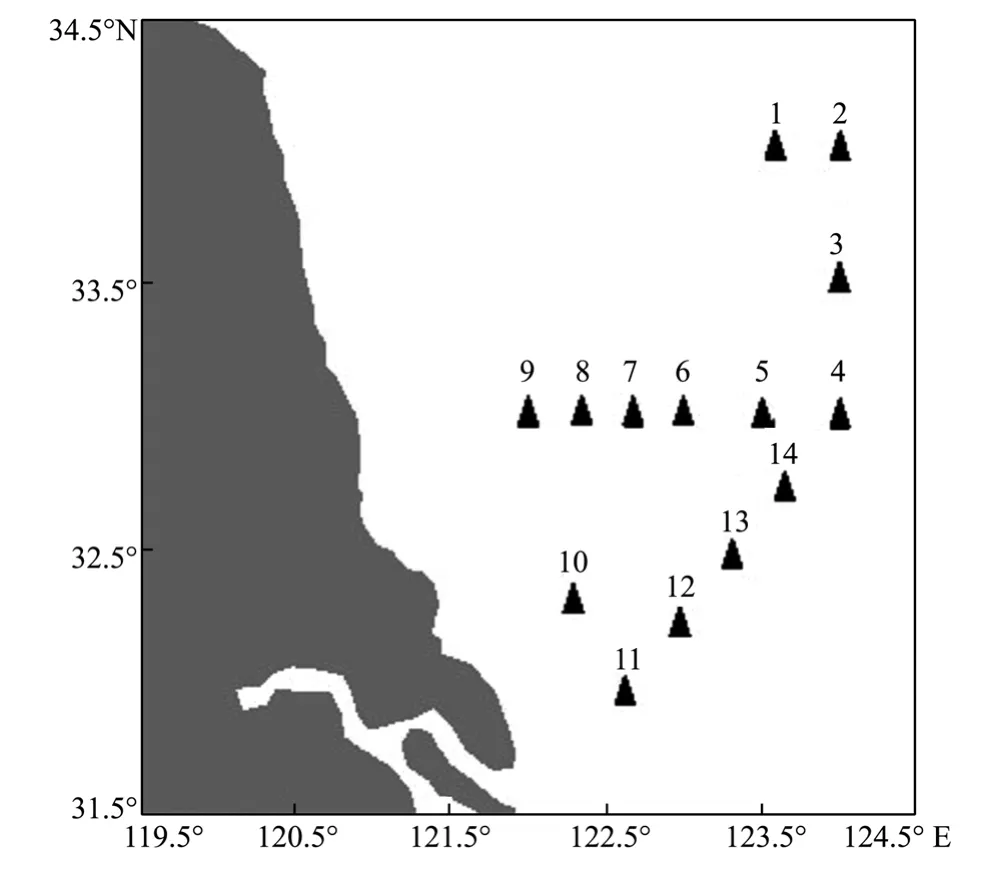
Fig.2 Samplinge stations in the Southern Yellow Sea.
An investigation cruise was carried out in the South Yellow Sea (April 21-25,2010) with sampling stations shown in Fig.2.Water samples were collected using 12 litre Niskin bottles mounted on a CTD/rosette system.At the selected stations,2.5 L samples (0,10 m) were concentrated according to the standard method (GB 12763.6-91.,1991) to 15-20 mL approximately for fluorescence measurements.
2.4 Instruments and Fluorescence Measurements
Fluorescence excitation-emission spectra measurements were completed by a fluorescence spectrometer(Fluorolog 3-11,JobinYvon,France) at room temperature.The spectral range (excitation 400 to 650 nm,emission 600 to 750 nm) aimed to reveal fluorescence of chlorophyllaand phycobiliprotein (phycocyanin and phycoerythrin) fluorescence,both wavelength intervals and slit width being 5 nm.A xenon lamp of 450 w was used as the light source with scanning speed 80 nm per second.The fluorescence of sample in a 1cm quartz cuvette was induced and then via the excitation monochromator then via the emission monochromator,and recorded with a photomultiplier tube for further digital processing.The recorded spectra were exported from the instrument’s software and rearranged in an ASCII format.Excitationemission spectra of two replicates were obtained,one of which was the training set to establish the technique,and the other was used to test the feasibility of the technique.Similar process was conducted for the mixed and the field samples.It was worth noting that the concentration of the algal culture fluid was within the linear range of the fluorometer in the case of the re-absorption that caused a change of the spectral shape.
3 Establishing the Discrimination Technique
3.1 Data Pre-Processing
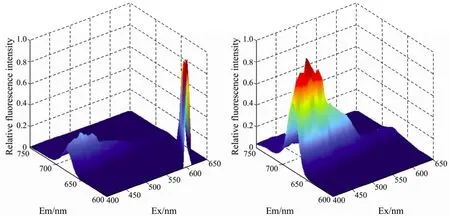
Fig.3 The original FEEMs of the phytoplankton (a) and those without Rayleigh scattering (b).
Such a matrix also yields signals from Rayleigh scattering (Fig.3).To obtain more detailed information on the fluorescence spectra of the algae,they should be removed.Here,we introduced the Delaunay triangulation method(Zeppet al.,2004) to interpolate linearly in the scattering areas concretely.Then,the 3-D data in a view format of 2-D by a specific procedure named ‘end to end’.Series multi-excitation and of both multi-emission spectra were obtained.Then the two types of spectra formed the fluorescence excitation-emission spectra that were also normalized to the maximum fluorescence intensity following the modewherexiis the data pointiin the spectrum,andwhich was used to reveal the dissimilarity between the spectral shapes.All the work were finished by the MATLABr2008a (Mathworks Inc.,Natick,MA,USA).
3.2 Feature Selection
The goal of the feature selection is to extract features for reliable intelligent modeling and provide give the basis of norm spectra database.For feature extraction,wavelet packet transform (WPT) (Mzaik and Jagadeesh,1994; Cherifet al.,2010; Radeet al.,2008) was introduced.It represents a generalization of the method of multiresolution decomposition; according to multi-resolution analysis,we havewhereWjis the wavelet subspace.TheWPTmakes further decomposition forWjand has more flexibility in frequency resolution.The general representation ofWPTdecomposition is:

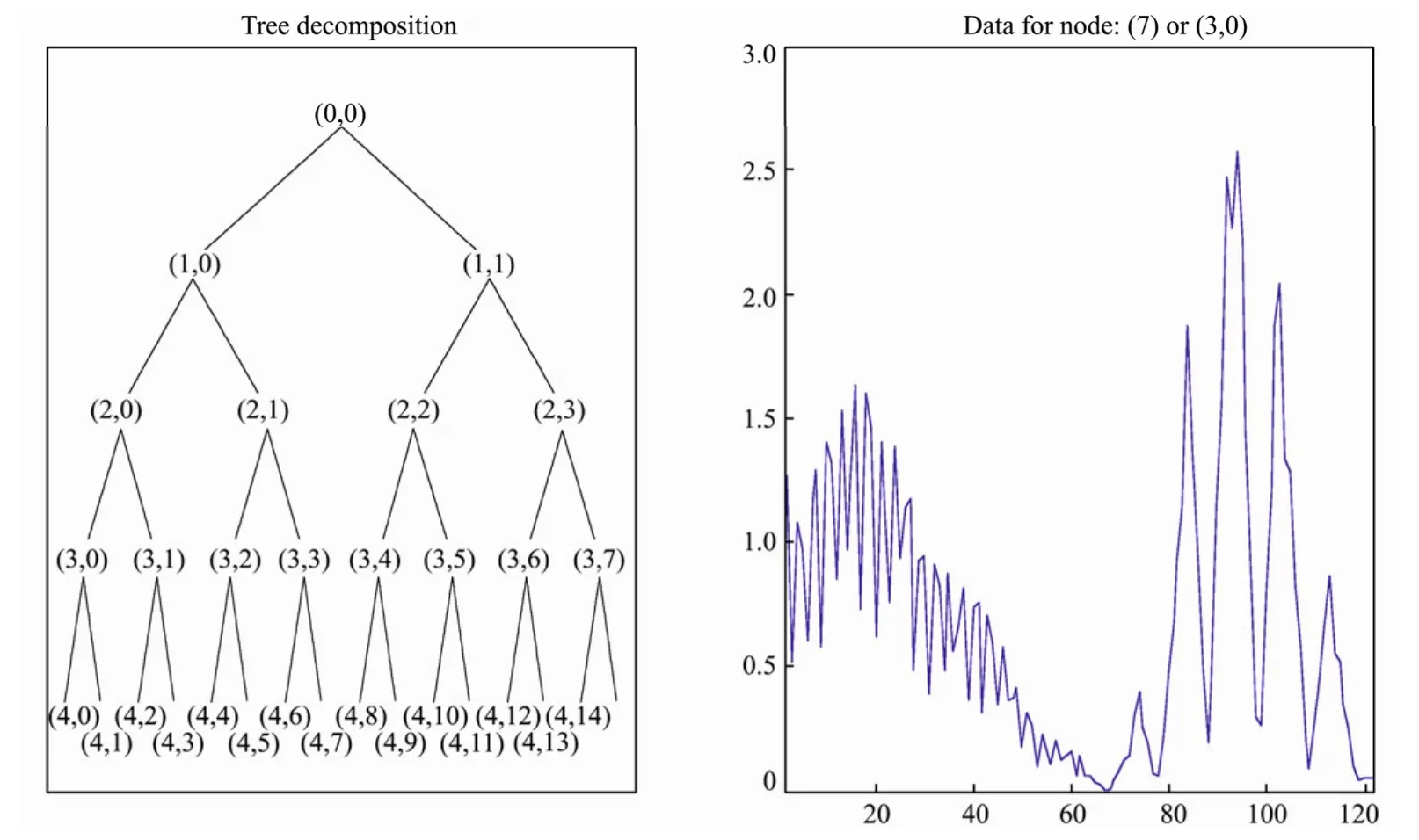
Fig.4 Decomposition structure by ‘coif-2’ wavelet packet.
Feature vectors are based on the coefficients vectors obtained by the decomposition.For each scale,there would be2m(m= 1,2,3,4)candidate coefficient vectors,concretely.Then,which feature vector is the best one containing as much information as possible to achieve the best classification? In this study,Bayes discrimination method was employed to select the optimal feature vectors from existing candidate coefficient vectors.Discrimination error rate was used as the judged criterion(detailed data not given).Finally,we chose the first coefficient vector (3,0) of the third level decomposition with the minimal error rate as the optimal feature spectra(OFS).
3.3 Establishment of the Norm Spectra Database and Identification of Phytoplankton Species
In the course of whole study,large numbers of spectra were obtained according to the different incubated conditions including illumination,temperature,growth stage,and these spectra were always similar for algae species coming from the same genus or same division due to the similar pigment composition.Actually,it was necessary to eliminate the disadvantage to the recognition efficiency resulting from the bulk similar spectra and use the least optimal spectra to achieve the best classification.
Here,Hierarchical cluster analysis was employed to classify all the optimal feature spectra (OFS) of the training samples to acquire the norm spectra.Single-linkage clustering with the Euclidean distance is the measurement of the spectral similarity between each pair of points on two OFS.The hierarchical cluster dendrogram is shown in Fig.5.Here,we use the OFS ofSkeletonema costatumas an example: The OFS ofS.costatumclustered in Fig.5(left) can be classified into five groups (right).However,the fifth group contains only one OFS (the second OFS).Comparatively speaking,the third and fourth OFS that have common features with the second OFS are classified into the second group.The original spectra of the second OFS is abnormal and will be eliminated from further consideration.Thus,the remaining spectra of other four groups were used as the norm spectra to establish the norm spectra database.
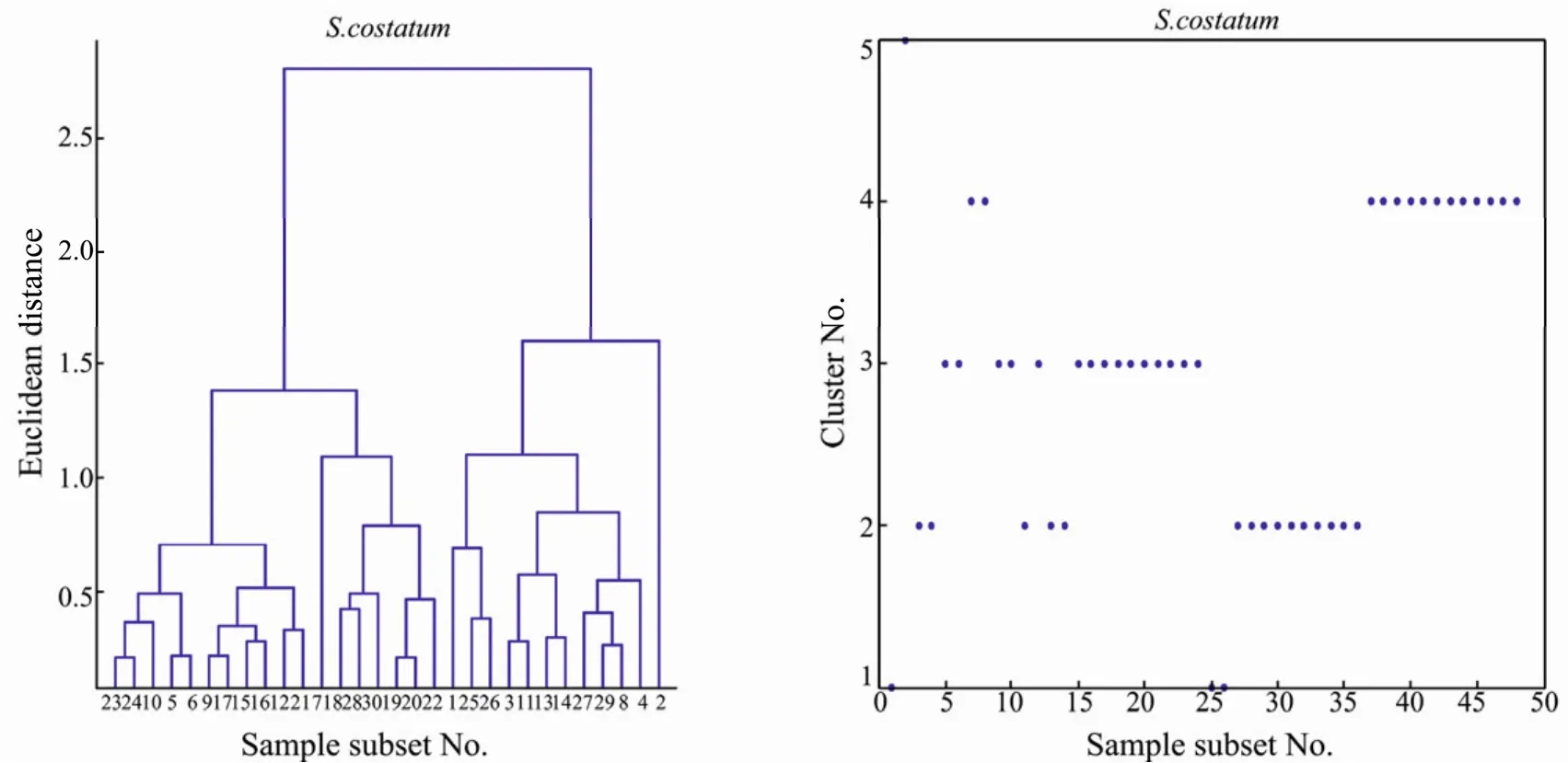
Fig.5 Sample hierarchical clustering graph (left) of the optimal feature spectra and assignment graph (right) of Sk.
Based on the norm spectra database,multiple linear regression analyses resolved by non-negative least square was used to complete the discrimination.Laboratory simulative mixtures and field samples were tested by the method.The regression model waswhereyrepresents the spectra of unknown species,xnis the norm spectra,b0³0,bnis the regression coefficient describing the contribution rate of the spectra from the norm spectra to the unknown spectra,and parametereis residual error between the real and estimated values ofythat should keep minimum.Important and detailed step was to compute the coefficientbnfrom the norm spectra against the unknown spectra in the whole identification process.What has been mentioned above was finished by MATLABr2008a.
4 Results and Discussion
4.1 Discrimination of the Simulative Mixtures
The results (Table 2) with simulative mixtures at the division level indicate that when the algae dominance achieves 60%,70%,80% and 90% of a random divisionrespectively,the average correct discrimination rates(CDRs) are 83.0%,99.1%,99.7% and 99.9% with average relative contents of 58.5%,68.4%,77.7% and 86.3%,respectively.That is to say when the algae species account for more than 60% of the total biomass,it would be identified correctly by the technique at the division level.Furthermore,the method could be used for assessing effectively the phytoplankton community composition qualitatively and quantitatively when marine ecosystem is in or out of red tide period.
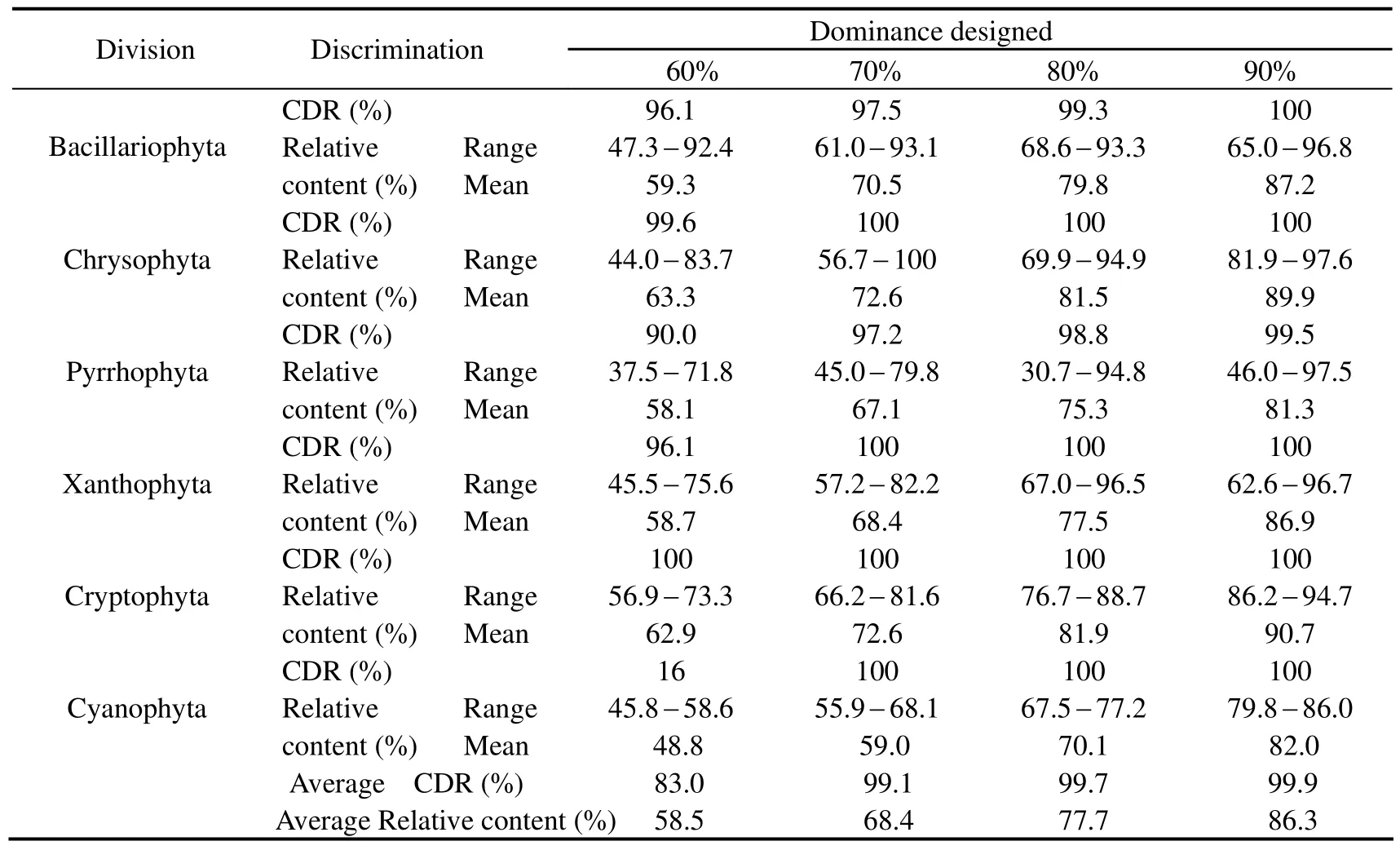
Table 2 Discrimination results of the simulative samples at the division level
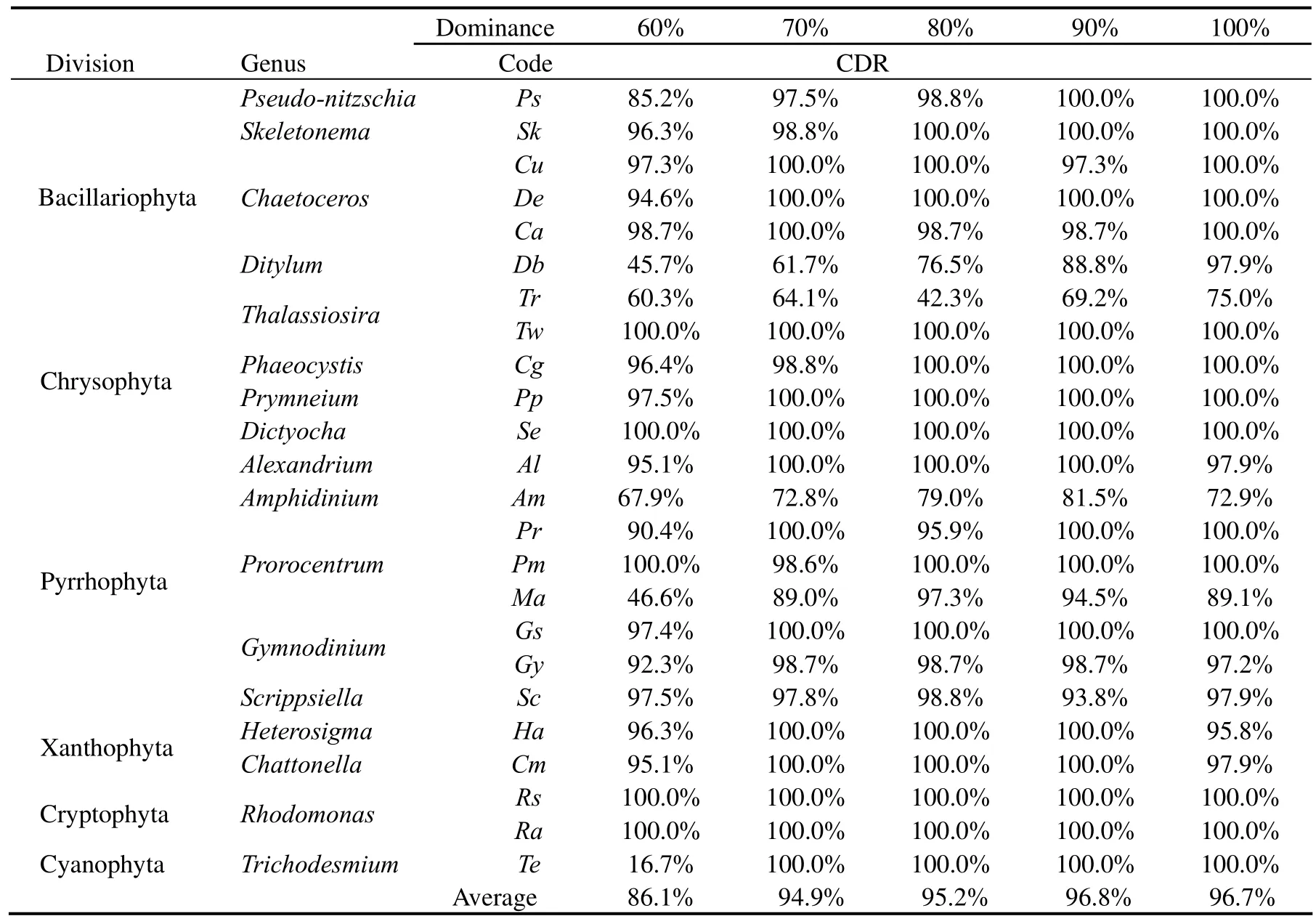
Table 3 Discrimination results of the simulative samples at the genus level
The results (Table 3) of simulative mixtures at the genus level indicate that when the algae dominance achieve 60%,70%,80%,90% and 100% of a random genus respectively,the average correct discrimination rates(CDRs) are 86.1%,94.9%,95.2%,96.8% and 96.7%,respectively.That is to say when the algae species account for above 60% of the total biomass,it would be identified correctly by the presented technique at the genus level.
4.2 Discrimination with the Laboratory Mixtures
Table 4 and Table 5 list the discrimination results with laboratory mixtures by the technique.Relevant details indicate that the majority of species are correctly identified at the division level with the mean CDR of 88.1% no matter what the mixture proportion it may be.The discrimination results were not satisfactory for Chrysophyta.This was perhaps because the number of mixture samples or these species were in unfavorable growing period.In the process of the discrimination,only the first dominantspecies was considered,and the majority of species were identified correctly with the mean CDR of 78.4% except thekareniaandphaeocystisat the genus level.In addition,during the discrimination,norm spectra database and feature spectra were also important influence factors.Further studies are needed.
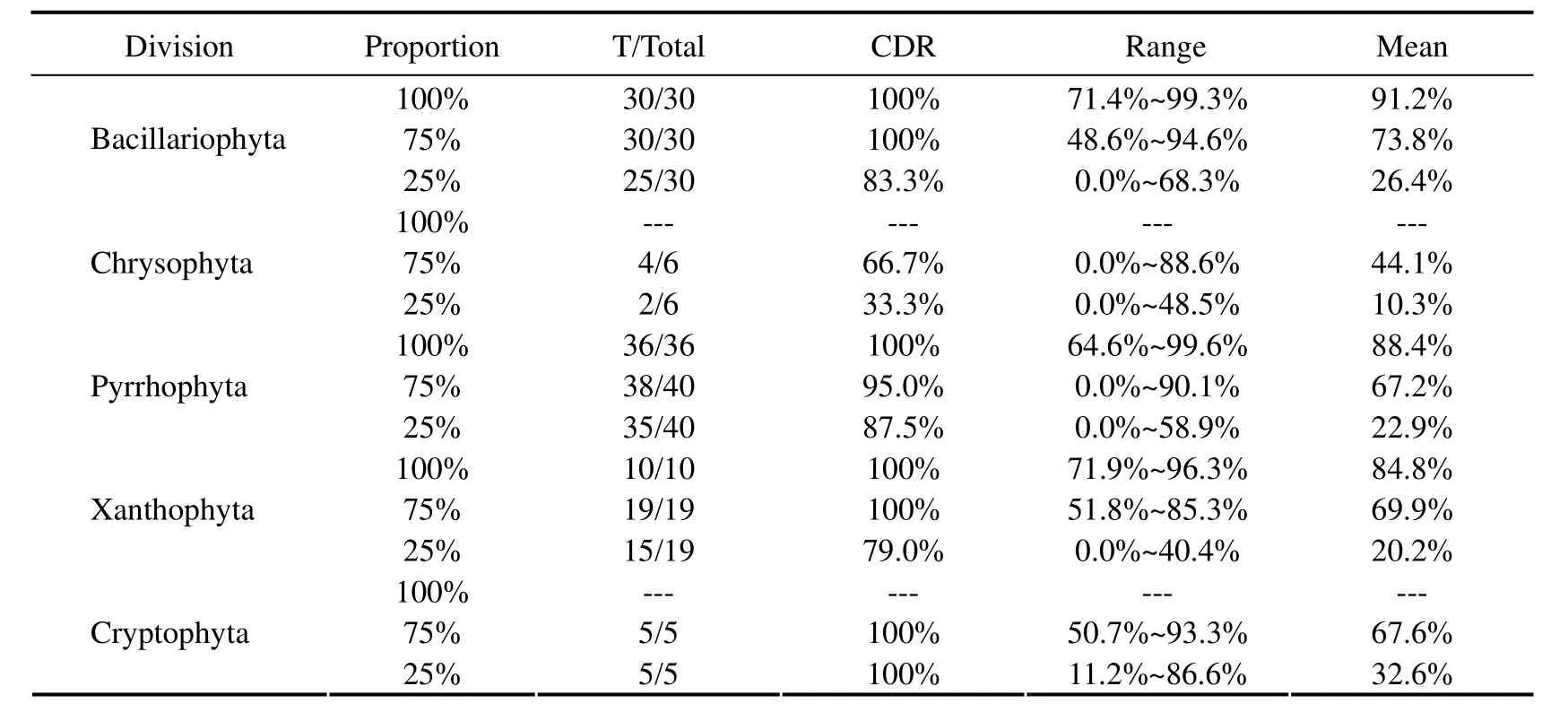
Table 4 Discrimination results with laboratory mixtures at the division level
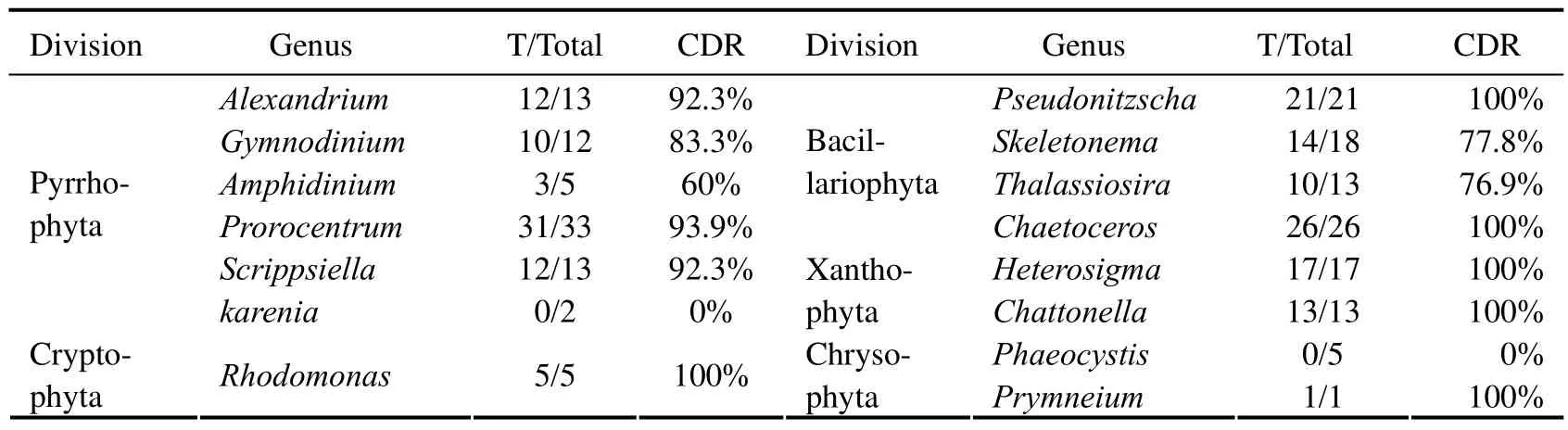
Table 5 Discrimination results of laboratory mixtures at the genus level
4.3 Discrimination with the Field Water Samples
4.3.1 Discrimination with the water samples from mesocosm experiments
From the results of the mesocosm experiments (Table 6),microscopic examination results show that 10 of the 12 samples are dominated by diatoms absolutely with the dominance of 76.2 - 99.9%,and the other two samples are dominated by dinoflagellate with the dominance of 56.7 -70.5%.Meanwhile,Chaetocerosis the absolutely dominant species with the dominance over 80% for five samples.Discrimination results by the technique show that 11 of 12 samples are correctly identified (CDR: 91.7%.) with the relative content of 38.4 - 93.9%,and 4 of 5 samples with single species dominance over 80% are correctly identified asChaetoceros(CDR: 80%).
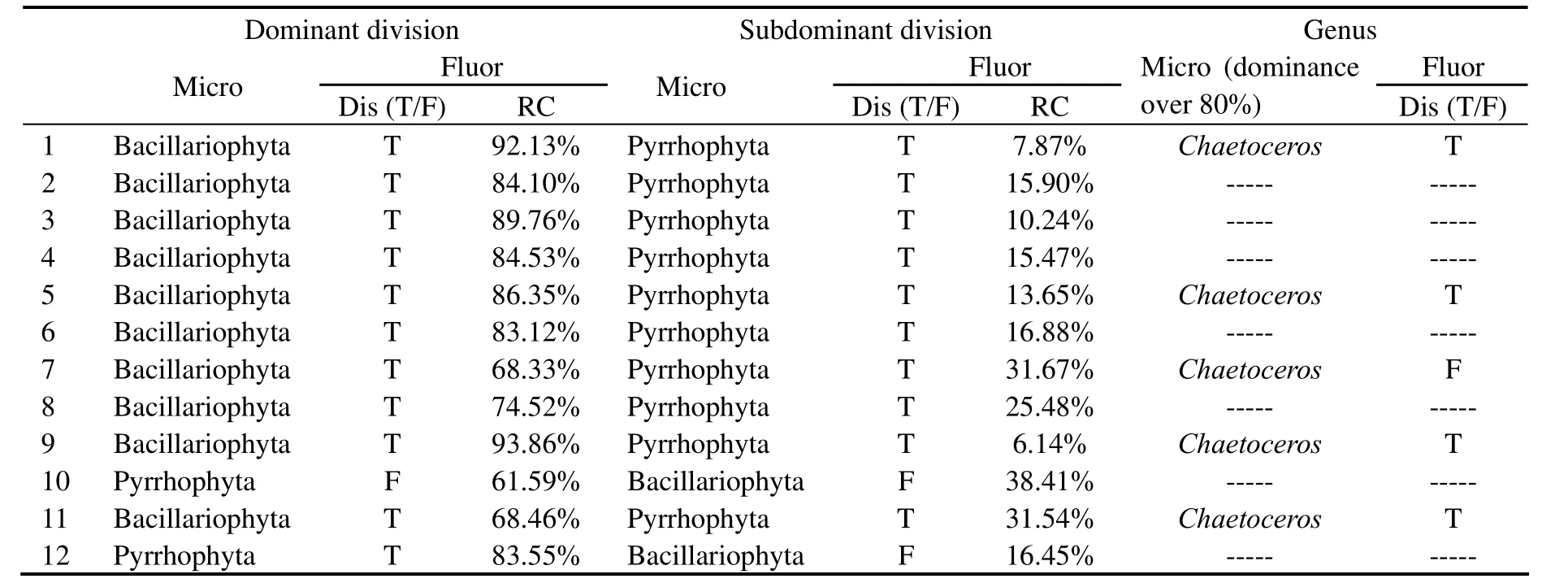
Table 6 Discrimination results with the water samples from mesocosm experiments
4.3.2 Discrimination with the water samples collected from Jiaozhou Bay
The fluorescence technique had been field-tested for 12 stations in Jiaozhou Bay (Table 7),the algal taxonomic groups found by microscopic examination for the twelve stations are dominated by large diatoms with an almost total dominance of 94.6~99.9%,Skeletonema costatumbeing the dominant species.Meanwhile,the algal community composition was estimated by the technique.It shows that diatoms are the absolutely dominant group with the dominance of 72.1~94.4% of the gross biomass in accordance with the microscopic analysis,and the average relative content is 84.1%.Moreover,there are three samples in whichSkeletonema costatumaccounts for more than 80% of the total phytoplankton according to the microscopic counting,and two of which are correctly identified asSkeletonemaby the fluorescence technique.
4.3.3 Discrimination with the water samples collected from the South Yellow Sea
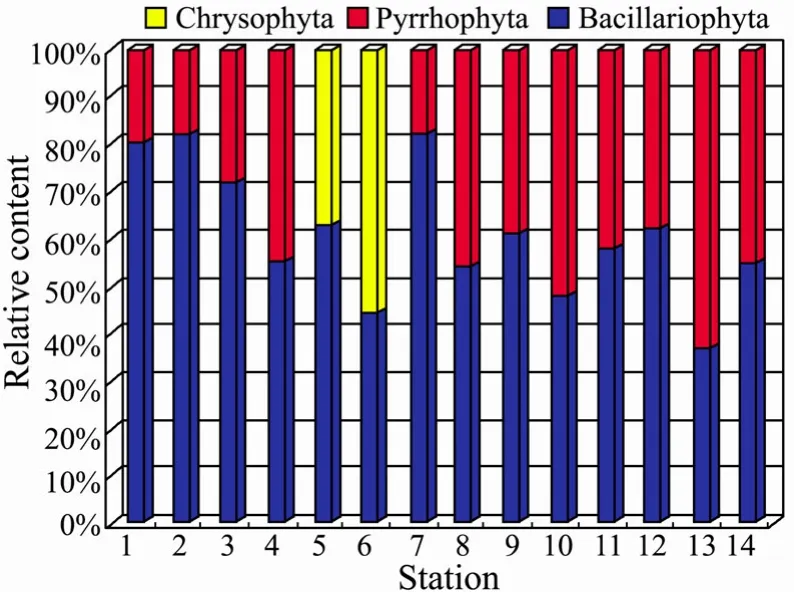
Fig.6 Relative abundance of dominant and co-dominant classes of phytoplankton in the South Yellow Sea (surface layer).
In the South Yellow Sea,the phytoplankton community is dominated by diatoms as revealed by the fluorescence technique (Table 8 and Table 9,Fig.6 and Fig.7),contributing up to 60.98% (surface layer) and 67.80% (subsurface) to the total phytoplankton biomass,respectively.Meanwhile,dinoflagellate species are also abundant with the contribution of 32.38% (surface layer) and 24.37%(subsurface) to the total biomass.The chrysophyte was tested occasionally with low contribution of 6.64% (surface layer) and 7.85% (subsurface) of the total biomass.
In brief,the qualitative and semi-quantitative discrimination results for the phytoplankton distributions show that the predominant algae group in the investigation areas is Bacillariophyta,followed by Pyrrhophyta,which isin agreement with those in previously published papers(Tian and Sun,2011; Wang,2001; Ji and Jin,2005) and the characteristics of the phytoplankton community composition in the China Sea (data from marine environment quality bulletin of China).
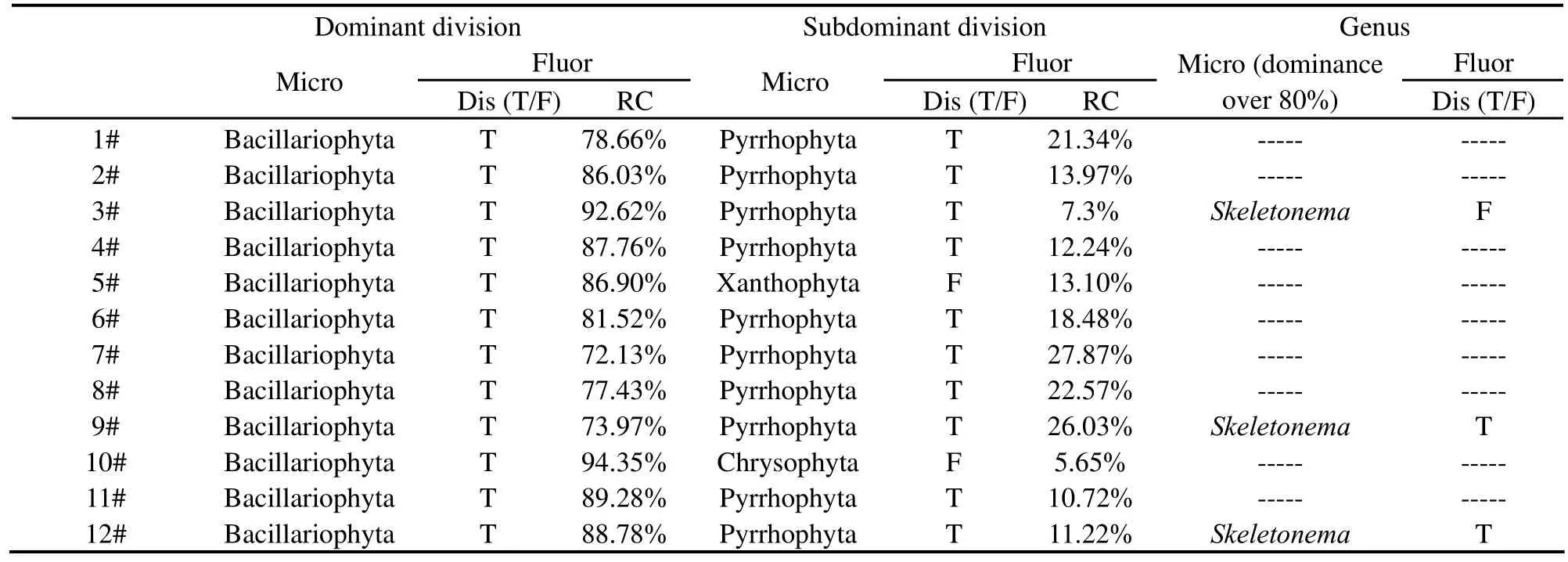
Table 7 Discrimination results with the water samples from Jiaozhou Bay

Table 8 Discrimination results with the water samples from the SouthYellow Sea (surface layer)
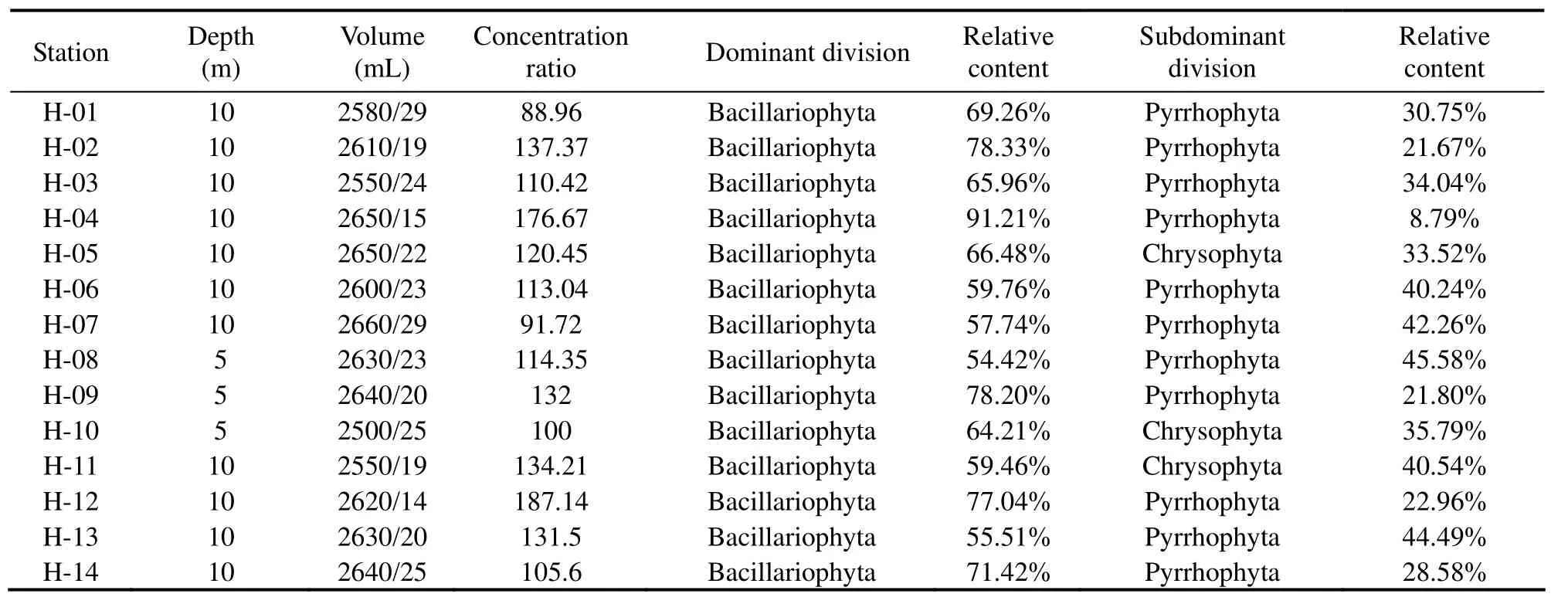
Table 9 Discrimination results with the water samples from the South Yellow Sea (subsurface layer)
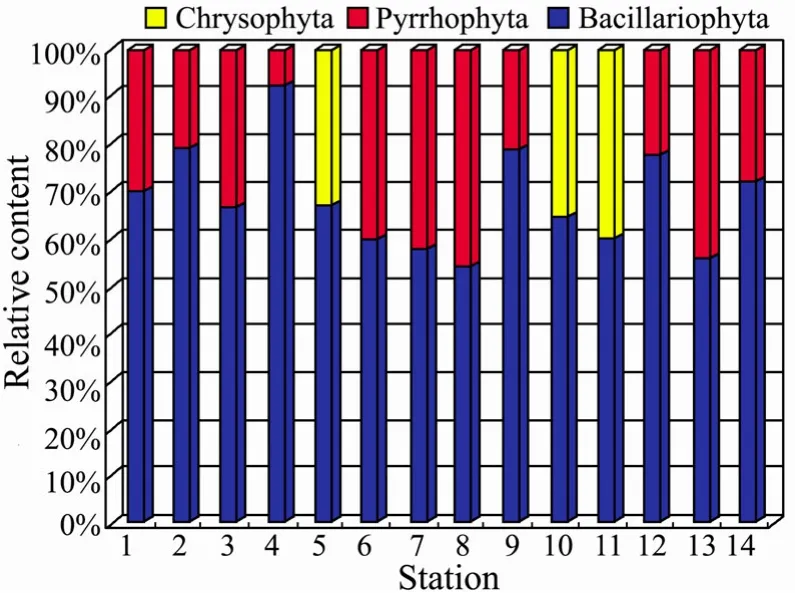
Fig.7 Relative abundance of dominant and co-dominant classes of phytoplankton in the South Yellow Sea (subsurface).
5 Conclusion
This study shows the feasibility of using FEEMs based on coif2-wavelet packet transform to analyze the phytoplankton community composition.The key points are the feature extraction and the establishment of norm spectra database which depend mostly on the reliability of the feature spectra.Also,the spectra should be representative,remarkable and less interferential.
Wavelet packet transform provids an entire spatial domain interpretation of the transform.The fluorescence spectra are decomposed byWPTand the feature spectra are extracted which can be used as input to classifiers for identification.
The proposed fluorescence technique not only can be used to estimate the phytoplankton community composition and the relative abundance of different classes at the division level,but also can be used for identifing the phytoplankton species causing harmful algae blooms at the genus level when the bloom happens.Moreover,the technique might be implemented into current and future ecological monitoring programs.
This work was supported by the National High-Tech Research and Development Program of China (863 Program) (No.2009AA063005) and the Natural Science Foundation of Shandong Province (No.ZR2009EM001).We would also like to thank the reviewers for many helpful comments that have made this a better paper.
Beutler,M.,Wiltshire,K.H.,Meyer,B.,Moldaenke,C.,Lüring,C.,Meyerhöfer,M.,Hansen,U.P.,and Dau,H,2002.A fluorometric method for the differentiation of algal populations in vivo and in situ.Photosynthesis Research,72: 39-53.DOI: 10.1023/A:1016026607048.
Boesch,D.F.,Anderson D.M.,Horner,R.A.,Shumway,S.E.,Tester,P.A.,and Whitledge,T.E,1997.Harmful algal blooms in coastal waters: Options for prevention,control and mitigation.Noaa coastal ocean program decision analysis series No.10.Noaa coastal office,Sliver spring,MD.P.+Appendix.31-32.
Cherif,L.H.,Debbal,S.M.,and Bereksi-Reguig,F.,2010.Choice of the wavelet analyzing in the phonocardiogram signal analysis using the discrete and the packet wavelet transform.Expert Systems with Applications,37: 913-918.DOI:10.1016/j.eswa.2009.09.036.
Hu,X.P,2008.Fluorescence discrimination technique based on weighted nonnegative least-squares for the phytoplankton in the coastal waters.Ph.D.Ocean University of China,58-67(in Chinese).
Ji,Z.,and Jin,N.,2005.The phytoplankton and zooplankton in the coastal sea of China and the relationship between them.http://www.paper.edu.cn.
Johnsen,G.,Samset,O.,Granskog,L.,and Sakshaug.E,1994.In vivo absorption characteristics in 10 classes of bloomforming phytoplankton: Taxonomic characteristics and responses to photoadaption by means of discriminant and HPLC analysis.Marine Ecology-Progress Series,105: 149-157.
Lu,L.,2007.Study on fluorescence spectra for identifying phytoplankton community.Ph.D.Ocean University of China,23-41 (in Chinese).
Mackey,M.D.,Mackey,D.J.,Higgins,H.W.,and Wright,S.W,1996.CHEMTAX-A program for estimating class abundances from chemical markers: application to HPLC measurements of phytoplankton.Marine Ecology-Progress Series,144: 265-283.
Millie,D.F.,Schofield,O.M.,Kirkpatrick,G.J.,Johnsen,G.,Tester,P.A.,and Vinyard,B.T,1997.Detection of harmful algal blooms using photopigments and absorption signatures:A case study of the Florida red tide dinoflagellate,Gymnodinium breve.Limnolgy Oceanography,42: 1240-1251.
Mzaik,T.,and Jagadeesh,J.M,1994.Wavelet-based detection of transients in biological signals.In:Wavelet Applications in Signal and Image Processing: II.Proceedings of the Society for Photo Instrumentation Engineering.Laine,A.F.,User,M.A.,eds.,105-119.
Pech-Pacheco,J.L.,and Alvarez-Borrego,J.,1998.Optical-digital system applied to the identification of five phytoplankton species.Marine Biology,132 (3):357-365.DOI:10.1007/s002270050402.
Rade,K.,and Dominik,E,2008.Methods for the anisotropic wavelet packet transform.Applied and Computational Harmonic Analysis,25: 295-314.
Seppaelae,J.,and Balode,M,1998.The use of spectral fluorescence methods to detect changes in the phytoplankton community.Hydrobiologia,363 (1-3): 207-217.DOI: 10.1023/A:1003129906730.
Specifications for oceanographic survey-part 6: marine biological survey (GB12763.6-91),1991.Administration of Technical Supervision of the People’s Republic of China.standards press of China,Beijing,8 pp.
Tian,W.,and Sun,J.,2011.Later spring phytoplankton community in Southern Yellow Sea in 2009.Marine Science,35(6): 19-23.
Wang,J.,2001.Study on phytoplankton in the Yellow Sea in Spring.Marine Fisheries Research,22 (1): 56-61.
Walsh,D.,Reeves,R.A.,Saul,D.J.,Gray,R.D.,Mackenzie,L.,Bergquist,P.R.,and Bergquist,P.L,1998.Heterogeneity of SSU and LSU rDNA sequences of Alexandrium species.Biochemical Systematics and Ecology,26 (5): 495-509.DOI:10.1016/S0305-1978(98)00006-4.
Yentsch,C.S.,and Yentsch,C.M,1979.Fluorescence spectral signatures characterization of phytoplankton populations by the use of excitation and emission-spectra.Journal of Marine Research,37: 471-483.
Yentsch,C.S.,and Phiney,D.A,1985.Spectral fluorescence:an ataxonomic tool for studying the structure of phytoplankton populations.Journal of Plankton Research,7 (5):617-632.DOI:10.1093/plankt/7.5.617.
Zepp,R.G.,Sheldon,W.N.,and Moran,M.A,2004.Dissolved organic fluorophores in southeastern US coastal waters: correction method for eliminating Rayleigh and Raman scattering peaks in excitation-emission matrices.Marine Chemistry,89 (1): 15-36.DOI:10.1016/j.marchem.2004.02.006.
Zhang,F.,Su,R.G.,Wang,X.L.,Wang,L.,and He,J.F.,2009.A fluorometric method for the discrimination of harmful algal bloom species developed by wavelet analysis.Journal of Experimental Marine Biology and Ecology,368 (1): 37-43.DOI:10.1016/j.jembe.2008.10.004.
杂志排行
Journal of Ocean University of China的其它文章
- Optimization of the Purification Methods for Recovery of Recombinant Growth Hormone from Paralichthys olivaceus
- Growth,Metabolism and Physiological Response of the Sea Cucumber,Apostichopus japonicus Selenka During Periods of Inactivity
- Purification and Characterization of a New Thermostable κ-Carrageenase from the Marine Bacterium Pseudoalteromonas sp. QY203
- Seasonal Changes in Food Uptake by the Sea Cucumber Apostichopus japonicus in a Farm Pond: Evidence from C and N Stable Isotopes
- What Depth Should Deep-Sea Water be Pumped up from in the South China Sea for Medicinal Research?
- Chemical Characteristics and Anticoagulant Activities of Two Sulfated Polysaccharides from Enteromorpha linza(Chlorophyta)
