Synthesis of PGMA Microspheres with Amino Groups for High-capacity Adsorption of Cr(VI) by Cerium Initiated Graft Polymerization*
2012-08-02LIPengfei李鹏飞YANGLiangrong杨良嵘HEXiuqiong何秀琼WANGJuan王娟KONGPeng孔鹏XINGHuifang邢慧芳andLIUHuizhou刘会洲
LI Pengfei (李鹏飞), YANG Liangrong (杨良嵘)**, HE Xiuqiong (何秀琼), WANG Juan (王娟), KONG Peng (孔鹏), XING Huifang (邢慧芳) and LIU Huizhou (刘会洲)**
Key Laboratory of Green Process and Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China
Synthesis of PGMA Microspheres with Amino Groups for High-capacity Adsorption of Cr(VI) by Cerium Initiated Graft Polymerization*
LI Pengfei (李鹏飞), YANG Liangrong (杨良嵘)**, HE Xiuqiong (何秀琼), WANG Juan (王娟), KONG Peng (孔鹏), XING Huifang (邢慧芳) and LIU Huizhou (刘会洲)**
Key Laboratory of Green Process and Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China
A novel polyglycidylmethacrylate (PGMA) microspheres with high adsorption capacity of Cr(VI) was prepared by cerium(IV) initiated graft polymerization of tentacle-type polymer chains with amino group on polymer microspheres with hydroxyl groups. The micron-sized PGMA microspheres were prepared by a dispersion polymerization method and subsequently modified by ring-opening reaction to introduce functional hydroxyl groups. The polymer microspheres were characterized by scanning electron microscopy (SEM) and Fourier transform infrared spectroscopy (FTIR). The results indicated that the polymer microspheres had an average diameter of 5 μm with uniform size distribution. The free amino group content was determined to be 5.13 mmol·g−1for g-PGMA-NH2microspheres by potentiometric and conductometric titration methods. The Cr(VI) adsorption results indicated that the graft polymerization of tentacle-type polymer chains on the polymer microspheres could produce adsorbents with high adsorption capacity (500 mg·g−1). The polymer microspheres with grafted tentacle polymer chains have potential application in large-scale removal of Cr(VI) in aqueous solution.
microsphere, PGMA, amino group, high-capacity, Cr(VI), Ce(IV)-initiated graft polymer
Chinese Journal of Chemical Engineering,20(1) 95—104 (2012)
1 INTRODUCTION
With the rapid development of global industrialization, Cr(VI) are generated as a byproduct in various industries and it creates serious environmental problems [1, 2]. More and more attention is being paid to wastewater containing Cr(VI) due to their tendency to accumulate in vital organs in human and animals. Researches [3-5] have indicated that Cr(VI) does not degrade into harmless products in the metabolic cycle but accumulate in the food chain causing great hazard to the living organs [6]. Thus, Cr(VI) is considered to be highly toxic to the biological production systems and human health, and US Environmental Protection Agency (EPA) recommends that the amount of Cr(VI) in drinking water should be less than 100 μg·L−1. Due to its high toxicity to biological systems, the removal of Cr(VI) from aqueous effluent requires advanced technologies [4] including chemical precipitation, reverse osmosis, electroparting and ion exchange [7-13]. Among them, ion exchange adsorption technology based on physical and chemical mechanisms is a conventional and efficient technique with wider application. It possesses many advantages such as high selectivity, low energy costs, less sludge volume produced and the meeting of strict discharge specifications [14].
To date, many kinds of adsorbents for wastewater treatment have been developed, such as activated carbon, activated alumina, coated silica gel, hydroxyapatite (HA)-based materials, peat moss, and raw rice bran [15]. However, after modification with cationic groups, the maximum adsorption capacity of these ion-exchange absorbents to the target Cr(VI) ions was not always improved as expected. For example, the maximum adsorption capacity, for Cr(VI) ions, of the ethylenediamine functionalized polymer, i.e., poly(methyl methacrylate-co-glycidyl methacrylate)-ethylenediamine [poly(GMA-co-MMA)-EDA], reported by Bayramoglu and Arica [16]. Pyridine strong base anion exchange resin with amide functional groups, reported by Neagu [17] and the hexadecyltrimethylammonium (HDTMA) modified nanozeolite A, reported by Tashauoei et al. [18] was found to be 22.93 mg·g−1[16], 94.0 mg·g−1[17], and 14.16 mg·g−1[18], respectively. Thus, to obtain novel polymer microspheres with high adsorption capacity for Cr(VI) ions is still facing great challenges.
Cerium (IV) initiated grafting has been used widely for surface modification of both natural and synthetic polymers, such as cellulose, starch, and poly(vinyl alcohol) [19, 20]. The substrate onto which the grafting occurs must contain hydroxyl groups (OH) that are transformed to free radicals because of the oxidation of ceric ions with functional OH [21, 22]. However, most of the work on cerium initiated graft polymerization are limited to water soluble substrates, and work on surface graft polymerization on polymer microspheres initiated by ceric ions have rarely been reported. Müller [23] and Ma et al. [20] demonstrated that Ce(IV) initiated grafting of polymer chains onto the surface of porous polymer beads to form tentacletype supports could sufficiently increase the binding capacity. The enhanced adsorption capacity of these grafted polymer sorbents would apparently arise fromtentacles extending away from the support surface a sufficient distance to allow Cr(VI) to penetrate the polymer layer and bind in multiple layers. Also with tentacle-type chains, the contact between the Cr(VI) and the base matrix support surface is markedly reduced, which minimizes undesirable interactions between the Cr(VI) and the base matrix surface. Thus, from this idea, we assumed this graft technique could be an efficient way to enhance the adsorption capacity of Cr(VI) on polymer microspheres. To our knowledge, a study on preparing PGMA-g-GMA microspheres for removal of Cr(VI) similar to this work has not been reported.
In this paper, we applied the cerium initiated graft polymerization method to obtain adsorbents with high adsorption capacity. The preparation of poly(glycidyl methacrylate) (PGMA) based ion exchangers includes three steps: (1) preparation of nonporous micron-sized PGMA microspheres by dispersion polymerization method, (2) hydrolysis of the above microspheres to produce PGMA microspheres with hydroxyl groups, named PGMA OH, (3) cerium initiated graft polymerization of a hydrophilic acrylate monomer on the PGMA OH to form a tentacle polymer layer followed by ring-opening reaction with EDA, named as PGMA with functional amino groups (g-PGMA NH2). Besides, the characterizations and adsorption properties of g-PGMA NH2microspheres for Cr(VI) were investigated.
2 EXPERIMENTAL
All chemicals used were purchased from Beijing Chemical Reagents Co. (Beijing, China) unless otherwise stated. Glycidyl methacrylate (GMA) purchased from Alfa Aesar was distilled under vacuum. 2,2-azobisisobutyronitrile (AIBN) and poly(vinyl pyrrolidone) (PVP K-30, MW=40000) were used as initiator and stabilizer, respectively. Ceric ammonium nitrate (CAN), ethylenediamine (EDA), nitric acid, sulfuric acid were of analytical grade were all used as received.
2.1 Preparation of monodisperse nonporous PGMA microspheres by dispersion polymerization method
The recipe used to prepare PGMA microspheres is given in Table 1. Dispersion polymerization wascarried out in a 250 ml, four necked, round-bottom flask equipped with an anchor-type agitator (50 mm in diameter), a reflux condenser and nitrogen inlet.
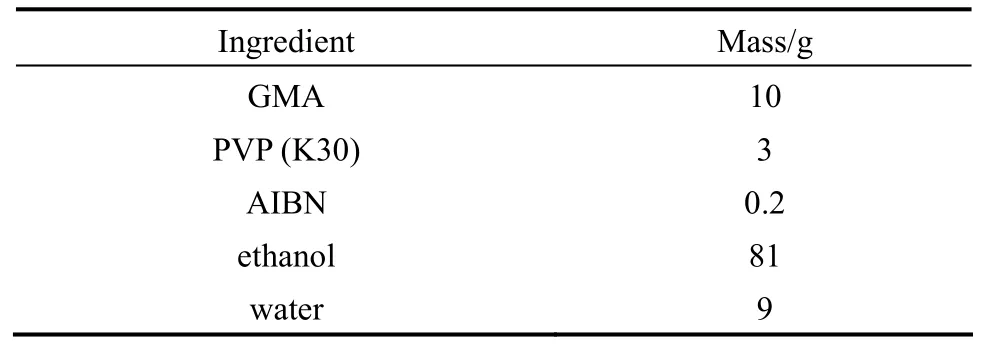
Table 1 Recipe for dispersion polymerization of monodisperse PGMA microspheres (N2, 70 °C, 24 h, 120 r·min−1)
PVP dissolved in a dispersion medium containing ethanol and water was introduced into the reaction flask. Then the monomer phase, prepared by dissolving AIBN in GMA, was mixed with the dispersion under stirring. After purging with nitrogen for 20 min, the flask was immersed in a preheated water bath at 70 °C and stirred (120 r·min−1) for 24 h. At the end of the reaction, the polymer microspheres were isolated by centrifugation and washed thoroughly with water. Finally, the microspheres were dried under vacuum at ambient temperature.
2.2 Hydrolysis of the above microspheres to produce PGMAOH
The PGMA microspheres were transformed into PGMAOH microspheres by hydrolysis in acidic condition. The PGMA microspheres (3 g) prepared by dispersion polymerization were added to a 100 ml 0.1aqueous solution for the hydrolysis reaction at 30 °C for 48 h. The resultant PGMA OH microspheres were separated by centrifugation and washed several times with water.
2.3 Preparation of g-PGMANH2microspheres with tentacle polymer layer by cerium initiated graft polymerization method
To carry out the graft polymerization, 1 g of PGMAOH microspheres was suspended in 80 ml of deionized water in a three-necked flask which was kept in a water bath at a temperature of 60 °C. The mixture was stirred and purged with nitrogen gas for 1 h in order to remove oxygen. Then 10 ml of 0.1 mol·L−1CAN solution in 1 mol·L−1nitric acid was added. After 30 min, 10 ml of GMA monomer was added, and the stirring was continued under nitrogen for 6 h. Then, the mixture was centrifuged and the microspheres were washed with ethanol and water to pH~7.0 to remove the excess GMA. After that 75 ml EDA and 50 ml H2O was added in a 250 ml flask to produce amino groups for the adsorption of Cr(VI). The mixture was stirred at 80 °C for 12 h. Then, the mixture was centrifuged and the microspheres were washed with ethanol and water to pH~7.0 to remove the excess EDA. The microspheres were denoted as g-PGMA NH2.
2.4 Chemical modification PGMA with EDA
EDA was used to react with PGMA particles to produce amino groups inside the particles. 2 g of PGMA particles was added in a 250-ml flask containing 50 ml H2O and 75 ml EDA. The mixture was stirred at 80 °C for 12 h. Then the mixture was centrifuged andthe particles were washed with water to remove the excess EDA. The particles were denoted as PGMA NH2.
2.5 Characterizations and adsorption properties of g-PGMANH2microspheres
The size, size distribution and surface morphology of the PGMA microspheres and g-PGMA NH2microspheres were examined with scanning electronic microscope (SEM, JEOL JSM-6700F, Japan). Particle size and size distribution were determined by measuring 300 microspheres from the SEM photographs. Two types of mean particle size were calculated: number average and mass average,is the number of microspheres, andParticle size distribution was expressed as the polydispersity index (PDI, PDI=dw/dn).
The attenuated total rejection (ATR) method was employed to analyzed qualitatively surface property of microspheres with an FTIR-ATR spectrophotometer (Bruker, Vector 22, Germany). The concentration of Cr(VI) in the aqueous solution was analyzed with inductively-coupled plasma spectrometer (ICP, Optima 7000DV, Perkin-Elmer, USA).
The adsorption of Cr(VI) ions from aqueous solution on the g-PGMA NH2microspheres was investigated using PGMA NH2microspheres as a control system. To examination of the adsorption ability of the two kinds of microspheres on Cr(VI), a stock solution of chromium(VI) at concentration of 10-300 mg·L−1was prepared by dissolving a known quantity of analytical grade potassium dichromate (K2Cr2O7) in ultrapure water. Batch adsorption studies were performed by mixing 0.01 g g-PGMA NH2microspheres with 50 ml K2Cr2O7solution of varying concentration from 10 to 300 mg·L−1in a 250 ml stoppered conical flask, stirred 120 r·min−1for 1 h at 298.15 K. 0.5 mg·L−1HCl and 0.5 mg·L−1NaOH solutions were used for pH adjustment. To investigate the effect of pH, 50 ml of 150 mg·L−1Cr(VI) with pH ranging from 1.5 to 8.5 (which was adjusted with H2SO4or NaOH at the beginning of the experiment and not controlled afterwards) was mixed with g-PGMA NH2microspheres for 24 h to reach equilibrium. For the adsorption kinetic studies, g-PGMA NH2microspheres was added into 50 ml of 150 mg·L−1Cr(VI), samples were taken for Cr(VI) concentration measurements at specific time intervals.
Adsorption isotherm studies were conducted by varying the initial Cr(VI) concentration from 40 to 300 mg·L−1at temperature of 298.15 K and pH value at 4.0-4.5. For the thermodynamics studies, 0.01 g g-PGMA NH2microspheres was added into 50 ml of 150 mg·L−1Cr(VI) with temperature ranging from 288.15 K to 338.15 K.
After the desired adsorption periods (about 1 h), g-PGMA NH2microspheres were separated from aqueous phases using centrifugation separation and the residual concentration of the Cr(VI) ions in the aqueous phases were measured by inductively coupled plasma (ICP).
2.6 Adsorption data analysis
The equilibrium adsorption capacity for each adsorbent, qe(mg·g−1), was determined by analyzing Cr(VI) concentration before and after the treatment and calculated by using

where C0and Ceare the initial and equilibrium Cr(VI) concentrations in the solution (mg·L−1), m is the adsorbent dosage (mg), and V is the volume of the solution (ml), the same hereinafter.
The Langmuir isotherm [Eq. (2)] and the Freundlich isotherm [Eq. (3)] was used to characterize the maximum adsorption capacity of adsorbents [24]:

where qmis the values of maximum absorbed per unit mass (mg·g−1), k is pseudo first order kinetics absorption rate constant (min−1), n and kf(g·mg−1·min−1) are constants.
The adsorption thermodynamic data obtained from batch experiments were analyzed by using the following van’t Hoff equation [24]:

where ΔH0and ΔS0are the values of standard enthalpy change, and standard entropy change, respectively. KCis the distribution coefficient.
3 RESULTS AND DISCUSSION
3.1 Preparation of g-PGMANH2microspheres
A great number of hydroxyl groups exist on the surfaces of PGMA OH microspheres. According to the redox initiation mechanism of cerium salt [25], the reaction between Ce(VI) ions and the hydroxyl groups on PGMA OH occurred first, forming a complex. Subsequently, oxidation with a single electron transfer occurred, and the complex was soon disproportionated, forming free radicals on the carbon atoms bearing the hydroxyl groups. Thus, the graft polymerization of GMA on PGMA OH microspheres was initiated. The ring-opening reaction proceeds with EDA as the reaction reagent, and the epoxy group on the g-PGMA was transformed into the amino group, resulting in the formation of g-PGMA NH2microspheres.

Figure 1 Preparation, surface modification and Ce(IV) initiated graft polymerization of PGMA microspheres
On the basis of Ma et al. [20], the entire process of preparing g-PGMA NH2microspheres is expressed schematically in Fig. 1.
Reaction mechanism is suggested as follows. Radical formation:

Monodisperse procusor PGMA microspheres were prepared by dispersion polymerization of GMA in ethanol/water medium (Fig. 1). Dispersion polymerization is a simple and effective method for preparing monodisperse spherical microspheres in the micron size range in a single step. In this work, GMA was selected because its epoxy groups could be easily converted into various functional groups [26], such as amines, alcohols, and aldehydes.
In our experiment, monodisperse crosslinked PGMA microspheres were obtained due to the hydrophilic surface-active epoxy groups in PGMA exerting a co-stabilizing effect on the stability of the surface of the primary microspheres. The epoxy groups facilitated the adsorption and grafting of PVP onto the oligomer chains leading to the brief period of forming the stable primary particle [27].
3.2 Characterization of PGMA microspheres and g-PGMANH2microspheres
Figure 2 displays the SEM micrograph of the PGMA [Fig. 2 (a)], g-PGMA NH2microspheres [Fig. 2 (b)] and the particle size distribution of g-PGMA NH2[Fig. 2 (c)]. The sphericity of the microspheres was excellent, and exhibited high monodispersity. The PGMA microspheres had an average size of 2.11 μm without coagula and the PDI was 1.02. A PDI of less than 1.05 is usually considered as being monodisperse [28].
Spherical PGMA microspheres were typically produced in an aqueous medium by suspension polymerization (the main disadvantage being the formed microspheres with several hundred micrometers and broad size distribution) [29] or emulsion polymerization (its main drawback being partial or complete hydrolysis of the reactive epoxy groups) [30]. In comparison with PGMA microspheres obtained by the above two methods in an aqueous phase, in which reactive epoxy groups are partially or completely hydrolyzed, the epoxy groups can retain almost completely in dispersion polymerization of GMA in the ethanol/water medium, as evidenced elsewhere [30, 31]. PGMA microspheres prepared with dispersion polymerization have micron-sized diameter and narrow size distribution, being comparable with emulsion polymerization and suspension polymerization.
After surface modification and graft polymerization of polymer chains, the average size of g-PGMA NH2microspheres was 6.40 μm without coagula and the PDI was 1.02, indicating that the microspheres remained monodisperse. It can be seen that the g-PGMA NH2microspheres have a special “tentacle type” surface structure which may increase the adsorption capacity of Cr(VI) [Fig. 2 (b)].

Figure 2 The SEM micrograph of the PGMA, g-PGMA NH2microspheres and particle size distribution of g-PGMA NH2microspheres
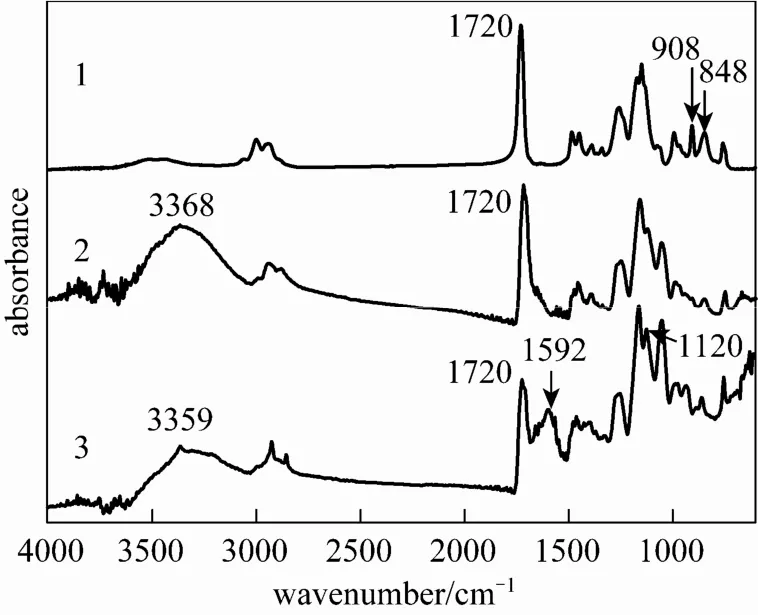
Figure 3 FTIR spectra of PGMA (1), PGMA OH (2) and g-PGMA NH2(3) microspheres
Figure 3 shows the FTIR-ATR spectra of PGMA (curve 1), PGMA OH (curve 2) and g-PGMANH2(curve 3) microspheres. The PGMA microspheres (1)(epoxy group). In the spectrum of PGMA OH microspheres (2), the characteristic absorbance bands appeared at 3368, 1720 (νCO). The first band can be ascribed to the stretching vibration of NH2in the hydroxyl group (νOH), the second band can be attributed to the vibration absorption of carbonyl (C O) in the aliphatic group, and the last band should correspond to the stretching vibration of CO in the primary alcohol group (νCO). The relative intensity ratio of I908/I1720and I848/I1720decreased to zero and the absorbance bands appeared at I3368and I1054, indicating complete conversion of epoxy groups to hydroxyl groups. In the spectrum of g-PGMA NH2microspheres (3), the characteristic absorbance bands appeared at 3359 (νNH), 1720, and 1592 cm−1(δNH). The first band can be ascribed to the stretching vibration of NH2in the amido group, the second band can be attributed to the vibration absorption of carbonyl (C O), and the last band should correspond to the deformation vibration absorption of NH2in the amido group. The presence of these characteristic bands showed that GMA had been grafted onto PGMA OH microspheres, and the grafted microspheres had formed via the graft polymerization of GMA initiated by cerium salt. In the spectrum ofgreatly, and some new bands appeared. The deformation and stretching vibration adsorptions of the primary amine groups (NH2) appeared at1592 and 3359 cm−1,respectively. Another newband at1120 cm−1should be attributed to the stretching vibration adsorption of the CAN bond of the primary amine groups (NH2). These spectrum variations showed that the amido groups of the g-PGMA NH2microspheres had been transformed into primary amine groups via the ring-opening reaction of epoxy groups by EDA, and the functional g-PGMANH2microspheres had been formed.
Figure 4shows the FTIR-ATR spectra of PGMANH2and g-PGMANH2microspheres. The P GMANH2microspheres showed the characteristic absorptio nbandsat1720cm−1(carbonyl groups) and 3359and1559cm−1.The relative intensityratio I3359/I1720in PGMANH2was lower than I3359/I1720of g-PGMA NH2microspheres, which implied that the amino group content was higher in g-PGMA NH2microspheres. The free amino group content of g-P GMANH2andmicrosphereswas estimated as 5.13 and 2.41 mmol·g−1respectively bypotentiometric and conductometric titration method, providing a supporting proof to the results of the FTIR-ATR method. So the g-PGMA NH2microspheres initiated by Ce(IV) are expected to have a good performance on adsorption of Cr(VI).
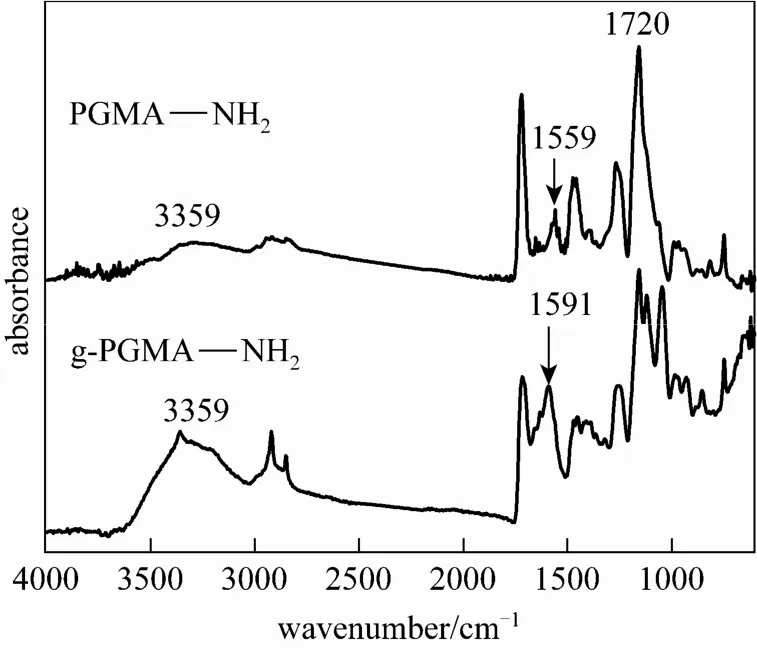
Figure 4 FTIR spectra of PGMA NH2and g-PGMA NH2microspheres
3.3 Effect of adsorption time on the adsorption properties and adsorption kinetics
Figure 5 indicates the changes of adsorption properties along with time. It can be seen that the rate of Cr(VI) adsorption on PGMA NH2and g-PGMA NH2microspheres was initially quite high, followed by a much slower adsorption, and approached gradually to equilibrium. The adsorption equilibrium time for PGMA NH2microspheres was about 15 min, and that for g-PGMA NH2microspheres was about 1 h.
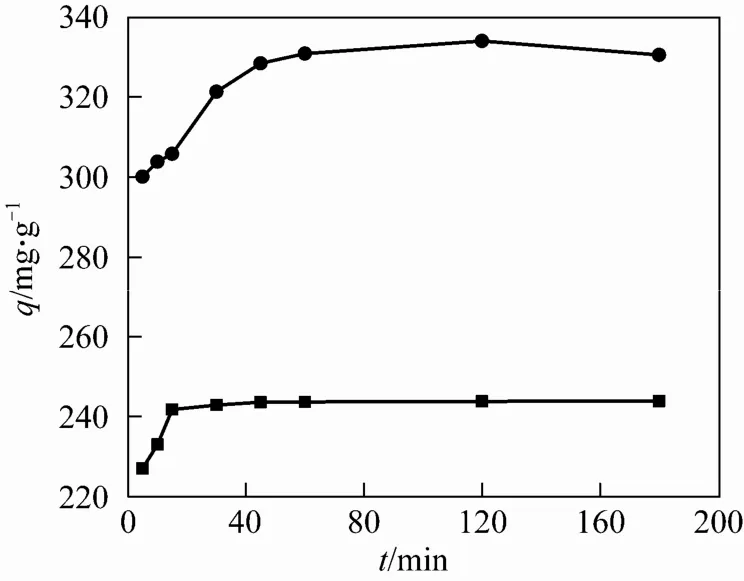
Figure 5 Effect of reaction time on the sorption of Cr(VI)● g-PGMANH2; ■ PGMA NH2
The results indicate that more time is needed to reach adsorption equilibrium for g-PGMA NH2microspheres than that for PGMA NH2microspheres. That is because these grafted polymer sorbents with special “tentacle type” surface structure which extends a sufficient distance away from the support surface renders extra resistance for Cr(VI) to penetrate the polymer layer and bind onto the adsorptive sites. However, compared with other ion exchangers reported in the literature [17, 32], less time was spent for g-PGMA NH2microspheres to reach adsorption equilibrium under similar conditions.
3.4 Effect of initial concentration on the adsorption of Cr(VI)
As shown in Fig. 6, the adsorption q increased with increasing metal ions concentration in the medium. The experimental Cr(VI) adsorption curve was very steep at low ion concentration and the adsorptionin the adsorption capacity of the g-PGMA NH2microspheres after Ce(IV) initiated grafting was observed for Cr(VI) ions.

Figure 6 Effect of initial metal ion concentration on the sorption of Cr(VI) by PGMA●
The Langmuir isotherm and Freundlich isotherm fitting results are presented in Table 2. It can be seen that the adsorption pattern for the microspheres followed the Langmuir isotherm better than the Freundlich isotherm. The Langmuir isotherm is valid for monolayer adsorption onto a surface containing a finite number of identical sites. However, Rivero et al. [33] reported that the adsorption of chromium by resin Lewatit MP-64 was properly described by the Freundlich isotherm, which may be attributed to the different characteristics for the resins.
From Table 2, the maximum adsorption capacitydry beads, respectively. Graft polymerization method initiated by Ce(IV) increased the adsorption capacity of the microspheres about 1.65-folds compared to theplain PGMA NH2microspheres. The higher adsorption capacity of 500 mg·g−1may be due to the tentacle type polymer chains on the polymer particles surface, which formed the special structure showed in SEM result in Fig. 2 (B). The polymer particles with grafted tentacle polymer chains have potential application in large-scale removal of Cr(VI).

Table 2 Parameters of Freundlich and Langmuir isotherms

Table 3 Adsorption capacities of various adsorbents for Cr(VI)
3.5 Comparison of adsorption capacity
The comparison of the adsorption capacity of g-PGMA NH2microspheres with PGMANH2microspheres and other adsorbents for the removal of Cr(VI) under similar conditions reported in the literature are summarized in Table 3, showing that the present g-PGMANH2microspheres are very promising for the removal of Cr(VI) in wastewater.
3.6 Effect of pH value on the adsorption properties

Figure 7 Effect of pH on Cr(VI) adsorption onto the1.5-8.5)● g-PGMANH2; ■ PGMA NH2
The effect of pH on the adsorption of Cr(VI) onto g-PGMANH2microspheres was studied in the pH value range 1.5-8.5. Fig. 7 shows that the maximumadsorption of Cr(VI) from aqueous solutions was observed at pH 1.5 for both of PGMA NH2and g-PGMA NH2microspheres. When pH>7, the removal of Cr(VI) from the solution decreased sharply as pH increased. The decrease in Cr(VI) adsorption capacity in more alkaline regions was related to electrostatic repulsion effects between the oppositely charged groups [46].
The effect of pH value on the adsorption efficiency is due to its influence on the surface properties of the PGMA NH2and g-PGMANH2microspheres as well as different species of Cr(VI) in aqueous solution. The chromate may be present in various forms such asin the solution phase as a function of pH and concentration.ions exist in the solution through out the experimental concentration range when pH>6.5, in the pH range from 0 to 6.5, H CrO−4andare predominant [3]. An anion-exchange resin will absorb chromate ions from aqueous solution according to the following reactions:

Another factor affecting this variation of adsorptive capacity in different pH may be the adsorption free energy of various chromium species [35].exist at different pH. At pH of 2.0-6.0, the predominant Cr(VI) species exist mainly in the monovalent4HCrO−form, which is then gradually converted to the divalentform as pH increases. The adsorption free energy ofandis −10.47 to −2.51 and −8.79 to −1.26 kJ·mol−1, respectively [35]. The adsorption free energy ofis lower than that ofConsequently,is more favo rably adsorbed than C rO24−at the same concentration. The removal of Cr(VI) at lower pH is mainly due to the adsorption of4HCrO−, which is expected to be adsorbed in larger quantities thanunder the same adsorption affinity. WhenHCrO−at concentration is much higher than4higher pH, the adsorption free energy oflower, and only under such a circumstance canadsorption be more favorable than
Hence, the suitable pH range is suggested to be 1.5-4.5. Additionally, Gode and Pehlivan [47] reported that the optimal pH range was 2-6. The predominant form of Cr(VI) after reduced by PGMA NH2and g-PGMA NH2microspheres isand the adsorption mechanism of the g-PGMANH2microspheres may relate to ion exchange and electrostatic attraction.
3.7 Adsorption thermodynamic studies
The effect of temperature on the adsorption of Cr(VI) by the g-PGMA NH2and PGMANH2microspheres were investigated between 288 and 328 K at pH 4.5. The effects of temperature on the sorption of Cr(VI) by g-PGMANH2and PGMANH2microspheres are shown in Fig. 8. In general, higher temperature is beneficial to the adsorption of Cr(VI), and 318 K is the suitable temperature for g-PGMA NH2microspheres.

Figure 8 Effect of temperature on the adsorption of Cr(VI)● g-PGMANH2; ■ PGMA NH2
Thermodynamic parameters showed in Table 4, such as free energy change (ΔG0), enthalpy change (ΔH0) and entropy change (ΔS0) can be calculated by Eqs. (4)-(6). The temperature of the mixture was ranged from 288 to 328 K. The negative ΔG indicates the adsorption on g-PGMA NH2and PGMANH2microspheres to be a spontaneous process. The positive ΔH0shows that the adsorption processes for g-PGMA NH2and PGMANH2microspheres are endothermic reactions. The reason why adsorption level for g-PGMANH2and PGMANH2microspheres increased when increasing temperature can be explained by the value of ΔH0, and the enhancement of adsorption capacity at higher temperatures may be attributed to enlargement of pore size and/or activation of the adsorbent surface.
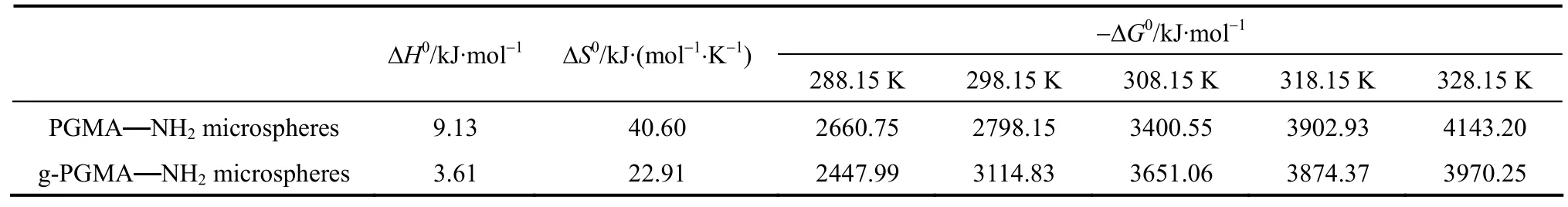
Table 4 Thermodynamic parameters for the adsorption of Cr(VI) on PGMA NH2and g-PGMA NH2microspheres
4 CONCLUSIONS
To facilitate the adsorption separation on a large scale, the preparation of polymer microspheres with high adsorption capacity is necessary. In this study, with an average size of 2.11 μm and surface functional hydroxyl groups were prepared by a disperse polymerization. Amino groups modified nonporous PGMA microspheres with high density tentacle polymer layer (g-PGMA NH2microspheres) were synthesized by cerium initiated surface graft polymerization method and ring-open reaction. SEM results showed that the g-PGMA NH2microspheres have a special “tentacle type” surface structure. The adsorption capacity ofother adsorbents reported in the past. The adsorption data for Cr(VI) onto g-PGMA NH2microspheres were well fitted to the Langmuir isotherm, and the adsorption process was endothermic and entropy favored in nature. The removal efficiency was highly pH dependent and the optimal adsorption occurred at pH 1.5. Adsorption mechanism studies suggested that the adsorption of Cr(VI) onto g-PGMA NH2microspheres involved electrostatic interaction and ion exchange. The g-PGMA NH2microspheres are ideal potential adsorbents which could be used in large scale removal of Cr(VI) from waste water.
REFERENCES
1 Agrawal, A., Kumar, V., Pandey, B.D., “Remediation options for the treatment of electroplating and leather tanning effluent containing chromium—A review”, Miner. Process. Extr. M.,27(2), 99-130 (2006).
2 Horton, R.N., Apel, W.A., Thompson, V.S., Sheridan, P.P., “Low temperature reduction of hexavalent chromium by a microbial enrichment consortium and a novel strain of Arthrobacter aurescens”, BMC Microbiol.,6, 1-8 (2006).
3 Mohan, D., Pittman, C.U., “Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water”, J. Hazard. Mater.,137(2), 762-811 (2006).
4 Mohan, D., Singh, K.P., Singh, V.K., “Removal of hexavalent chromium from aqueous solution using low-cost activated carbons derived from agricultural waste materials and activated carbon fabric cloth”, Ind. Eng. Chem. Res.,44(4), 1027-1042 (2005).
5 Mohan, D., Singh, K.P., Singh, V.K., “Trivalent chromium removal from wastewater using low cost activated carbon derived from agricultural waste material and activated carbon fabric cloth”, J. Hazard. Mater.,135(1-3), 280-295 (2006).
6 Qiu, J.Y., Wang, Z.Y., Li, H.B., Xu, L., Peng, J., Zhai, M.L., Yang, C., Li, J.Q., Wei, G.S., “Adsorption of Cr(VI) using silica-based adsorbent prepared by radiation-induced grafting”, J. Hazard. Mater.,166(1), 270-276 (2009).
7 Hankins, N.P., Lu, N., Hilal, N., “Enhanced removal of heavy metal ions bound to humic acid by polyelectrolyte flocculation”, Sep. Purif. Technol.,51(1), 48-56 (2006).
8 Hasany, S.M., Ahmad, R., “The potential of cost-effective coconut husk for the removal of toxic metal ions for environmental protection”, J. Environ. Manage,81(3), 286-295 (2006).
9 Pascal, V., Laetitia, D., Joel, L., Marc, S., Serge, P., “New concept to remove heavy metals from liquid waste based on electrochemical pH-switchable immobilized ligands”, Appl. Surf. Sci.,253(6), 3263-3269 (2007).
10 Polat, H., Erdogan, D., “Heavy metal removal from waste waters by ion flotation”, J. Hazard. Mater.,148(1-2), 267-273 (2007).
11 Wang, J.S.F., Wai, C.M., “Transporting metal ions using reverse micelles in alcohol modified supercritical carbon dioxide”, J. Supercrit. Fluid,40(2), 176-182 (2007).
12 Sengupta, A.K., Clifford, D., “Important process variables in chromate ion-exchange”, Environ. Sci. Technol.,20(2), 149-155 (1986).
13 Hegazy, E.S.A., Kamal, H., Maziad, N., Dessouki, A. M., “Membranes prepared by radiation grafting of binary monomers for adsorption of heavy metals from industrial wastes”, Nucl. Instrum. Methods Phys. Res. B,151(1-4), 386-392 (1999).
14 Gode, F., Pehlivan, E., “A comparative study of two chelating ion-exchange resins for the removal of chromium(III) from aqueous solution”, J. Hazard. Mater.,100(1-3), 231-243 (2003).
15 Zhao, Y.G., Shen, H.Y., Pan, S.D., Hu, M.Q., Xia, Q.H., “Preparation and characterization of amino-functionalized nano-Fe3O4magnetic polymer adsorbents for removal of chromium(VI) ions”, J. Mater. Sci.,45, 5291-5301 (2010).
16 Bayramoglu, G., Arica, M.Y., “Ethylenediamine grafted poly(glycidyl-methacrylate-co-methylmethacrylate) adsorbent for removal of chromate anions”, Sep. Purif. Technol.,45(3), 192-199 (2005).
17 Neagu, V., “Removal of Cr(VI) onto functionalized pyridine copolymer with amide groups”, J. Hazard. Mater.,171(1-3), 410-416 (2009).
18 Tashauoei, H.R., Attar, H.M., Kamali, M., Amin, M.M., Aein, M.N.,“Removal of hexavalent chromium(VI) from aqueous solutions using surface modified nanozeolite A”, Int. J. Environ. Res.,4(3), 491-500 (2010).
19 Bamford, C.H., Allamee, K.G., “Polymer surface functionalization and grafting by a simple and inexpensive method”, Macromol. Rapid Commun.,15(4), 379-384 (1994).
20 Ma, Z.Y., Guan, Y.P., Liu, X.Q., Liu, H.Z., “Synthesis of magnetic chelator for high-capacity immobilized metal affinity adsorption of protein by cerium initiated graft polymerization”, Langmuir,21(15), 6987-6994 (2005).
21 Mino, G., Kaizerman, S., “A new method for the preparation of graft copolymers-polymerization initated by ceric ion redox systems”, J. Polym. Sci.,31(122), 242-243 (1958).
22 Odian, G., Kho, J.H.T., “Ceric lon initiated graft polymerization onto poly(vinl alcohol)”, J. Macromol. Sci. Chem.,A4(2), 317-330 (1970).
23 Muller, W., “New ion-exchangers for the chromatography of biopolymers”, J. Chromatog.,510, 133-140 (1990).
24 Sheha, R.R., El-Zahhar, A.A., “Synthesis of some ferromagnetic composite resins and their metal removal characteristics in aqueous solutions”, J. Hazard. Mater.,150(3), 795-803 (2008).
25 Arslan, H., Hazer, B., “Ceric ion initiation of methyl methacrylate using polytetrahydrofuran diol and polycaprolactone diol”, Eur. Polym. J.,35(8), 1451-1455 (1999).
26 Ma, Z.Y., Guan, Y.P., Liu, H.Z., “Synthesis and characterization of micron-sized monodisperse superparamagnetic polymer particles with amino groups”, J. Polym. Sci. A Polym. Chem.,43(15),3433-3439 (2005).
27 Chen, C.H., Lee, W.C., “Preparation of methyl methacrylate and glycidyl methacrylate copolymerized nonporous particles”, J. Polym. Sci. A Polym. Chem.,37(10), 1457-1463 (1999).
28 Chen, M.Q., Serizawa, T., Kishida, A., Akashi, M., “Graft copolymers having hydrophobic backbone and hydrophilic branches. XXIII. Particle size control of poly(ethylene glycol)-coated polystyrene nanoparticles prepared by macromonomer method”, J. Polym. Sci. A Polym. Chem.,37(13), 2155-2166 (1999).
29 Tang, M., Cao, X.J., Liu, Z.Z., Wu, X.Y., Gance, D., “Synthesis of glycidyl methacrylate-based matrix and its application in affinity chromatography of urokinase”, Process Biochem.,34(8), 857-862 (1999).
30 Zurkova, E., Bouchal, K., Zdenkova, D., Pelzbauer, Z., Svec, F., Kalal, J., “Preparation of monodisperse reactive styrene-glycidyl methacrylate latexes by the emulsifier-free dispersion co-polymerization technique”, J. Polym. Sci. A Polym. Chem.,21(10), 2949-2960 (1983).
31 Horak, D., Shapoval, P., “Reactive poly(glycidyl methacrylate) microspheres prepared by dispersion polymerization”, J. Polym. Sci. A Polym. Chem.,38(21), 3855-3863 (2000).
32 Wojcik, G., Neagu, V., Bunia, I., “Sorption studies of chromium(VI) onto new ion exchanger with tertiary amine, quaternary ammonium and ketone groups”, J. Hazard. Mater.,190(1-3), 544-552 (2011).
33 Rivero, M.J., Primo, O., Ortiz, M.I., “Modelling of Cr(VI) removal from polluted groundwaters by ion exchange”, J. Chem. Technol. Biotechnol.,79(8), 822-829 (2004).
34 Neagu, V., Mikhalovsky, S., “Removal of hexavalent chromium by new quaternized crosslinked poly(4-vinylpyridines)”, J. Hazard. Mater.,183(1-3), 533-540 (2010).
35 Hu, J., Chen, G.H., Lo, I.M.C., “Removal and recovery of Cr(VI) from wastewater by maghemite nanoparticles”, Water Rese.,39(18), 4528-4536 (2005).
36 Park, H.J., Tavlarides, L.L., “Adsorption of chromium(VI) from aqueous solutions using an imidazole functionalized adsorbent”, Ind. Eng. Chem. Res.,47(10), 3401-3409 (2008).
37 Singh, K.K., Rastogi, R., Hasan, S.H., “Removal of Cr(VI) from wastewater using rice bran”, J. Colloid Interf. Sci.,290(1), 61-68 (2005).
38 Melo, J.S., D’Souza, S.F., “Removal of chromium by mucilaginous seeds of Ocimum basilicum”, Bioresource Technol.,92(2), 151-155 (2004).
39 Sarin, V., Pant, K.K., “Removal of chromium from industrial waste by using eucalyptus bark”, Bioresource Technol.,97(1), 15-20 (2006).
40 Dupont, L., Guillon, E., “Removal of hexavalent chromium with a lignocellulosic substrate extracted from wheat bran”, Environ. Sci. Technol.,37(18), 4235-4241 (2003).
41 Gupta, V.K., Shrivastava, A.K., Jain, N., “Biosorption of chromium(VI) from aqueous solutions by green algae Spirogyra species”, Water Res.,35(17), 4079-4085 (2001).
42 Arica, M.Y., Tuzun, I., Yalcin, E., Ince, O., Bayramoglu, G., “Utilisation of native, heat and acid-treated microalgae Chlamydomonas reinhardtii preparations for biosorption of Cr(VI) ions”, Process Biochem. istry,40(7), 2351-2358 (2005).
43 Hu, J., Lo, I.M.C., Chen, G.H., “Fast removal and recovery of Cr(VI) using surface-modified jacobsite (MnFe204) nanoparticles”, Langmuir,21(24), 11173-11179 (2005).
44 Janos, P., Hula, V., Bradnova, P., Pilarova, V., Sedlbauer, J., “Reduction and immobilization of hexavalent chromium with coal- and humate-based sorbents”, Chemosphere,75(6), 732-738 (2009).
45 Bai, S., Abraham, T. E., “Biosorption of Cr (VI) from aqueous solution by Rhizopus nigricans”, Bioresource Technol.,79(1), 73-81 (2001).
46 Chiarle, S., Ratto, M., Rovatti, M., “Mercury removal from water by ion exchange resins adsorption”, Water Res.,34(11), 2971-2978 (2000).
47 Gode, F., Pehlivan, E., “Removal of Cr(VI) from aqueous solution by two Lewatit-anion exchange resins”, J. Hazard. Mater.,119(1-3), 175-182 (2005).
2011-08-16, accepted 2011-10-10.
* Supported by the National Natural Science Foundation of China (21106162), the National Key Natural Science Foundation of China (21136009), the National High Technology Research and Development Program of China (2009CB219904), and the State Key Laboratory of Chemical Engineering (SKL-ChE-11A04).
** To whom correspondence should be addressed. E-mail: lryang@home.ipe.ac.cn; hzliu@home.ipe.ac.cn
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- The Research Progress of CO2Capture with Ionic Liquids*
- Simultaneous Removal of Thiophene and Dibenzothiophene by Immobilized Pseudomonas delafieldii R-8 cells*
- Polymer/Ceramic Composite Membranes and Their Application in Pervaporation Process
- Effects of Additives and Coagulant Temperature on Fabrication of High Performance PVDF/Pluronic F127 Blend Hollow Fiber Membranes via Nonsolvent Induced Phase Separation
- Solvothermal Synthesis and Optical Performance of One-dimensional Strontium Hydroxyapatite Nanorod*
- Ternary System of Fe-based Ionic Liquid, Ethanol and Water for Wet Flue Gas Desulfurization*
